Nasal equine herpesvirus type 1 (EHV-1) shedding is essential for virus transmission during outbreaks. Cell-associated viremia is a prerequisite for the most severe disease outcomes, abortion and equine herpesvirus myeloencephalopathy (EHM). Thus, protection from viremia is considered essential for preventing EHM. Ab4ΔORF2 vaccination prevented EHV-1 challenge virus replication in the upper respiratory tract in fully protected horses. Consequently, these neither shed virus nor developed cell-associated viremia. Protection from virus shedding and viremia during challenge infection in combination with reduced virulence at the time of vaccination emphasizes ORF2 deletion as a promising modification for generating an improved EHV-1 vaccine. During this challenge infection, full protection was linked to preexisting local and systemic EHV-1-specific antibodies combined with rapidly increasing intranasal IgG4/7 antibodies and lack of nasal type I interferon and chemokine induction. These host immune parameters may constitute markers of protection against EHV-1 and be utilized as indicators for improved vaccine development and informed vaccination strategies.
KEYWORDS: equine herpesvirus, immunization, infectious disease, neutralizing antibodies
ABSTRACT
Equine herpesvirus type 1 (EHV-1) outbreaks continue to occur despite widely used vaccination. Therefore, development of EHV-1 vaccines providing improved immunity and protection is ongoing. Here, an open reading frame 2 deletion mutant of the neuropathogenic EHV-1 strain Ab4 (Ab4ΔORF2) was tested as a vaccine candidate. Three groups of horses (n = 8 each) were infected intranasally with Ab4ΔORF2 or the parent Ab4 virus or were kept as noninfected controls. Horses infected with Ab4ΔORF2 had reduced fever and nasal virus shedding compared to those infected with Ab4 but mounted similar adaptive immunity dominated by antibody responses. Nine months after the initial infection, all horses were challenged intranasally with Ab4. Previously noninfected horses (control/Ab4) displayed clinical signs, shed large amounts of virus, and developed cell-associated viremia. In contrast, 5/8 or 3/8 horses previously infected with Ab4ΔORF2 or Ab4, respectively, were fully protected from challenge infection as indicated by the absence of fever, clinical disease, nasal virus shedding, and viremia. All of these outcomes were significantly reduced in the remaining, partially protected 3/8 (Ab4ΔORF2/Ab4) and 5/8 (Ab4/Ab4) horses. Protected horses had EHV-1-specific IgG4/7 antibodies prior to challenge infection, and intranasal antibodies increased rapidly postchallenge. Intranasal inflammatory markers were not detectable in protected horses but quickly increased in control/Ab4 horses during the first week after infection. Overall, our data suggest that preexisting nasal IgG4/7 antibodies neutralize EHV-1, prevent viral entry, and thereby protect from disease, viral shedding, and cell-associated viremia. In conclusion, improved protection from challenge infection emphasizes further evaluation of Ab4ΔORF2 as a vaccine candidate.
IMPORTANCE Nasal equine herpesvirus type 1 (EHV-1) shedding is essential for virus transmission during outbreaks. Cell-associated viremia is a prerequisite for the most severe disease outcomes, abortion and equine herpesvirus myeloencephalopathy (EHM). Thus, protection from viremia is considered essential for preventing EHM. Ab4ΔORF2 vaccination prevented EHV-1 challenge virus replication in the upper respiratory tract in fully protected horses. Consequently, these neither shed virus nor developed cell-associated viremia. Protection from virus shedding and viremia during challenge infection in combination with reduced virulence at the time of vaccination emphasizes ORF2 deletion as a promising modification for generating an improved EHV-1 vaccine. During this challenge infection, full protection was linked to preexisting local and systemic EHV-1-specific antibodies combined with rapidly increasing intranasal IgG4/7 antibodies and lack of nasal type I interferon and chemokine induction. These host immune parameters may constitute markers of protection against EHV-1 and be utilized as indicators for improved vaccine development and informed vaccination strategies.
INTRODUCTION
Equine herpesvirus type 1 (EHV-1) is prevalent in horse populations worldwide and has significant health, welfare, and economic impacts (1, 2). Current vaccines do not provide complete protection from EHV-1 infection and disease, especially against the neurologic manifestation, equine herpesvirus myeloencephalopathy (EHM) (3–5). Outbreaks of EHV-1 and EHM occur despite widely used vaccination (6, 7). Consequently, new vaccine candidates for protection from EHV-1 infection and disease have been studied (8–10), and various immune parameters have been explored in horses in an attempt to elucidate protective immunity against EHV-1.
Most reports on vaccination and protection against EHV-1 focused on systemic immunity (3–5, 10, 11). Commonly, serum neutralizing (SN) antibodies were used to evaluate EHV-1-specific host immune responses. Increases in SN titers were induced in response to most vaccines within 1 month after initial vaccination or boost (4, 12, 13). A reduction in severity of clinical signs and nasal shedding was commonly observed after vaccination (3, 5, 13–15). However, full protection from EHV-1-induced disease, including virus shedding and cell-associated viremia was only achieved in a few vaccinated horses, and EHV-1 SN antibodies or seroconversion did not predict protection in these trials (3, 14, 15).
More recent studies characterized the host immune mechanisms after EHV-1 infection or vaccination in more detail by differentiation of EHV-1-specific antibody isotypes and evaluation of antibody responses against different glycoprotein antigens of the virus (3–5, 16). These reports consistently identified EHV-1-specific, short-lived immunoglobulin G1 (IgG1) and longer-lasting IgG4/7 as major systemic antibody responses induced by different EHV-1 vaccines and vaccination regimes. In contrast, EHV-1 vaccination induced IgG3/5 or IgG6 inconsistently and to a lesser degree than the aforementioned IgG isotypes (3–5). IgG1 and IgG4/7 specific for EHV-1 envelope glycoproteins C and D (gC and gD, respectively) also dominated the host immune response after vaccination with an inactivated vaccine, and the glycoprotein-specific IgG4/7 antibodies correlated highly with EHV-1 SN titers (17). Experimental infection with EHV-1 strain Ab4 likewise induced serum antibody responses against gB, gC, and gD with IgG4/7 antibodies carrying the long-lasting adaptive immunity (18, 19).
In addition to antibody responses, EHV-1-specific T cells are considered important for protection from cell-associated viremia and EHM pathogenesis (1, 5, 20–23). The induction of EHV-1-specific interferon-γ (IFN-γ)-secreting T cells in the circulation was observed after vaccination and infection, typically occurring after antibody responses were detected, and T cell responses were often transient (5, 17, 18, 24–27). Nevertheless, repeated exposure to EHV-1 stimulated the expansion of T cells in some approaches, although T cell frequencies in the circulation remained low (5, 17, 26, 27).
Local immunity at the site of EHV-1 entry, the upper respiratory tract, is critical for early immune recognition, induction of adaptive immunity, and consequently, protection from disease (28) but has only been analyzed sporadically (12, 29, 30). Recently, we demonstrated the induction of EHV-1-specific IgG1 and IgG4/7 isotypes in nasal secretions after experimental EHV-1 Ab4 infections of susceptible horses, while only small amounts of EHV-1-specific IgG3/5, IgG6, and IgA were produced (18, 19). In addition, interferon-α (IFN-α) and inflammatory mediators, such as CC chemokine motif ligand 2 (CCL2) and soluble CD14 (sCD14), were rapidly upregulated at the viral entry site after EHV-1 infection, and IFN-α secretion highly correlated with nasal shedding of infectious virus (18, 19). Nevertheless, local immunity against EHV-1 is likely complex and has only been evaluated recently in protected horses. After intranasal challenge with Ab4, protection from clinical disease, virus shedding, and cell-associated viremia were highly correlated with preexisting and rapidly increasing intranasal EHV-1-specific IgG4/7 antibody amounts and with the absence of nasal IFN-α and inflammatory mediator induction. These observations suggested neutralization of EHV-1 by antibodies in the upper respiratory tract (31).
Due to the medical and economic impact of EHV-1, vaccine development for EHV-1 is ongoing. Different modified live virus (MLV) vaccines were explored, because these mimic EHV-1 infection more closely and were expected to provide improved protection against EHV-1 compared to inactivated vaccines (3, 4, 32). Many MLV vaccines are based on attenuated EHV-1 strains to improve safety. Attenuations often involved loss of genes that constituted virulence factors of EHV-1. For example, the MLV vaccine strain RacH is devoid of open reading frame 1 (ORF1), ORF2, and ORF67, and in addition, other genes are mutated relative to virulent viruses. The RacH strain is used in the EHV-1 MLVs commercially available in the United States and Europe (Rhinomune [Boehringer Ingelheim] and Prevaccinol [MSD Tiergesundheit], respectively). In experimental infection studies of horses, deletions of ORF1/2, ORF1/71, or ORF2 of the neuropathogenic EHV-1 strain Ab4 led to reduction of clinical disease and viral shedding, confirming ORF1 and ORF2 as virulence factors (18, 19, 33). The current EHV-1 strain Ab4 contains intact genes of most known virulence factors, in contrast to the attenuated MLV strain RacH (34).
Here, we further explored an ORF2 deletion mutant of Ab4 (Ab4ΔORF2) as a vaccine candidate for EHV-1, focusing on the protective effects of this single gene deletion. Nine months prior to this study, horses were experimentally infected with the vaccine candidate Ab4ΔORF2 or the parent Ab4 virus (19). Infection with Ab4ΔORF2 resulted in reduced virulence evident by reduction of fever and virus shedding compared to Ab4. EHV-1-specific antibodies in serum and nasal secretions were similarly induced by infection with Ab4ΔORF2 or Ab4. While EHV-1-specific antibodies remained high for several months after infection, neither Ab4ΔORF2 nor Ab4 induced substantial amounts of EHV-1-specific T cells in peripheral blood of the horses (19). Reduced virulence in combination with high immunogenicity suggested that Ab4ΔORF2 could be a promising vaccine candidate.
In the present study, all horses enrolled in our earlier report (19) were challenged with neuropathogenic EHV-1 Ab4 to further evaluate the vaccine candidate Ab4ΔORF2 and to characterize preexisting immunity correlating with protection against EHV-1 and host immune markers that can distinguish protected horses from those that are susceptible to EHV-1 infection.
RESULTS
Previous infection with Ab4ΔORF2 protects from clinical disease, virus shedding, and cell-associated viremia after challenge with EHV-1 Ab4.
A total of 24 adult Icelandic horses were challenged by intranasal infection with the neuropathogenic EHV-1 strain Ab4. Nine months prior to the challenge infection performed here, the horses were infected intranasally with the vaccine candidate Ab4ΔORF2 or the parent Ab4 virus or served as noninfected controls (n = 8 per group). At the time of initial infection, horses in the two virus-infected groups were fully susceptible to EHV-1 infection, while the control group did not show any signs of infection, as previously described (19) and summarized in Table S1 in the Supplemental Material. This resulted in three experimental challenge groups: (i) Ab4ΔORF2/Ab4, (ii) Ab4/Ab4, and (iii) control/Ab4.
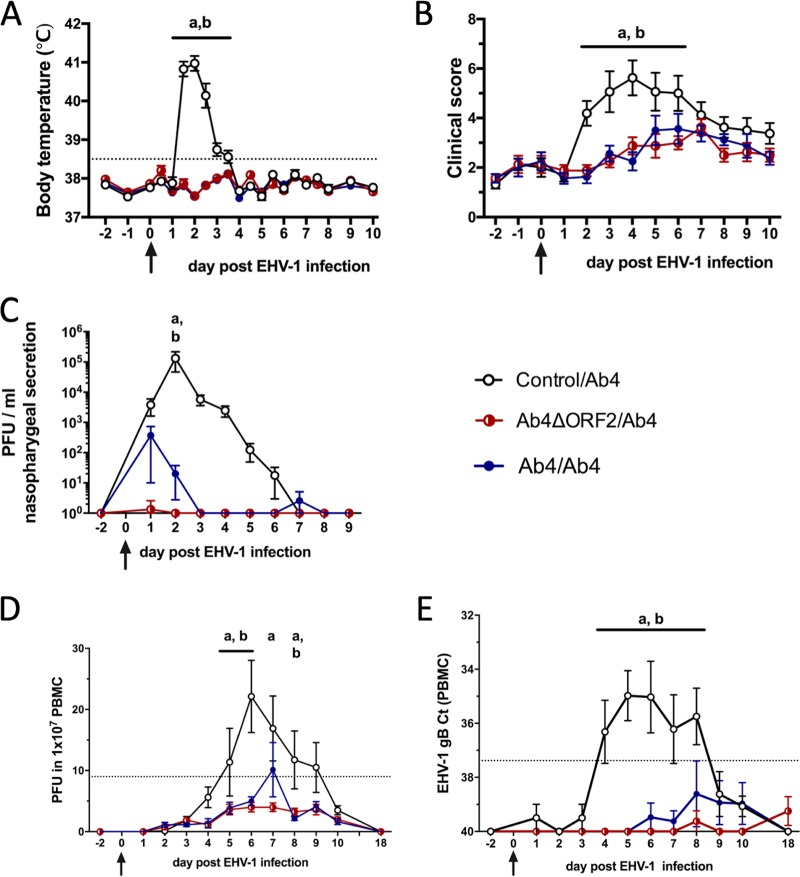
Intranasal Ab4 challenge infection of horses in the control/Ab4 group induced characteristic signs of respiratory EHV-1 infection with a high fever at 36 to 84 hours postinfection (p.i.) (Fig. 1A) and increased respiratory signs within the first week p.i. as indicated by clinical scores (Fig. 1B). One horse in the control/Ab4 group also exhibited mild hind limb ataxia on days 4 and 5 p.i. (neurologic score 2/5) without loss of tail or anal tone. Signs of EHM resolved in this horse without treatment by day 6 p.i. All horses in the control/Ab4 group shed infectious virus peaking on day 2 p.i. in their nasal secretions as detected by virus isolation (Fig. 1C). Cell-associated viremia was detected in peripheral blood mononuclear cells (PBMC) from infected horses in the control/Ab4 group between days 5 and 9 p.i. or days 4 and 8 p.i. by virus isolation (Fig. 1D) or real-time PCR (Fig. 1E), respectively.
FIG 1.
Clinical disease and virus detection after EHV-1 challenge infection. A total of 24 horses were challenged intranasally with 1 × 107 PFU neuropathogenic EHV-1 Ab4 on day 0 (arrows). Nine months prior to challenge, these horses were initially infected intranasally with Ab4ΔORF2 (n = 8) or Ab4 (n = 8) or kept as controls without infection (n = 8). (A) Body temperature; (B) clinical score (range, 0 to 22) calculated as the sum of numerical scores for nasal discharge, ocular discharge, lymph node enlargement, ataxia, depression, and reduced appetite; (C) virus shedding (PFU/ml) in nasal secretions detected by virus isolation; (D) viremia detected by virus isolation in PBMC; (E) viremia detected by real-time PCR and shown as cycle threshold (CT) values. Mean and standard errors are plotted for each group. Dotted horizontal lines represent a cutoff of fever at 38.5°C (A), EHV-1 detection at 9 PFU (D), or 37.38 CT (E). Significant differences between groups are marked as a (control/Ab4 versus Ab4ΔORF2/Ab4) and b (control/Ab4 versus Ab4/Ab4).
After Ab4 challenge, body temperatures (Fig. 1A) and clinical scores (Fig. 1B) did not change in the Ab4ΔORF2/Ab4 and Ab4/Ab4 groups. Nasal EHV-1 shedding was markedly reduced in horses in the previously infected groups, with the highest virus amounts detected on day 1 p.i. (Fig. 1C). In fact, 7/8 horses in the Ab4ΔORF2/Ab4 and 5/8 in the Ab4/Ab4 group did not shed any infectious virus in their nasal secretions (Table 1). Likewise, previous infection protected most horses from cell-associated viremia after EHV-1 challenge (Fig. 1D and E).
TABLE 1.
Protection of horses based on body temperature, nasal shedding of infectious virus, and cell-associated viremia after challenge infection with EHV-1a
| Horse information |
Maximum feverb |
Maximum sheddingc |
Maximum viremiad |
|||||
|---|---|---|---|---|---|---|---|---|
| EHV-1 protection status | ID | Group | °C | Hours p.i. | PFU/ml | Day p.i. | CTe | Day p.i. |
| Fully protected | 1 | Ab4ORF2/Ab4 | No fever | NA | No shedding | NA | No viremia | NA |
| 8 | Ab4ORF2/Ab4 | No fever | NA | No shedding | NA | No viremia | NA | |
| 14 | Ab4ORF2/Ab4 | No fever | NA | No shedding | NA | No viremia | NA | |
| 18 | Ab4ORF2/Ab4 | No fever | NA | No shedding | NA | No viremia | NA | |
| 23 | Ab4ORF2/Ab4 | No fever | NA | No shedding | NA | No viremia | NA | |
| 6 | Ab4/Ab4 | No fever | NA | No shedding | NA | No viremia | NA | |
| 11 | Ab4/Ab4 | No fever | NA | No shedding | NA | No viremia | NA | |
| 24 | Ab4/Ab4 | No fever | NA | No shedding | NA | No viremia | NA | |
| Partially protected | 2 | Ab4ORF2/Ab4 | No fever | NA | No shedding | NA | 36.96 | 8 |
| 12 | Ab4ORF2/Ab4 | 38.9 | NA | No shedding | NA | No viremia | NA | |
| 17 | Ab4ORF2/Ab4 | No fever | NA | 10 | 1 | No viremia | NA | |
| 4 | Ab4/Ab4 | No fever | NA | 30 | 1 | 35.82 | 6 | |
| 10 | Ab4/Ab4 | No fever | NA | No shedding | NA | 36.96 | 7 | |
| 13 | Ab4/Ab4 | No fever | NA | 140 | 2 | No viremia | NA | |
| 16 | Ab4/Ab4 | No fever | NA | 2,900 | 1 | No viremia | NA | |
| 21 | Ab4/Ab4 | No fever | NA | No shedding | NA | 30.20 | 8 | |
| Susceptible | 3 | Control/Ab4 | 41.2 | 36 | 19,500 | 3 | 31.25 | 6 |
| 5 | Control/Ab4 | 41.4 | 48 | 700,000 | 2 | 30.43 | 4 | |
| 7 | Control/Ab4 | 41.1 | 60 | 29,500 | 2 | 30.13 | 6 | |
| 9 | Control/Ab4 | 40.9 | 48 | 8,000 | 2 | 35.43 | 8 | |
| 15 | Control/Ab4 | 41.6 | 48 | 240,000 | 2 | 33.71 | 5 | |
| 19 | Control/Ab4 | 41.3 | 36 | 7,550 | 3 | 32.59 | 6 | |
| 20 | Control/Ab4 | 41.4 | 48 | 52,500 | 2 | 31.32 | 7 | |
| 22 | Control/Ab4 | 41.2 | 36 | 28,000 | 2 | 32.89 | 8 | |
NA, not applicable.
Body temperature >38.5°C considered a fever.
Virus isolation in nasal secretion (PFU/ml).
Detection by real-time PCR of EHV-1 gB in PBMC.
CT, cycle threshold.
Overall, 5/8 horses in the Ab4ΔORF2/Ab4 and 3/8 in the Ab4/Ab4 groups did not develop any fever, clinical disease, nasal EHV-1 shedding, and cell-associated viremia after challenge infection with EHV-1 Ab4 (Table 1) and were thus fully protected from EHV-1 infection. The remaining 3/8 (Ab4ΔORF2/Ab4) and 5/8 (Ab4/Ab4) horses only developed a mild fever or shed small amounts of EHV-1 in their nasal secretions and/or developed mild viremia for 1 to 3 days. These 8 horses were considered partially protected. None of the 16 horses in the Ab4ΔORF2/Ab4 and Ab4/Ab4 groups showed any depression or signs of respiratory or neurologic disease. Horses in these two groups are referred to as protected groups from here on.
Inflammatory mediator secretion in the upper respiratory tract in response to EHV-1 infection.
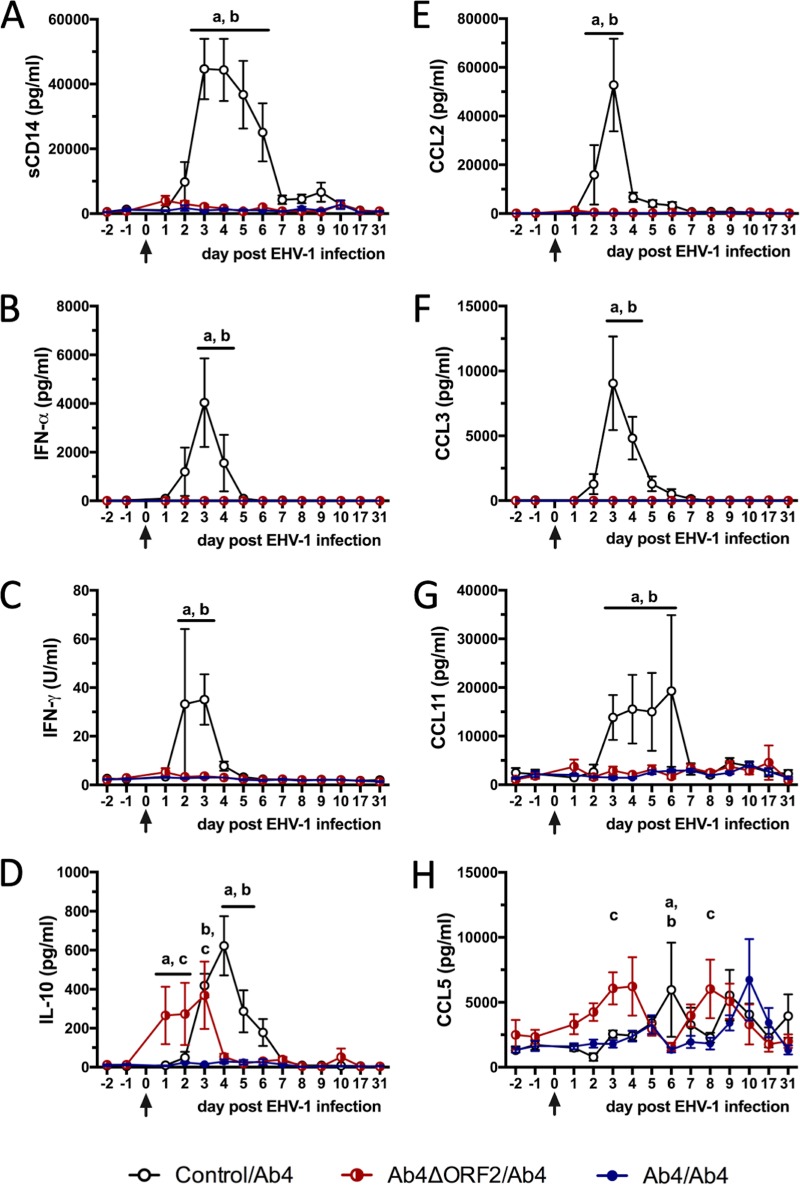
EHV-1 infection of susceptible horses in the control/Ab4 group resulted in the rapid increase of a number of inflammatory cytokines and chemokines in nasal secretions (Fig. 2). These included sCD14 with highest concentrations on days 3 to 4 p.i. (Fig. 2A), IFN-α (Fig. 2B) and IFN-γ which peaked on day 3 p.i. (Fig. 2C), and also anti-inflammatory interleukin-10 (IL-10) with a peak on day 4 p.i. (Fig. 2D). IL-4 and IL-17 were not secreted into nasal secretions in response to EHV-1 infection (data not shown). In addition, CC motif chemokine ligands (CCL) were detected in nasal secretions of the control/Ab4 group. CCL2 and CCL3 were undetectable prior to infection; they were first detected on day 2 p.i., reached highest concentrations on day 3 p.i., and returned to baseline thereafter (Fig. 2E and F). CCL11 was detectable in very low concentrations before infection and was increased on days 3 to 6 p.i. On day 7 p.i. and afterward, CCL11 concentrations returned to baseline (Fig. 2G). In contrast, CCL5 was present in low concentrations in nasal secretions before infection and displayed wave-like increases with highest concentrations on days 6 and 9 p.i. in the control/Ab4 group (Fig. 2H).
FIG 2.
Inflammatory mediators in nasal secretions after EHV-1 challenge infection. Three groups of horses (n = 8/group) were challenged intranasally with neuropathogenic EHV-1 Ab4 (arrows). Control/Ab4 horses were susceptible at the time of challenge, while horses in the previously infected Ab4ΔORF2/Ab4 and Ab4/Ab4 groups were protected from clinical disease. Cytokines and chemokines were quantified in the nasal secretions of all horses using fluorescent bead-based multiplex assays detecting sCD14 (A), IFN-α (B), IFN-γ (C), IL-10 (D), CCL2 (E), CCL3 (F), CCL11 (G), and CCL5 (H). Mean and standard errors are shown for each group. Significant differences between groups are marked as a (control/Ab4 versus Ab4ΔORF2/Ab4), b (control/Ab4 versus Ab4/Ab4), and c (Ab4ΔORF2/Ab4 versus Ab4/Ab4).
In the Ab4ΔORF2/Ab4 and Ab4/Ab4 groups, challenge infection did not induce upregulation of intranasal sCD14, IFN-α, IFN-γ, CCL2, CCL3, or CCL11. Horses in the Ab4ΔORF2/Ab4 group displayed an early increase of nasal IL-10 secretion between days 1 and 3 p.i., which was higher than in the other two groups on days 1 to 2 p.i. (P < 0.05; Fig. 2D). Horses in the Ab4ΔORF2/Ab4 group also upregulated intranasal CCL5 by day 3 p.i. and again on day 8 p.i., which resulted in higher CCL5 concentrations than in the Ab4/Ab4 group on both days (P < 0.05; Fig. 2H). Horses in the Ab4/Ab4 group did not display an increase of IL-10 in their nasal secretions, and CCL5 concentrations were only increased on day 10 p.i. compared to preinfection but were still similar to those in nasal secretions of the other groups.
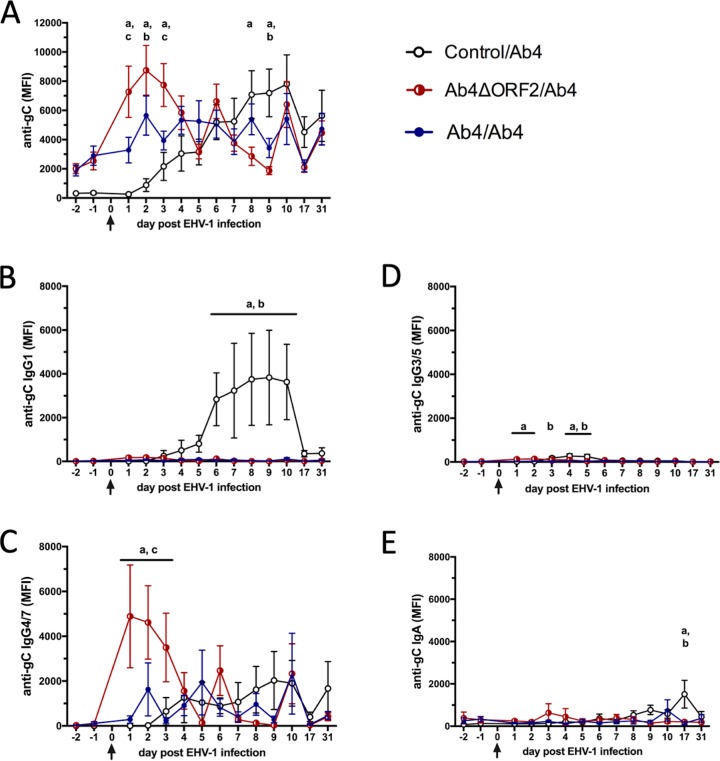
Intranasal immunity in protected horses is characterized by immediate increases of EHV-1-specific IgG4/7 antibodies.
An EHV-1-specific multiplex assay was used to measure antibody responses to the envelope glycoproteins gB, gC, and gD of EHV-1. Antibody responses against all three glycoprotein targets were similar throughout the study period, and thus, only anti-gC results are shown here. EHV-1-susceptible horses in the control/Ab4 group did not have EHV-1-specific antibodies in their nasal secretions prior to challenge infection with Ab4. After EHV-1 infection, antibodies increased between days 2 and 10 p.i., decreased afterward, and were still detected at day 31 p.i. (Fig. 3A). The early increase of intranasal EHV-1-specific antibodies consisted predominantly of IgG1 (Fig. 3B) and IgG4/7 (Fig. 3C). IgG3/5 was increased early at small amounts following Ab4 challenge (Fig. 3D). After day 10 p.i., EHV-1-specific IgG4/7 antibodies were maintained in the nasal secretions of the control/Ab4 group (Fig. 3C). Nasal EHV-1-specific IgA was induced in small amounts and increased late, and only in the control horses (Fig. 3E).
FIG 3.
Anti-EHV-1 gC antibodies in nasal secretions before and after EHV-1 challenge infection. Three groups of horses (n = 8/group) were challenged intranasally with 1 × 107 PFU of the neuropathogenic EHV-1 Ab4 on day 0 (arrows). EHV-1 gC-specific antibodies were quantified in nasal secretions by fluorescent bead-based multiplex assays. Anti-gC total Ig (A), IgG1 (B), IgG4/7 (C), IgG3/5 (D), and IgA (E) are shown as median fluorescent intensities (MFI). Mean and standard errors are plotted for each group. Significant differences between groups are marked as a (control/Ab4 versus Ab4ΔORF2/Ab4), b (control/Ab4 versus Ab4/Ab4), and c (Ab4ΔORF2/Ab4 versus Ab4/Ab4).
In contrast to the control/Ab4 group, protected horses (Ab4ΔORF2/Ab4 and Ab4/Ab4) had preexisting intranasal EHV-1 gC-specific antibodies with a mean of 1,500 to 2,000 median fluorescent intensities (MFI) prior to Ab4 challenge infection (Fig. 3A). After the Ab4 challenge infection, EHV-1-specific antibodies increased rapidly in the nasal secretions of both protected groups. Within 1 day of infection, the Ab4ΔORF2/Ab4 group had a 3- to 4-fold MFI increase in anti-gC total Ig and a 40- to 100-fold MFI increase in IgG4/7 antibodies (Fig. 3C). Intranasal EHV-1-specific Ig and IgG4/7 antibodies in the Ab4ΔORF2/Ab4 group were significantly greater than in the control/Ab4 group on days 1 to 3 p.i. (P < 0.01) and were also higher than in the Ab4/Ab4 group on almost all of these days (P < 0.05) (Fig. 3A and C). Afterward, intranasal EHV-1 anti-gC Ig and IgG4/7 antibody values in the Ab4ΔORF2/Ab4 group decreased until day 5 p.i. and then fluctuated around 3,000 and 1,000 MFI, respectively, until the end of the study (Fig. 3A and C). The Ab4/Ab4 group had a less pronounced increase in EHV-1 anti-gC antibody values. These also consisted of IgG4/7, were different from control horses on day 2 p.i. only (P < 0.05), and were similar to the Ab4ΔORF2/Ab4 group from day 4 p.i. on (Fig. 3A and C). Intranasal EHV-1-specific IgG1, IgG3/5, and IgA antibodies were present in the nasal secretions of the Ab4ΔORF2/Ab4 and Ab4/Ab4 groups in small amounts without major increases after challenge infection (Fig. 3B, D, and E).
Preexisting nasal anti-gC total Ig and IgG4/7 antibodies correlated with protection from fever, clinical disease, nasal EHV-1 shedding, and cell-associated viremia (Table 2).
TABLE 2.
Spearman-rank correlations of preexisting (day –2) anti-gC Ig and IgG4/7 antibodies in nasal secretion and serum with protection from EHV-1 infection outcomes
| Parameter | Nasal anti-gC Ig | Nasal anti-gC IgG4/7 | Serum anti-gC Ig | Serum anti-gC IgG4/7 |
|---|---|---|---|---|
| Fevera | R = –0.622 | R = –0.539 | R = –0.747 | R = –0.767 |
| P = 0.0012 | P = 0.0065 | P < 0.0001 | P < 0.0001 | |
| Clinical diseaseb | R = –0.443 | R = –0.404 | R = –0.482 | R = –0.465 |
| P = 0.0304 | P = 0.0503 | P = 0.0172 | P = 0.0222 | |
| Virus sheddingc | R = –0.806 | R = –0.748 | R = –0.816 | R = –0.805 |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | |
| Cell-associated viremiad | R = 0.670 | R = 0.607 | R = 0.759 | R = 0.758 |
| P = 0.0001 | P = 0.0017 | P < 0.0001 | P < 0.0001 |
Body temperature at 60 h p.i. (fever peak).
Clinical score on day 4 p.i.
Virus isolation in nasal secretion (PFU) on day 2 p.i.
EHV-1 real-time PCR for the gB gene (cycle threshold [CT]) in PBMC on day 5 pi.
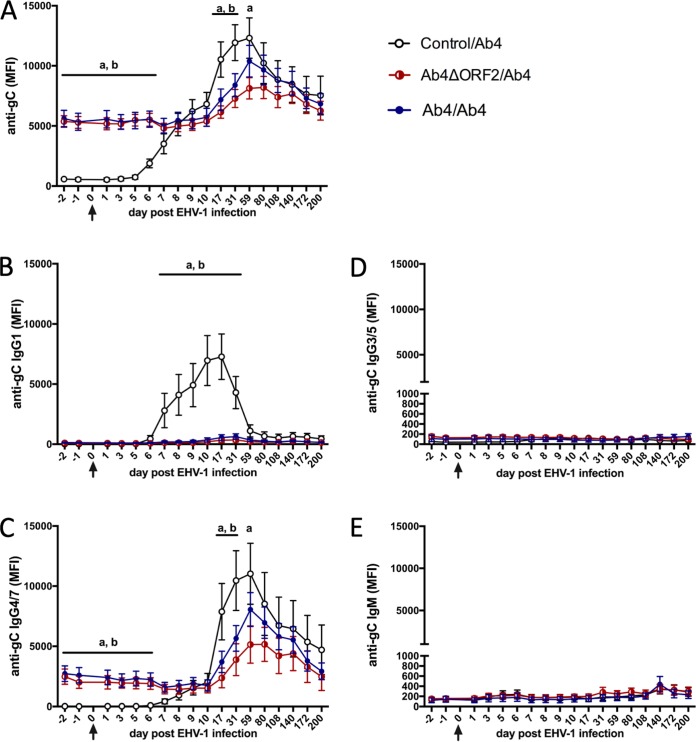
Preexisting IgG4/7 antibodies in serum correlate with protection from EHV-1 challenge.
Horses in the Ab4ΔORF2/Ab4 and Ab4/Ab4 groups had significantly higher serum EHV-1-specific antibodies consisting of IgG4/7 (Fig. 4A and C) than control horses (control/Ab4) before Ab4 challenge infection (P < 0.05). Both preexisting anti-gC total Ig and IgG4/7 antibodies correlated highly with protection from fever, clinical disease, nasal EHV-1 shedding, and cell-associated viremia (Table 2). After Ab4 challenge infection, EHV-1-specific total Ig and IgG4/7 values remained unchanged for the first 10 days p.i., then increased between days 17 and 59 p.i., and declined slowly until day 200 p.i. (Fig. 4A and C). After day 59 p.i., the two protected groups had similar EHV-specific serum Ig and IgG4/7 amounts as the control/Ab4 group. Anti-gC IgG1 was not majorly elevated in the two protected groups (Fig. 4A). EHV-1-specific IgG3/5 (Fig. 4D) and IgM (Fig. 4E) were present in serum at small amounts and similar in all groups.
FIG 4.
Anti-EHV-1 gC antibodies in serum before and after Ab4 challenge infection in EHV-1-susceptible and -protected horses. A total of 24 horses were challenged intranasally with neuropathogenic EHV-1 Ab4 (arrows). Horses in control/Ab4 were fully susceptible to EHV-1 infection. Horses in the previously infected Ab4ΔORF2/Ab4 and Ab4/Ab4 group were protected from disease. EHV-1 gC-specific antibodies were quantified in serum by fluorescent bead-based multiplex assays. Total Ig (A), IgG1 (B), IgG4/7 (C), IgG3/5 (D), and IgM (E) were measured and are shown as median fluorescent intensity (MFI). Mean and standard errors are plotted for each group. Significant differences between groups are marked as a (control/Ab4 versus Ab4ΔORF2/Ab4) and b (control/Ab4 versus Ab4/Ab4).
In contrast, horses in the susceptible control/Ab4 group did not have detectable EHV-1-specific antibodies in serum prior to EHV-1 challenge infection. After challenge, these horses seroconverted with EHV-1-specific Ig detected in serum starting on day 6 p.i. The highest anti-gC antibodies were reached on day 59 p.i. and then declined until the end of the study on day 200 p.i. (Fig. 4A). Initially, large amounts of EHV-1-specific IgG1 were induced in the control/Ab4 group (Fig. 4B), starting on day 7 p.i. and detectable until day 59 p.i. EHV-1-specific IgG4/7 was first detected on day 8 p.i., mimicked overall anti-gC total Ig, and accounted for the long-lasting antibody responses until day 200 p.i., which was similar to the findings in protected horses described above (Fig. 4C). The amounts of EHV-1-specific Ig and IgG4/7 antibodies in the serum of the control/Ab4 group exceeded those in serum of previously infected horses on days 17 to 59 p.i. (Ab4ΔORF2/Ab4, P < 0.01) or days 17 to 31 p.i. (Ab4/Ab4, P < 0.05).
Cellular immune responses to EHV-1 challenge infection.
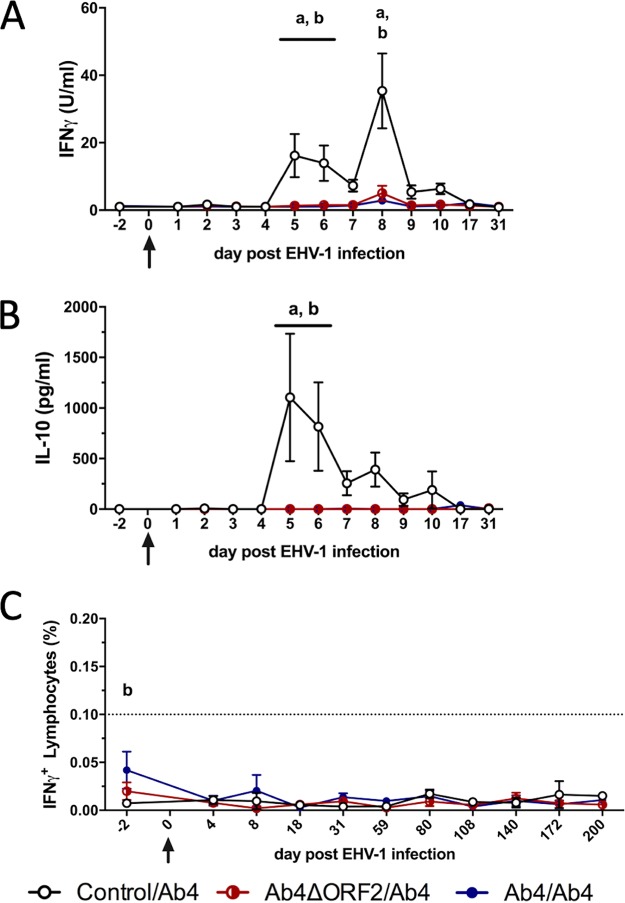
Cytokine secretion and EHV-1-specific T cells were evaluated using EHV-1 restimulation of PBMC in vitro. PBMC from horses in the control/Ab4 group secreted IFN-γ and IL-10 starting on day 5 p.i. (Fig. 5A and B), simultaneously with the onset of viremia (Fig. 1D and E). IFN-γ secretion in the control/Ab4 group was higher than that from previously infected horses in the Ab4ΔORF2/Ab4 and Ab4/Ab4 groups on days 5, 6, and 8 p.i. (P < 0.001, Fig. 5A). IL-10 secretion peaked on days 5 and 6 p.i. (Fig. 5B) when it was higher than in horses of the other two groups (P < 0.001). In contrast, IFN-γ and IL-10 secretion from restimulated PBMC was not induced or was very low in the Ab4ΔORF2/Ab4 and Ab4/Ab4 groups (Fig. 5A and B). Horses in these protected groups also showed significantly reduced cell-associated viremia (Fig. 1D and E) or did not develop viremia at all (Table 1). IFN-α secretion from PBMC was induced by restimulation of PBMC at all time points and was similar in all three groups (data not shown). IL-4 or IL-17 secretion was not detected in any of the groups (data not shown).
FIG 5.
Cellular immune responses to EHV-1 infection. Three groups of horses (n = 8/group) challenged intranasally with neuropathogenic EHV-1 Ab4 (day 0) 9 months after being noninfected controls (control/Ab4), after primary intranasal infection with Ab4 devoid of open reading frame 2 (Ab4ΔORF2/Ab4), or with Ab4 (Ab4/Ab4). PBMC were isolated and restimulated in vitro with EHV-1 (Ab4). Cytokine production (A and B) was analyzed in supernatants using a fluorescent bead-based multiplex assay. Secreted IFN-γ (A) and IL-10 (B) are shown. EHV-1-stimulated PBMC were also fixed, stained for intracellular IFN-γ, and analyzed using flow cytometry. Total EHV-1-specific IFN-γ-expressing lymphocytes are displayed (C). The dotted horizontal line (C) represents a cutoff of 0.1% IFN-γ+ lymphocytes. PBMC controls kept in medium alone typically result in values below this cutoff value. All values (A to C) are cell culture medium control corrected. Mean and standard errors are plotted. Significant differences between groups are marked as a (control/Ab4 versus Ab4ΔORF2/Ab4) and b (control/Ab4 versus Ab4/Ab4).
Adaptive EHV-1-specific T cell responses were analyzed by flow cytometric detection of IFN-γ in peripheral lymphocytes. PBMC of most horses contained less than 0.05% IFN-γ-producing lymphocytes in response to EHV-1 in vitro throughout the study (Fig. 5C). Differences in peripheral EHV-1-specific T cell percentages were not observed between the three groups, with the exception of higher numbers of preexisting EHV-1-specific T cells in the Ab4/Ab4 group in comparison to the control/Ab4 group on day 2 before challenge infection (P < 0.01).
DISCUSSION
In this report, we confirmed protection against EHV-1 challenge 9 months after intranasal infection with the vaccine candidate Ab4ΔORF2 and characterized key parameters of local and systemic immunity in EHV-1-susceptible and -protected horses.
Horses previously infected intranasally with Ab4ΔORF2 or the neuropathogenic parent strain Ab4 were all protected from clinical disease when challenged with Ab4. In addition, fully protected horses did not develop any fever, nasal virus shedding, or viremia after challenge. Partially protected horses showed one or two of these EHV-1 infection parameters at significantly lower levels and shorter duration than EHV-1-susceptible horses. In contrast, the magnitude and duration of fever, clinical signs, virus shedding, and cell-associated viremia in the control/Ab4 group in the present study resembled those of susceptible horses described previously (18, 19, 35). Most horses in the Ab4ΔORF2/Ab4 group (5/8) and some in the Ab4/Ab4 group (3/8) were fully protected against Ab4 challenge, suggesting that the ability of the Ab4ΔORF2 deletion mutant virus to induce protective immunity is at least as good as protection provided by infection with the virulent Ab4 parent virus. During initial infection, the Ab4ΔORF2 vaccine candidate displayed significantly reduced virulence as indicated by reduced initial fever and low nasal shedding in horses infected with the deletion mutant virus (19). Overall, these characteristics of attenuation, reduced virulence, and immunogenicity in combination with the ability to generate robust protective immunity indicate that Ab4ΔORF2 is a promising vaccine candidate and warrants further examination. For example, Ab4ΔORF2 could be tested for systemic inoculation to explore its potential of induction of immunity and protection while circumventing nasal replication of the virus. If used as an intranasal vaccine, future modifications of the Ab4ΔORF2 vaccine candidate are needed for improving its safety to obtain an MLV vaccine that does not provoke nasal shedding or result in cell-associated viremia after the initial vaccine administration.
Immunity after EHV-1 vaccination is considered short-lasting (1). Challenge infections for several previously tested EHV-1 vaccines or vaccine candidates were performed after 3 months or less (3, 4, 8, 12–14, 36), and EHV-1 vaccination recommendations were adjusted accordingly. For example, the American Association of Equine Practitioners recommends vaccination against EHV-1 every 6 months (37). Here, we challenged horses with EHV-1 9 months after initial intranasal infection with either Ab4 or the Ab4ΔORF2 vaccine candidate. We found that Ab4ΔORF2 provided similar or better protection than the parent Ab4 strain and longer-lasting immunity than previously tested vaccines. However, most EHV vaccines are administered intramuscularly, in contrast to the intranasal application of Ab4ΔORF2 prior to our challenge. Intranasal application of Ab4ΔORF2 contributed to local immune development at the virus entry site (19) and likely extended the duration of immunity and protection observed here. Similarly, increased protective immunity after challenge was reported previously after intranasal application of other EHV-1 vaccines or vaccine candidates (15, 38). However, protection from viremia by the latter candidates was not achieved (15) or not verified (38).
At the time of the Ab4 challenge, EHV-1-specific intranasal antibodies were still detectable in the upper respiratory tract, and large amounts of serum IgG4/7 antibodies were found in the circulation of protected horses. These preexisting antibodies originated from the prior infection with either Ab4ΔORF2 or Ab4 (19), suggesting the induction of EHV-1-specific long-lived plasma cells that were continuously secreting antibodies in both groups. At the time of Ab4 challenge, some of these plasma cells may have homed and resided in the upper respiratory tissues, contributing to local antibody levels in nasal secretions of protected horses. Alternatively, the serum EHV-1-specific IgG4/7 pool may provide a constant source of antibodies that are transported through the respiratory mucosa into the nasal secretion.
Mucosal antibodies are considered essential for neutralization of respiratory viruses (38, 39). Here, preexisting intranasal EHV-1-specific IgG4/7 antibodies likely neutralized EHV-1 immediately after intranasal challenge and thereby prevented infection of respiratory epithelial cells and all downstream effects of EHV-1 infection, including cell-associated viremia in fully protected horses. The inhibition of viral entry into respiratory epithelial cells in fully protected horses was further supported by the complete absence of detectable infectious virus in their nasal secretions 24 h p.i. and afterward. It was also supported by the lack of antiviral IFN-α and inflammatory mediator upregulation at the local infection site in the protected groups. These intranasal immune markers provide danger signals in response to EHV-1 entry and promote host immune induction in susceptible horses (18, 19). If viral entry is prevented by preexisting neutralizing intranasal antibodies, innate immune induction signals related to cellular infection are not induced. IFN-α, inflammatory cytokines, and chemokines are consequently not secreted in horses that are fully protected against EHV-1 as observed here and in a recent report by Perkins et al. (31). Although the lack of EHV-1 entry in fully protected horses still needs to be experimentally confirmed and directly shown, the evidence from this and the former study (31) strongly suggest a robust potential of intranasal IgG4/7 antibodies to effectively neutralize EHV-1 at the viral entry site and to prevent all outcomes of infection.
After Ab4 challenge infection of susceptible horses, intranasal EHV-1-specific antibody production started within a few days, increased slowly, and peaked by day 10 p.i., which was similar to previously reported local antibody induction by EHV-1 infection (18, 19). In contrast, challenge infection of the protected groups resulted in an immediate increase of EHV-1-specific antibodies in the upper respiratory tract. This suggested that neutralized EHV-1, despite not entering local epithelial cells, was still immunogenic and stimulated local EHV-1-specific memory B cells at the infection site to differentiate into new antibody-producing plasma blasts. Alternatively, and as mentioned above, the rapid increase in intranasal EHV-1-specific antibodies in protected horses possibly originated from an active transport of preexisting circulating antibodies from serum. During the first 3 days postchallenge, intranasal EHV-1-specific IgG4/7 responses in the Ab4ΔORF2/Ab4 group were also higher than in the Ab4/Ab4 group, suggesting superior local immune stimulation in horses previously infected with Ab4ΔORF2.
Overall, our data support the notion that preexisting intranasal EHV-1-specific IgG4/7 antibodies together with rapidly increasing local IgG4/7 responses after EHV-1 challenge effectively prevent viral entry into cells of the respiratory epithelium. Most likely, blocking viral entry prevented viral replication in the upper respiratory tract and virus shedding. These protective properties emphasize the value of Ab4ΔORF2 as a vaccine candidate that may be able to improve herd immunity. With increased numbers of fully protected horses in the population, virus transmission from horse to horse should become limited by the lack of susceptible hosts available. Maximizing the number of fully protected horses in the horse population is thus invaluable for improved immunity to convey prevention from EHV-1 infection and also for containment of EHV-1 during EHM outbreaks (28, 40, 41).
Cell-mediated immunity and EHV-1-specific cytotoxic T cells are considered important for protection from EHM and abortion by controlling cell-associated viremia (1, 28, 42). Although systemic cellular immunity to EHV-1 did not fully protect horses from experimental infection, it predicted protection from EHM in one study (23) or EHV-1-induced abortion in another (21). Allen et al. (23) also showed a correlation of high cytotoxic T lymphocyte precursor numbers with a low-magnitude EHV-1 viremia. More recent EHV-1 infection studies of naive or susceptible horses indicated that peripheral EHV-1-specific T cell responses overall are low and of slow onset (19, 43), similar to the low peripheral T cell numbers induced here. Nevertheless, EHV-1-specific Th1 cells were shown to support sustained IgG4/7 antibody production despite low numbers of these cells being detected in peripheral blood (17). This suggests that EHV-1-specific T cells reside mostly in lymphatic tissue and, even in fully protected horses, they occur in low frequencies in peripheral blood. Peripheral EHV-1-specific T cells are thus difficult to detect and cannot be considered a reliable marker of host immunity and protection.
Viremia is essential for infection of vascular endothelial cells in the spinal cord. Vascular endothelial cell infection causes subsequent entry of EHV-1 into the central nervous system, neuronal damage, and development of EHM (1, 23, 44, 45). Our present study showed complete protection from EHV-1 infection in horses with low peripheral EHV-1-specific T cell frequencies despite previous infection and effective immunity against EHV-1. However, cellular immune responses may be critical to control EHV-1 infected cells, viremia, and severe clinical manifestations once the virus has established cell-associated viremia. In contrast, antibodies at the local infection site neutralized EHV-1, prevented infection of the respiratory epithelium, and thereby effectively prevented or minimized viremia in the two protected groups. It can be concluded that intranasal EHV-1-specific IgG4/7 antibodies, which protect against viral entry and/or virus replication and, consequently, viremia, may also protect horses against EHM.
CONCLUSIONS
Intranasal infection with Ab4ΔORF2 or Ab4 9 months prior to EHV-1 challenge protected the majority of horses from clinical disease, virus shedding, and cell-associated viremia after challenge infection. The attenuated vaccine candidate Ab4ΔORF2 protected a higher number of horses completely from EHV-1 infection, clinical disease, virus shedding, and viremia than infection with its parent strain Ab4. Improved protection was linked to a stronger, rapidly increasing nasal IgG4/7 response and the absence of inflammatory mediator upregulation in the upper respiratory tract after challenge. In contrast, EHV-1 infection of susceptible horses induced characteristic patterns of secreted nasal antiviral and inflammatory mediators (IFN-α, IFN-γ, sCD14, CCL2, CCL3, CCL11) within a few days p.i. and simultaneously with virus shedding, while intranasal EHV-1-specific IgG4/7 antibodies increased slowly. Preexisting EHV-1-specific IgG4/7 antibodies in serum were found to be robust markers of protection from challenge infection. Our data support EHV-1-specific IgG4/7 antibodies as valuable biomarkers and correlates of protection for improving vaccination and management strategies to limit EHV-1 outbreaks.
MATERIALS AND METHODS
Horses, groups, and initial EHV-1 infection with Ab4 or Ab4ΔORF2.
A total of 24 horses from the Cornell University herd of Icelandic horses with controlled EHV-1 status were enrolled in this study (11, 17). Nine months prior to the EHV-1 challenge infection study described here, all horses were randomly assigned to three groups (n = 8) and were experimentally infected with EHV-1 Ab4/8 (46) or an ORF2 gene deletion mutant of Ab4ΔORF2 as previously described in detail (19). Briefly, one group was not infected with EHV-1 (control). A second group was infected with the neuropathogenic EHV-1 strain Ab4/8 (47). The third group was infected with the new vaccine candidate Ab4ΔORF2 (48). Further details on the horses and experimental groups are given in Table 3. During the initial infection with Ab4 or the Ab4ΔORF2 vaccine candidate, the horses developed clinical disease, shed EHV-1 in their nasal secretion, and developed viremia. However, Ab4ΔORF2 was less virulent than its parent Ab4 virus as shown by the absence of the initial fever peak and significantly reduced EHV-1 shedding in the nasal secretions (19). Immune induction was markedly similar between the Ab4ΔORF2 and Ab4 infected groups, confirming the unmodified immunogenicity of the ORF2 deletion mutant virus (19).
TABLE 3.
Summary of the experimental groups
| Group/horses | Control/Ab4 | Ab4ΔORF2/Ab4 | Ab4/Ab4 |
|---|---|---|---|
| Initial infectiona,b | Not infected | Ab4ΔORF2 | Ab4 |
| Challenge infectionb | Ab4 | Ab4 | Ab4 |
| Horses (n) | 8 per group | 8 per group | 8 per group |
| Sex | 4 mares and 4 geldings per group | 4 mares and 4 geldings per group | 4 mares and 4 geldings per group |
| Median age (yrs) (range)c | 3 (3–4) | 4 (3–5) | 4 (3–4) |
The initial infection of the horses was described in detail by Schnabel et al. (19).
Intranasal infection with 1 × 107 PFU.
Age at challenge infection.
After release from the initial EHV-1 infection and prior to the EHV-1 challenge infection described here, all horses were kept on pasture, separated by group, at an isolated facility at Cornell University. The horses had no contact with other horses in the United States prior to and for the duration of this study. The facility had restricted access for people to avoid infection with common U.S. pathogens and to maintain the controlled EHV-1 status of the Icelandic herd. Grass hay was fed ad libitum during the winter months. Horses were vaccinated annually against rabies, tetanus, West Nile virus, and eastern and western encephalitis virus and regularly dewormed as a group but were not vaccinated or treated otherwise.
EHV-1 challenge infection.
The EHV-1 challenge infection was performed 9 months after the initial EHV-1 infection. All 24 horses were challenged by intranasal infection with 1 × 107 PFU of the neurogenic strain Ab4/8 using a mucosal atomizer device (Wolfe Tory Medical, Salt Lake City, UT) as previously described in detail (19). Challenge infection resulted in three experimental groups determined by the initial infection: control/Ab4, Ab4ΔORF2/Ab4, and Ab4/Ab4 (Table 3). Two days prior to infection, all horses were moved into one isolation barn with individual box stalls and one shared air space to acclimate. Isolation and biosecurity precautions were performed as previously described (19) with the exception of handling all horses as one group in this study. During the study, horses did not have any nose-to-nose contact but were randomly assigned to stalls independent of their group. Horse handlers did not change protective biosecurity clothing while sampling horses on a given day, with the only exception being changing gloves after taking a nasal sample from one horse before going to the next stall. Horses were released from the isolation barn after sampling on day 10 p.i. Horses in group Ab4ΔORF2/Ab4 were kept on a separated pasture without contact with other horses. The other two groups were housed together on the same pasture.
The experimental EHV-1 infection and all sample collections for this study were carried out in accordance with the recommendation in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee at Cornell University (protocol 2011-0011). The study also followed the Guide for Care and Use of Animals in Agricultural Research and Teaching. All efforts were made to minimize suffering of the animals, for example, by short sedation. All horses survived and were kept at the facility at Cornell University as research horses after the end of this experimental study.
Samples.
Blood and nasopharyngeal secretion samples were obtained and processed as previously described (19). Blood samples included heparinized samples for PBMC isolation and those without anticoagulant for serum collection. Nasal swab samples were taken for virus isolation and to determine soluble immune parameters in nasal secretions. Nasal swabs were transferred into a tube with 1 ml of sterile phosphate-buffered saline (PBS) solution immediately after sampling. Baseline blood and nasal secretion samples were taken 2 days before (day –2) and/or the day prior to EHV-1 challenge infection (day –1). Afterward, blood and nasal secretion samples were taken daily for 10 days postchallenge infection (days 1 to 10 p.i.) and on days 18, 31, 59, 80, and 108 p.i. Additional blood samples were taken on days 140, 172, and 200 p.i.
Clinical evaluation.
Clinical scoring was performed on day –2, day –1, immediately before EHV-1 challenge infection (day 0), and in the mornings of days 1 to 10 p.i. Clinical scoring, including gait evaluation, was performed as previously described (19) according to the system described by Furr and Reed (49). Body temperatures were taken at the same time points before samples were obtained and also in the evenings of days 0 to 7 p.i. A fever was defined as a rectal temperature of >38.5°C. The clinicians taking samples and performing the scoring were not aware of the group assignments of the horses during the prior initial EHV-1 infection.
PBMC isolation.
PBMC were isolated from heparinized blood by density gradient centrifugation (Ficoll-Paque Plus, GE Healthcare, Piscataway, NJ) within a few hours after blood collection as previously described (19).
Cell-associated viremia.
PCR was performed using PBMC to determine cell-associated viremia as previously described (11, 19). PBMC aliquots of 9 × 106 cells were snap-frozen on each sampling day. DNA from an equivalent of 3.42 × 105 snap-frozen PBMC per sample was used to determine viral genome copy numbers by quantitative PCR targeting the gB gene (50). The quantitative PCR was performed at the Animal Health Diagnostic Center at Cornell University.
Virus isolation from nasal secretions and PBMC.
Virus isolation from nasal secretions and PBMC was performed in a plaque assay to quantify shedding of infectious virus and viremia as previously described in detail (18, 19). Virus isolation from nasal samples was determined as PFU/ml nasal secretion. Viremia was determined as PFU/1 × 107 PBMC.
Quantification of EHV-1-specific antibodies in nasal secretions and serum.
Antibodies specific for EHV-1 gC, gD, and gB were measured by a fluorescent bead-based EHV-1 multiplex assay as previously described (19). In brief, a monoclonal anti-IL-4 antibody (IL-4 monoclonal antibody [MAb] 25) was first coupled to beads 35, 36, and 33 (Luminex Corp., Austin, TX) Beads 35, 36, and 33 were then incubated with IL-4/gC, IL-4/gD, and IL-4/gB, respectively. The beads were then incubated with samples or assay controls. Serum was diluted 1:400, and nasal secretion samples were measured undiluted. A polyclonal biotinylated anti-IgG (H+L) detection antibody (Jackson Immunoresearch Laboratories, West Grove, PA) followed by streptavidin-phycoerythrin (Invitrogen, Carlsbad, CA) was used to analyze total EHV-1-specific antibodies. For isotyping, biotinylated monoclonal antibodies against IgG1, IgG1/3, IgG4/7, IgG3/5, IgG6, IgA, and IgM were used (11, 17, 19). The assay was analyzed in a Luminex 200 analyzer (Luminex Corp.), and antibody values were expressed as median fluorescence intensities (MFI). Antibody patterns against EHV-1 gB, gC, and gD were highly similar to each other in this study and as previously described (17, 19). Thus, only anti-gC antibody results are shown here.
Cellular in vitro restimulation assay.
EHV-1 restimulation of PBMC was performed with the EHV-1 strain Ab4 at a multiplicity of infection (MOI) of 1 for 48 h as previously described in detail (5, 11, 17–19).
Cytokine and chemokine detection by multiplex assays.
Nasal secretion samples and cell culture supernatants from EHV-1 restimulated PBMC were evaluated using an equine cytokine multiplex assay detecting IFN-α, IL-4, IL-10, IL-17, and IFN-γ as previously described (51). The equine cytokine multiplex assay is available through the Animal Health Diagnostic Center at Cornell University. IL-4, IL-10, and IFN-α were reported in pg/ml, and IL-17 and IFN-γ were reported as U/ml.
In addition, cytokines, CCL2, CCL3, CCL5, CCL11, and sCD14 in nasal secretion samples were measured using an equine chemokine multiplex assay, based on monoclonal antibodies against equine CCL2, CCL3, CCL5, and CCL11 (52), and an sCD14 assay (53). These intranasal mediators were reported in pg/ml.
Flow cytometric analysis of EHV-1-specific T cells.
Tri-color staining and flow cytometric evaluation of EHV-1-specific T cells were performed as previously described (5, 19, 54, 55). Briefly, PBMC were restimulated with EHV-1 ex vivo in the presence of brefeldin A to block cytokine secretion. Cells were harvested, divided in aliquots, and triple stained for either cell surface CD4 and CD8 and intracellular IFN-γ production or intracellular IL-10, IL-4, and IL-17A. The cells were analyzed in a fluorescence-activated cell sorter (FACS) Canto II flow cytometer (BD Biosciences, San Diego, CA). Using FlowJo software version 10.2 (FlowJo LLC, Ashland, OR, USA), an analysis gate was set on the small lymphocytes. Cells were analyzed for cytokine production. The percentages of EHV-1-specific IFN-γ positive lymphocytes after restimulation were reduced by those of respective medium controls and evaluated as previously described in detail (5, 11, 17–19). Due to the small percentages of IFN-γ-positive lymphocytes, a separate analysis of CD4- and CD8-positive IFN-γ-producing cells was not performed. IL-4, IL-10, and IL-17 expression above the respective medium controls was also not detected in any of the samples.
Statistical analysis.
D’Agostino-Pearson normality tests indicated that values on most days were not normally distributed. All clinical, viral, antibody, and cellular immune parameters were compared using repeated-measure analyses of variance (ANOVAs) with Tukey’s post tests for multiple comparisons between the three groups (control/Ab4, Ab4ΔORF2/Ab4, Ab4/Ab4). Clinical parameters and virus detection were analyzed until days 10 and 18 p.i., respectively (Fig. 1). Statistical analyses of the nasal mediators (Fig. 2), nasal antibodies (Fig. 3), and secreted cytokines from restimulated PBMC (Fig. 5A and B) were limited to day –2 to day 31 p.i. Correlations of prechallenge infection values of EHV-1 gC-specific serum Ig with infection outcomes (fever, clinical disease, virus shedding, and viremia at the times these reached peak values in infected horses) were analyzed using Spearman rank correlations. P values of <0.05 were considered significant. The statistical analysis was performed, and graphs were created using GraphPad Prism 7 for Mac OS X, version 7e.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by the Agriculture and Food Research Initiative competitive grant no. 2015-67015-23091, “The Effect of ORF1 and ORF2 Gene Expression on Innate and Adaptive Immunity and Protection against Equine Herpes Virus Type 1 (EHV-1),” supported by the U.S. Department of Agriculture (USDA), National Institute of Food and Agriculture (NIFA). Additional funding for the horses in this study was provided by the Harry M. Zweig Memorial Fund for Equine Research at Cornell University. Monoclonal antibody development for horse cell surface markers, Ig isotype markers, cytokines, and chemokines was supported by USDA/NIFA grants 2005-01812, “The US Veterinary Immune Reagent Network,” and 2015-67015-23072, “Equine Immune Reagents: Development of Monoclonal Antibodies to Improve the Analysis of Immunity in Horses.”
For their great help with horse handling, sample acquisition, and processing, we thank Christina Watts, Alexandra Brunet, Denise Branecky, and Karynn Kilts. We furthermore thank Kevin Yager and his CARE team for excellent horse care and barn management and Laura Goodman for performing the PCR to detect viremia.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01011-19.
REFERENCES
- 1.Lunn DP, Davis-Poynter N, Flaminio M, Horohov DW, Osterrieder K, Pusterla N, Townsend HGG. 2009. Equine herpesvirus-1 consensus statement. J Vet Intern Med 23:450–461. doi: 10.1111/j.1939-1676.2009.0304.x. [DOI] [PubMed] [Google Scholar]
- 2.Pusterla N, Mapes S, Akana N, Barnett C, MacKenzie C, Gaughan E, Craig B, Chappell D, Vaala W. 2015. Prevalence factors associated with equine herpesvirus type 1 infection in equids with upper respiratory tract infection and/or acute onset of neurological signs from 2008 to 2014. Vet Rec 178:70. [DOI] [PubMed] [Google Scholar]
- 3.Goehring LS, Wagner B, Bigbie R, Hussey SB, Rao S, Morley PS, Lunn DP. 2010. Control of EHV-1 viremia and nasal shedding by commercial vaccines. Vaccine 28:5203–5211. doi: 10.1016/j.vaccine.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 4.Goodman L, Wagner B, Flaminio M, Sussman K, Metzger S, Holland R, Osterrieder N. 2006. Comparison of the efficacy of inactivated combination and modified-live virus vaccines against challenge infection with neuropathogenic equine herpesvirus type 1 (EHV-1). Vaccine 24:3636–3645. doi: 10.1016/j.vaccine.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 5.Goodman LB, Wimer C, Dubovi EJ, Gold C, Wagner B. 2012. Immunological correlates of vaccination and infection for equine herpesvirus 1. Clin Vaccine Immunol 19:235–241. doi: 10.1128/CVI.05522-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.USDA-APHIS. 2007. Equine herpesvirus myeloencephalopathy: a potentially emerging disease. https://www.aphis.usda.gov/animal_health/emergingissues/downloads/ehv1final.pdf.
- 7.Equine Disease Communication Center. 2019. Disease outbreak alerts. http://www.equinediseasecc.org/alerts/outbreaks. Accessed 1 September 2019.
- 8.Matsumura T, O’Callaghan DJ, Kondo T, Kamada M. 1996. Lack of virulence of the murine fibroblast adapted strain, Kentucky A (KyA), of equine herpesvirus type 1 (EHV-1) in young horses. Vet Microbiol 48:353–365. doi: 10.1016/0378-1135(09)59999-3. [DOI] [PubMed] [Google Scholar]
- 9.Soboll G, Hussey SB, Whalley JM, Allen GP, Koen MT, Santucci N, Fraser DG, Macklin MD, Swain WF, Lunn DP. 2006. Antibody and cellular immune responses following DNA vaccination and EHV-1 infection of ponies. Vet Immunol Immunopathol 111:81–95. doi: 10.1016/j.vetimm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura T, Kondo T, Sugita S, Damiani AM, O’Callaghan DJ, Imagawa H. 1998. An equine herpesvirus type 1 recombinant with a deletion in the gE and gI genes is avirulent in young horses. Virology 242:68–79. doi: 10.1006/viro.1997.8984. [DOI] [PubMed] [Google Scholar]
- 11.Wagner B, Perkins G, Babasyan S, Freer H, Keggan A, Goodman LB, Glaser A, Torsteinsdóttir S, Svansson V, Björnsdóttir S. 2017. Neonatal immunization with a single IL-4/antigen dose induces increased antibody responses after challenge infection with equine herpesvirus type 1 (EHV-1) at weanling age. PLoS One 12:e0169072. doi: 10.1371/journal.pone.0169072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breathnach CC, Yeargan MR, Sheoran AS, Allen GP. 2010. The mucosal humoral immune response of the horse to infective challenge and vaccination with equine herpesvirus-1 antigens. Equine Vet J 33:651–657. doi: 10.2746/042516401776249318. [DOI] [PubMed] [Google Scholar]
- 13.Van de Walle GR, May MA, Peters ST, Metzger SM, Rosas CT, Osterrieder N. 2010. A vectored equine herpesvirus type 1 (EHV-1) vaccine elicits protective immune responses against EHV-1 and H3N8 equine influenza virus. Vaccine 28:1048–1055. doi: 10.1016/j.vaccine.2009.10.123. [DOI] [PubMed] [Google Scholar]
- 14.Heldens JG, Hannant D, Cullinane AA, Prendergast MJ, Mumford JA, Nelly M, Kydd JH, Weststrate MW, van den Hoven R. 2001. Clinical and virological evaluation of the efficacy of an inactivated EHV1 and EHV4 whole virus vaccine (Duvaxyn EHV1,4). Vaccination/challenge experiments in foals and pregnant mares. Vaccine 19:4307–4317. doi: 10.1016/S0264-410X(01)00131-1. [DOI] [PubMed] [Google Scholar]
- 15.Patel JR, Bateman H, Williams J, Didlick S. 2003. Derivation and characterisation of a live equid herpes virus-1 (EHV-1) vaccine to protect against abortion and respiratory disease due to EHV-1. Vet Microbiol 91:23–39. doi: 10.1016/S0378-1135(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 16.Ruitenberg KM, Love DN, Gilkerson JR, Wellington JE, Whalley JM. 2000. Equine herpesvirus 1 (EHV-1) glycoprotein D DNA inoculation in horses with pre-existing EHV-1/EHV-4 antibody. Vet Microbiol 76:117–127. doi: 10.1016/S0378-1135(00)00237-6. [DOI] [PubMed] [Google Scholar]
- 17.Wagner B, Goodman LB, Babasyan S, Freer H, Torsteinsdóttir S, Svansson V, Björnsdóttir S, Perkins GA. 2015. Antibody and cellular immune responses of naïve mares to repeated vaccination with an inactivated equine herpesvirus vaccine. Vaccine 33:5588. doi: 10.1016/j.vaccine.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Wimer CL, Schnabel CL, Perkins G, Babasyan S, Freer H, Stout AE, Rollins A, Osterrieder N, Goodman LB, Glaser A, Wagner B. 2018. The deletion of the ORF1 and ORF71 genes reduces virulence of the neuropathogenic EHV-1 strain Ab4 without compromising host immunity in horses. PLoS One 13:e0206679. doi: 10.1371/journal.pone.0206679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnabel CL, Wimer CL, Perkins G, Babasyan S, Freer H, Watts C, Rollins A, Osterrieder N, Wagner B. 2018. Deletion of the ORF2 gene of the neuropathogenic equine herpesvirus type 1 strain Ab4 reduces virulence while maintaining strong immunogenicity. BMC Vet Res 14. doi: 10.1186/s12917-018-1563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kydd JH, Townsend HGG, Hannant D. 2006. The equine immune response to equine herpesvirus-1: the virus and its vaccines. Vet Immunol Immunopathol 111:15–30. doi: 10.1016/j.vetimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Kydd JH, Wattrang E, Hannant D. 2003. Pre-infection frequencies of equine herpesvirus-1 specific, cytotoxic T lymphocytes correlate with protection against abortion following experimental infection of pregnant mares. Vet Immunol Immunopathol 96:207–217. doi: 10.1016/j.vetimm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Kydd JH, Smith KC, Hannant D, Livesay GJ, Mumford JA. 1994. Distribution of equid herpesvirus-1 (EHV-1) in respiratory tract associated lymphoid tissue: implications for cellular immunity. Equine Vet J 26:470–473. doi: 10.1111/j.2042-3306.1994.tb04052.x. [DOI] [PubMed] [Google Scholar]
- 23.Allen GP. 2008. Risk factors for development of neurologic disease after experimental exposure to equine herpesvirus-1 in horses. Am J Vet Res 69:1595–1600. doi: 10.2460/ajvr.69.12.1595. [DOI] [PubMed] [Google Scholar]
- 24.Allen G, Yeargan M, Costa LR, Cross R. 1995. Major histocompatibility complex class I-restricted cytotoxic T-lymphocyte responses in horses infected with equine herpesvirus 1. J Virol 69:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paillot R, Daly JM, Juillard V, Minke JM, Hannant D, Kydd JH. 2005. Equine interferon gamma synthesis in lymphocytes after in vivo infection and in vitro stimulation with EHV-1. Vaccine 23:4541–4551. doi: 10.1016/j.vaccine.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Paillot R, Ellis S, Daly J, Audonnet J, Minke J, Davispoynter N, Hannant D, Kydd J. 2006. Characterisation of CTL and IFN-γ synthesis in ponies following vaccination with a NYVAC-based construct coding for EHV-1 immediate early gene, followed by challenge infection. Vaccine 24:1490–1500. doi: 10.1016/j.vaccine.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Paillot R, Daly JM, Luce R, Montesso F, Davis-Poynter N, Hannant D, Kydd JH. 2007. Frequency and phenotype of EHV-1 specific, IFN-γ synthesising lymphocytes in ponies: the effects of age, pregnancy and infection. Dev Comp Immunol 31:202–214. doi: 10.1016/j.dci.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Kydd JH, Slater J, Osterrieder N, Lunn DP, Antczak DF, Azab W, Balasuriya U, Barnett C, Brosnahan M, Cook C, Damiani A, Elton D, Frampton A, Gilkerson J, Goehring L, Horohov D, Maxwell L, Minke J, Morley P, Nauwynck H, Newton R, Perkins G, Pusterla N, Soboll-Hussey G, Traub-Dargatz J, Townsend H, Van de Walle GR, Wagner B. 2012. Third International Havemeyer Workshop on Equine Herpesvirus type 1: EHV-1 Havemeyer workshop report. Equine Vet J 44:513–517. doi: 10.1111/j.2042-3306.2012.00604.x. [DOI] [PubMed] [Google Scholar]
- 29.Kydd JH, Smith KC, Hannant D, Livesay GJ, Mumford JA. 1994. Distribution of equid herpesvirus-1 (EHV-1) in the respiratory tract of ponies: implications for vaccination strategies. Equine Vet J 26:466–469. doi: 10.1111/j.2042-3306.1994.tb04051.x. [DOI] [PubMed] [Google Scholar]
- 30.Breathnach CC, Yeargan MR, Timoney JF, Allen GP. 2006. Detection of equine herpesvirus-specific effector and memory cytotoxic immunity in the equine upper respiratory tract. Vet Immunol Immunopathol 111:117–125. doi: 10.1016/j.vetimm.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Perkins G, Babasyan S, Stout AE, Freer H, Rollins A, Wimer CL, Wagner B. 2019. Intranasal IgG4/7 antibody responses protect horses against equid herpesvirus-1 (EHV-1) infection including nasal virus shedding and cell-associated viremia. Virology 531:219–232. doi: 10.1016/j.virol.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Kim SK, Shakya AK, O’Callaghan DJ. 2016. Immunization with attenuated equine herpesvirus 1 strain KyA induces innate immune responses that protect mice from lethal challenge. J Virol 90:8090–8104. doi: 10.1128/JVI.00986-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soboll Hussey G, Hussey SB, Wagner B, Horohov DW, Van de Walle GR, Osterrieder N, Goehring LS, Rao S, Lunn DP. 2011. Evaluation of immune responses following infection of ponies with an EHV-1 ORF1/2 deletion mutant. Vet Res 42:23. doi: 10.1186/1297-9716-42-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hübert PH, Birkenmaier S, Rziha HJ, Osterrieder N. 1996. Alterations in the equine herpesvirus type-1 (EHV-1) strain RacH during attenuation. Zentralbl Veterinarmed B 43:1–14. doi: 10.1111/j.1439-0450.1996.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 35.Hussey G, Goehring LS, Lunn DP, Hussey SB, Huang T, Osterrieder N, Powell C, Hand J, Holz C, Slater J. 2013. Experimental infection with equine herpesvirus type 1 (EHV-1) induces chorioretinal lesions. Vet Res 44:118. doi: 10.1186/1297-9716-44-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel JR, Heldens J. 2005. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)—epidemiology, disease and immunoprophylaxis: a brief review. Vet J 170:14–23. doi: 10.1016/j.tvjl.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 37.American Association of Equine Practitioners. 2015. Equine herpesvirus (rhinopneumonitis). https://aaep.org/guidelines/vaccination-guidelines/risk-based-vaccination-guidelines/equine-herpesvirus-rhinopneumonitis. Accessed 1 September 2019.
- 38.Patel JR, Földi J, Bateman H, Williams J, Didlick S, Stark R. 2003. Equid herpesvirus (EHV-1) live vaccine strain C147: efficacy against respiratory diseases following EHV types 1 and 4 challenges. Vet Microbiol 92:1–17. doi: 10.1016/S0378-1135(02)00358-9. [DOI] [PubMed] [Google Scholar]
- 39.Bagga B, Cehelsky JE, Vaishnaw A, Wilkinson T, Meyers R, Harrison LM, Roddam PL, Walsh EE, DeVincenzo JP. 2015. Effect of preexisting serum and mucosal antibody on experimental respiratory syncytial virus (RSV) challenge and infection of adults. J Infect Dis 212:1719–1725. doi: 10.1093/infdis/jiv281. [DOI] [PubMed] [Google Scholar]
- 40.Pusterla N, Hussey GS. 2014. Equine herpesvirus 1 myeloencephalopathy. Vet Clin North Am Equine Pract 30:489–506. doi: 10.1016/j.cveq.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Nugent J, Paillot R. 2009. Equine herpesvirus myeloencephalopathy: unravelling the enigma. Vet J 180:271–272. doi: 10.1016/j.tvjl.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Osterrieder N, Van de Walle GR. 2010. Pathogenic potential of equine alphaherpesviruses: the importance of the mononuclear cell compartment in disease outcome. Vet Microbiol 143:21–28. doi: 10.1016/j.vetmic.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Wimer CL, Damiani A, Osterrieder N, Wagner B. 2011. Equine herpesvirus type-1 modulates CCL2, CCL3, CCL5, CXCL9, and CXCL10 chemokine expression. Vet Immunol Immunopathol 140:266–274. doi: 10.1016/j.vetimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Edington N, Bridges CG, Patel JR. 1986. Endothelial cell infection and thrombosis in paralysis caused by equid herpesvirus-1: equine stroke. Arch Virol 90:111–124. doi: 10.1007/BF01314149. [DOI] [PubMed] [Google Scholar]
- 45.Pusterla N, David Wilson W, Madigan JE, Ferraro GL. 2009. Equine herpesvirus-1 myeloencephalopathy: a review of recent developments. Vet J 180:279–289. doi: 10.1016/j.tvjl.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Goodman LB, Loregian A, Perkins GA, Nugent J, Buckles EL, Mercorelli B, Kydd JH, Palù G, Smith KC, Osterrieder N, Davis-Poynter N. 2007. A point mutation in a herpesvirus polymerase determines neuropathogenicity. PLoS Pathog 3:e160. doi: 10.1371/journal.ppat.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nugent J, Birch-Machin I, Smith KC, Mumford JA, Swann Z, Newton JR, Bowden RJ, Allen GP, Davis-Poynter N. 2006. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J Virol 80:4047–4060. doi: 10.1128/JVI.80.8.4047-4060.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma G, Feineis S, Osterrieder N, Van de Walle GR. 2012. Identification and characterization of equine herpesvirus type 1 pUL56 and its role in virus-induced downregulation of major histocompatibility complex class I. J Virol 86:3554–3563. doi: 10.1128/JVI.06994-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furr M, Reed S. 2015. Equine neurology. John Wiley & Sons, Chichester, United Kingdom: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118993712. [Google Scholar]
- 50.Elia G, Decaro N, Martella V, Campolo M, Desario C, Lorusso E, Cirone F, Buonavoglia C. 2006. Detection of equine herpesvirus type 1 by real time PCR. J Virol Methods 133:70–75. doi: 10.1016/j.jviromet.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Wagner B, Freer H. 2009. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol 127:242–248. doi: 10.1016/j.vetimm.2008.10.313. [DOI] [PubMed] [Google Scholar]
- 52.Schnabel CL, Wemette M, Babasyan S, Freer H, Baldwin C, Wagner B. 2018. C-C motif chemokine ligand (CCL) production in equine peripheral blood mononuclear cells identified by newly generated monoclonal antibodies. Vet Immunol Immunopathol 204:28–39. doi: 10.1016/j.vetimm.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Wagner B, Ainsworth DM, Freer H. 2013. Analysis of soluble CD14 and its use as a biomarker in neonatal foals with septicemia and horses with recurrent airway obstruction. Vet Immunol Immunopathol 155:124–128. doi: 10.1016/j.vetimm.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Wagner B, Burton A, Ainsworth D. 2010. Interferon-gamma, interleukin-4 and interleukin-10 production by T helper cells reveals intact Th1 and regulatory TR1 cell activation and a delay of the Th2 cell response in equine neonates and foals. Vet Res 41:47. doi: 10.1051/vetres/2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perkins GA, Goodman LB, Wimer C, Freer H, Babasyan S, Wagner B. 2014. Maternal T-lymphocytes in equine colostrum express a primarily inflammatory phenotype. Vet Immunol Immunopathol 161:141–150. doi: 10.1016/j.vetimm.2014.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.