SAMHD1 is a phosphohydrolase and reduces cellular dNTP concentrations, which impairs poxviral DNA replication. The simian SIV accessory protein Vpx promotes degradation of SAMHD1, leading to increased cellular dNTP concentrations. Vpx addition enables poxviral DNA replication in human dendritic cells (DCs), as well as the expression of viral late proteins, which is normally blocked. SAMHD1 function during modified vaccinia virus Ankara (MVA) infection of human DCs was studied with recombinant MVA-vpx expressing Vpx. Infection of human DCs with MVA-vpx decreased SAMHD1 protein amounts, enabling MVA DNA replication and expression of late viral genes. Unexpectedly, type I interferon expression was blocked after MVA-vpx infection. MVA-vpx might be a good tool to study SAMHD1 depletion during poxviral infections and to provide insights into poxvirus-host interactions.
KEYWORDS: MVA, SAMHD1, dendritic cells, vaccinia virus, vpx
ABSTRACT
Attenuated poxviruses like modified vaccinia virus Ankara (MVA) are promising vectors for vaccines against infectious diseases and cancer. However, host innate immune responses interfere with the viral life cycle and also influence the immunogenicity of vaccine vectors. Sterile alpha motif (SAM) domain and histidine-aspartate (HD) domain-containing protein 1 (SAMHD1) is a phosphohydrolase and reduces cellular deoxynucleoside triphosphate (dNTP) concentrations, which impairs poxviral DNA replication in human dendritic cells (DCs). Human immunodeficiency virus type 2 (HIV-2) and simian immunodeficiency virus (SIV) encode an accessory protein called viral protein X (Vpx) that promotes proteasomal degradation of SAMHD1, leading to a rapid increase in cellular dNTP concentrations. To study the function of SAMHD1 during MVA infection of human DCs, the SIV vpx gene was introduced into the MVA genome (resulting in recombinant MVA-vpx). Infection of human DCs with MVA-vpx led to SAMHD1 protein degradation and enabled MVA-vpx to replicate its DNA genome and to express genes controlled by late promoters. Late gene expression by MVA-vpx might improve its vaccine vector properties; however, type I interferon expression was unexpectedly blocked by Vpx-expressing MVA. MVA-vpx can be used as a tool to study poxvirus-host interactions and vector safety.
IMPORTANCE SAMHD1 is a phosphohydrolase and reduces cellular dNTP concentrations, which impairs poxviral DNA replication. The simian SIV accessory protein Vpx promotes degradation of SAMHD1, leading to increased cellular dNTP concentrations. Vpx addition enables poxviral DNA replication in human dendritic cells (DCs), as well as the expression of viral late proteins, which is normally blocked. SAMHD1 function during modified vaccinia virus Ankara (MVA) infection of human DCs was studied with recombinant MVA-vpx expressing Vpx. Infection of human DCs with MVA-vpx decreased SAMHD1 protein amounts, enabling MVA DNA replication and expression of late viral genes. Unexpectedly, type I interferon expression was blocked after MVA-vpx infection. MVA-vpx might be a good tool to study SAMHD1 depletion during poxviral infections and to provide insights into poxvirus-host interactions.
INTRODUCTION
Attenuated poxviruses like modified vaccinia virus Ankara (MVA) are promising vectors for vaccines against infectious diseases and cancer. MVA is highly attenuated due to deletions of viral genes, several of which counteract the host innate immune response to the infection. Furthermore, MVA is replication deficient in human cells due to defective maturation of viral particles in mammalian cells, although viral protein synthesis is not affected (1).
Innate immune responses of host cells restrict the life cycle of poxviruses and have a high impact on the immunogenicity of attenuated vaccine vectors. Dendritic cells (DCs) are professional antigen-presenting cells and are the only cell type capable of activating naive T cells. Sterile alpha motif (SAM) domain and histidine-aspartate (HD) domain-containing protein 1 (SAMHD1) is an innate antiviral immune factor, first discovered to limit proviral DNA synthesis of lentiviruses, particularly in nondividing myeloid cells such as macrophages and DCs (2–4). SAMHD1 is a phosphohydrolase and converts deoxynucleoside triphosphates (dNTPs) to inorganic phosphate and 2′-deoxynucleoside, resulting in the reduction of cellular dNTP concentrations (5, 6). Human immunodeficiency virus type 2 (HIV-2) and simian immunodeficiency virus (SIV) encode an accessory protein called viral protein X (Vpx) that overcomes SAMHD1-induced dNTP depletion. Vpx promotes proteasomal degradation of SAMHD1, leading to a rapid increase in cellular dNTP concentrations and enabling reverse transcription of lentiviruses (2, 3, 7, 8).
The poxvirus strain Western Reserve (WR) is unable to replicate in human nondividing cells because the levels of dNTPs are too low for the replication of viral DNA (9). When added, packaged in virus-like particles (VLPs) prior to poxviral infection, Vpx slightly increased the replication of WR in human myeloid cells by increasing the availability of dNTP substrate (9). Poxviruses encode enzymes such as ribonucleotide reductase (RNR) and thymidine kinase (TK) that supply dNTPs for viral DNA polymerase and enhance DNA replication (9). Consequentially, WR with a deleted TK gene was more restricted by SAMHD1, while the addition of Vpx restored viral replication (9). SAMHD1 restriction of poxviral replication most likely explains previous findings that MVA DNA synthesis and the subsequent expression of late viral proteins are blocked in human DCs (10–12). Cellular dNTP concentrations vary among cell types and are low in nondividing cells (13, 14), but they also vary among species. Mouse cells, including DCs, contain high dNTP levels and do not restrict poxvirus late protein synthesis (15, 16). Therefore, the induction of immune responses elicited by poxviruses might differ between mice and humans. DCs have two mechanisms for presenting antigens to CD8+ cytotoxic T cells (CTL), involving either antigens synthesized endogenously (direct presentation) or the uptake of exogenous antigens (cross-presentation). In mice, it has been shown that although late gene products are expressed by DCs endogenously, CTL responses against recombinant MVA in vivo are mainly caused by the cross-presentation of antigens, which are taken up by DCs from infected cells (17). In humans, CTL responses directed against late gene products are detectable, although late gene products are not synthesized in human DCs. This implies that cross-presentation and not direct presentation is the main antigen presentation mechanism for MVA-derived products in humans. Therefore, expression of late gene products in DCs, which could lead to direct presentation, might enhance the immunogenicity of MVA in humans (12).
In order to study the function of SAMHD1 during MVA infection of human DCs, the vpx gene was introduced into the MVA genome. This modification led to SAMHD1 protein degradation after infection and allowed MVA to replicate its DNA and to express genes controlled by late promoters in human DCs. Insights into the effects of MVA-vpx infection on host cells could contribute to understanding the antiviral effects of SAMHD1 and the molecular functions of virus-host interactions.
RESULTS
Generation and characterization of MVA recombinants expressing the SIV-PBj vpx gene.
Vaccinia virus (VACV) replication is restricted by SAMHD1 in human dendritic cells (9). The HIV-2 and SIV strain PBj (SIV-PBj) accessory gene vpx has been described to counteract SAMHD1 function by protein degradation (18). Therefore, we were interested in genetically linking vpx expression with MVA. This should restore late gene expression of the recombinant MVA in human DCs, which might enhance vector immunogenicity.
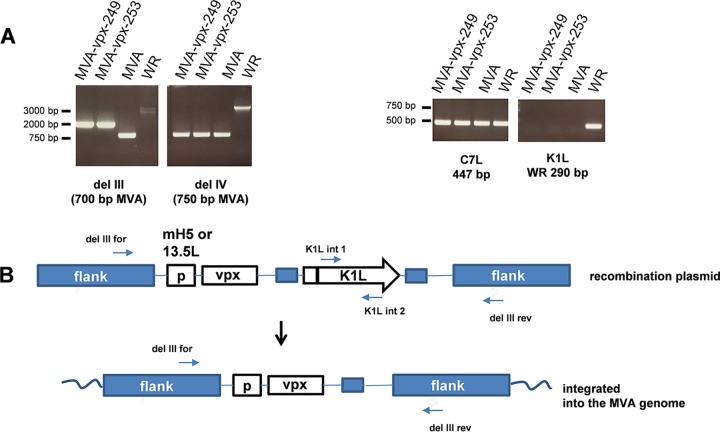
Two MVA recombinants were generated by insertion of a vpx expression cassette into the region of deletion III of MVA (19). The timing of vpx expression during infection was designed to be either very early or early late, depending on the promoter used to generate the recombinant viruses. Expression of vpx was under the control of the early/late promoter mH5 (20) in the recombinant virus MVA-vpx-249 and of the early 13.5L promoter in MVA-vpx-253 (21). The genetic structure of the recombinant MVAs was confirmed by PCR analyses of viral DNA prepared from infected DF-1 cells (Fig. 1A). The gene structure and the primers annealing to MVA sequences are indicated (Fig. 1B). Analysis of the deletion III region confirmed the insertion of sequences into this site (Fig. 1A). In addition, neither deletion IV nor the C7L gene (important for late gene expression [22]) was altered, and the K1L gene, which was used for the selection of recombinant viruses, was deleted (Fig. 1A).
FIG 1.
Characterization of the recombinant MVA-vpx genome. (A) PCR analysis of DNA from the indicated viruses. In MVA-vpx-249, vpx expression is controlled by the mH5 (early/late) promoter, and in MVA-vpx-253 it is controlled by the early 13.5L promoter. The genes were inserted into the region of deletion III of MVA. (B) Primers used for the analysis of the deletion III region and the K1L gene. Sequence information is given in the Materials and Methods. Schematic representation of the recombinant MVA. P, promoter. Analysis of the deletion III region confirmed the insertion of sequences into this site. Deletion IV and the C7L gene were not altered, and the K1L gene was deleted.
Vpx expression by the two recombinant viruses was monitored in lysates of HeLa cells infected at a multiplicity of infection (MOI) of 10 by Western blotting using an antibody directed against Vpx (Fig. 2). As expected, the MVA recombinants produced Vpx protein with different kinetics. At 2 h postinfection (hpi), Vpx expression in MVA-vpx-253-infected cells was higher than that in MVA-vpx-249-infected cells, corresponding to the expression under the control of the early 13.5L promoter. MVA-vpx-249-infected cells showed a Vpx expression peak at very late time points (16 h), as expected for the early/late promoter control.
FIG 2.
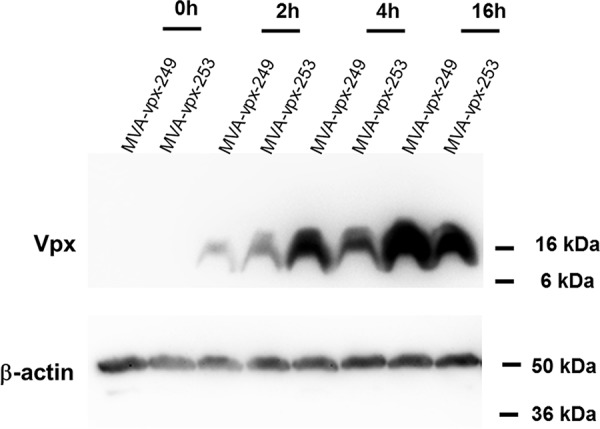
Western blot analysis of Vpx expression. HeLa cells were infected with MVA-vpx-249 or MVA-vpx-253 at an MOI of 10 and then harvested at the indicated time points. Proteins were separated by SDS-PAGE, and Western blot analysis was performed with an antibody directed against Vpx. Vpx expression by MVA-vpx-249 is controlled by an early/late promoter and that of MVA-vpx-253 by an early promoter. Vpx has a molecular weight of 16 kDa. Equal loading was confirmed by detection of β-actin. Vpx expression in MVA-vpx-253-infected cells was higher at early time points (2 h) than that in MVA-vpx-249-infected cells, which showed a Vpx expression peak at very late time points (16 h).
Vpx expression restores late protein expression of MVA in human DCs.
Next, it was examined whether Vpx expression by MVA would enable late gene expression in human DCs. Late gene expression of MVA is blocked in infected human DCs, presumably because DNA replication, a prerequisite for intermediate and late gene expression, is constrained by the depletion of intracellular dNTPs by SAMHD1 (9–11).
Human peripheral CD14-positive cells were cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) to differentiate into DCs. After 6 days of culture, cells were infected with the different viruses at an MOI of 3 and then harvested 6 h after infection, and protein expression was determined by Western blotting. As indicated in Fig. 3, late gene expression, exemplified by detection of the late-expressed B5 protein, was restored in DCs infected with MVA expressing Vpx. The expression of the late B5 protein could be detected in MVA-vpx-infected cell lysate (Fig. 3, lanes 3 and 4). Increased B5 expression correlated with the faintly detectable Vpx expression and a decreased amount of SAMHD1 protein (Fig. 3). In contrast, B5 expression could not be detected in DCs infected with MVA (Fig. 3).
FIG 3.
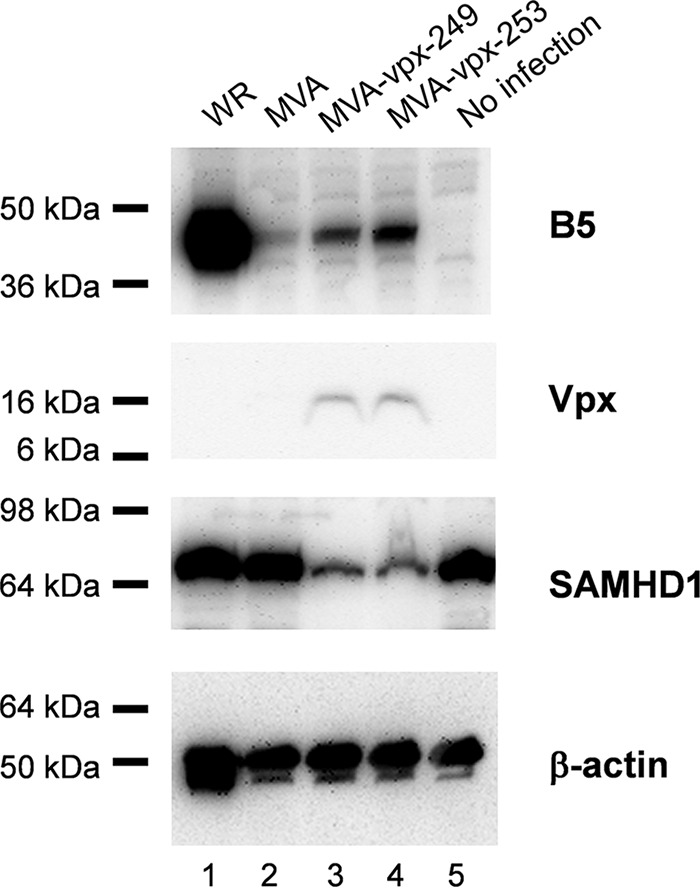
The late B5 protein is expressed in MVA-vpx-infected DCs and correlates with Vpx expression and a decrease in SAMHD1 protein. Human DCs were infected with VACV WR, MVA, MVA-vpx-249, and MVA-vpx-253 at an MOI of 3. Cells were harvested at 6 h after infection, proteins were separated by SDS-PAGE, and Western blot analysis was performed with an antibody directed against B5, a late-expressed VACV protein. In addition, Vpx and SAMHD1 expression were analyzed with specific antibodies. Equal loading was confirmed by detection of β-actin. Experiments were performed three times, and representative results are shown.
Vpx expression by MVA increases viral DNA replication.
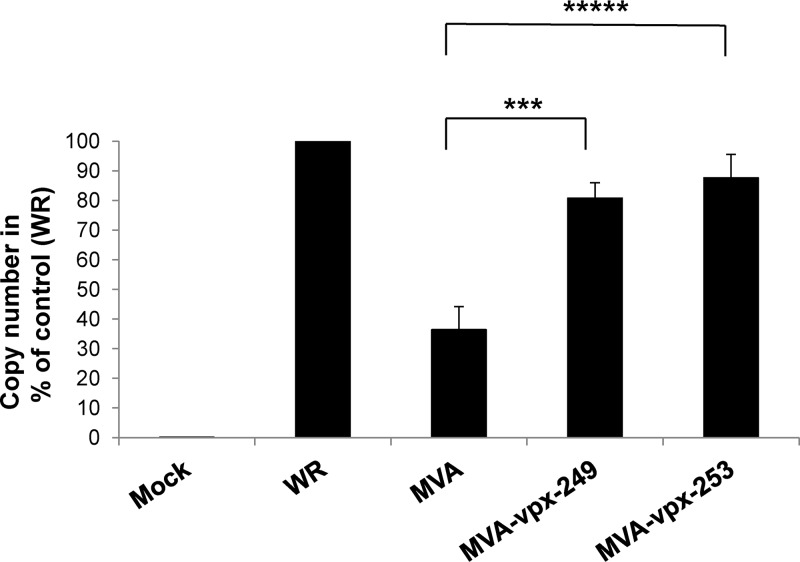
Vpx-mediated SAMHD1 depletion should raise the dNTP levels in the DCs and thereby enable viral DNA replication after infection of these cells. Vaccinia virus DNA replication was analyzed using quantitative real-time PCR (qPCR) of the highly conserved rpo18 gene (23). The template DNA was isolated from infected human DCs (MOI 3) 24 h after infection and the same amount of template DNA was used for each reaction. Figure 4 shows the average values obtained from two experiments. The number of copies obtained after WR infection was set to 100%. The results show that MVA DNA replication is inhibited in human DCs to 30% of WR replication (10). Insertion of vpx into the MVA genome was able to increase viral DNA replication to 80 to 90% of WR replication indicating that Vpx expression restored the DNA replication of MVA (Fig. 4).
FIG 4.
Insertion of vpx into the MVA genome increased viral DNA replication in DCs. Viral DNA replication was analyzed by quantitative real-time PCR of the highly conserved rpo18 gene. The template DNA was isolated from infected human DCs (MOI = 3) 24 h after infection, and 37.5 μg template DNA was used for each reaction. The amount of DNA measured in samples from WR-infected cells was set to 100%. Average values obtained from four experiments are given with standard deviations, and the P values are indicated. ***, P = 0.002 between MVA and MVA-vpx-249 infection; ****, P < 0.0001 between MVA and MVA-vpx-253 infection. P values were calculated with GraphPad Prism 7.04 software as unpaired two-tailed t tests.
Characterization of MVA-vpx-infected human DCs.
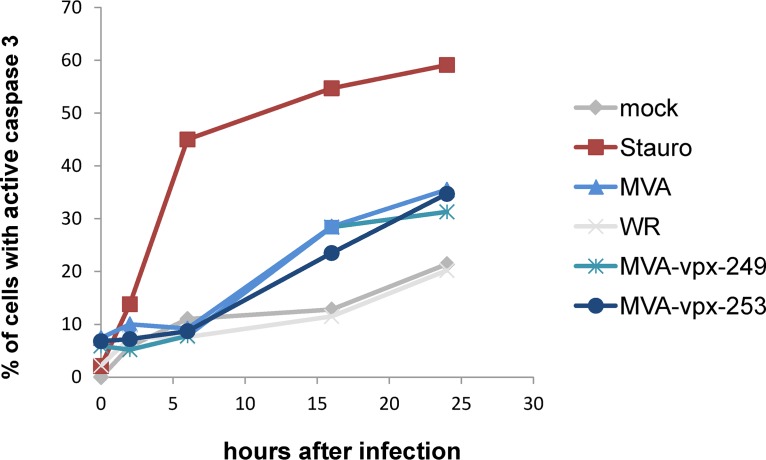
The induction of apoptosis after viral infection is a potent mechanism for aborting viral replication and also influences viral vector immunogenicity. Phagocytosis of apoptotic bodies by antigen-presenting cells (APCs) leads to cross-presentation of foreign antigens and priming of cytotoxic T cells. Vaccinia virus (VACV) encodes several antiapoptotic proteins that inhibit apoptosis induction (24). Therefore, induction of apoptosis in infected DCs was analyzed by performing a kinetic measurement of caspase 3 activation by flow cytometry. The presence of the activated effector caspase 3 is a marker for the cell’s entry into the apoptotic pathway. As illustrated in Fig. 5, expression of Vpx by MVA did not influence apoptosis induction. However, as described previously, DCs infected with WR showed a decreased apoptosis induction compared to those infected with MVA or MVA-vpx (Fig. 5) (25).
FIG 5.
Expression of Vpx by MVA does not influence apoptosis induction. The induction of apoptosis was measured using flow cytometry as caspase 3 activation. Human DC cells were infected at an MOI of 3 with the four viruses and then harvested at the indicated time points, permeabilized, and stained with an antibody directed against active caspase 3. Cells treated with 1 μM staurosporine (Stauro) were used as a positive control. The percentages of active caspase 3-positive cells are indicated.
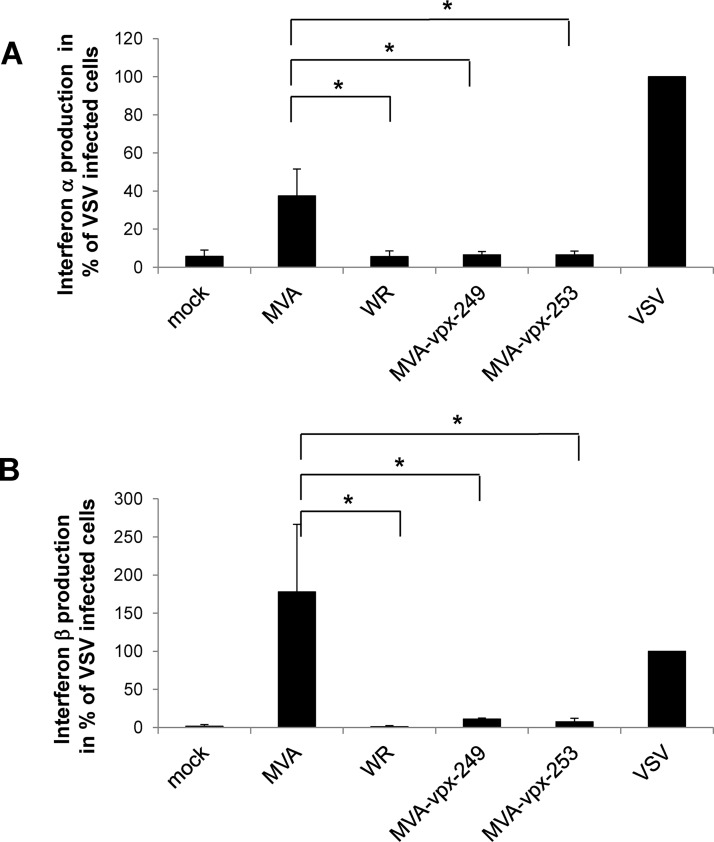
It has been previously described that MVA infection of mice and murine DCs induces significant type I interferon (IFN) responses, whereas infection with VACV WR, which expresses IFN inhibitors that are deleted in MVA, does not (26). The Vpx protein, on the other hand, has been described to interact with interferon regulatory factor 5 (IRF5) and inhibit its function (27). Therefore, we tested the induction of interferon alpha (IFN-α) and interferon beta (IFN-β) after poxviral infection of human DCs. Infection with the vesicular stomatitis virus M2 strain (VSV-M2) at an MOI of 1 was used as a positive control and showed the capacity of the cells to produce type I IFNs (Fig. 6 A and B). MVA infection induced the release of IFN-α DCs infected with VACV WR and the MVA-vpx viruses had the same phenotype and did not induce IFN-α at detectable levels (Fig. 6A). In addition, IFN-β induction was suppressed by Vpx expression (Fig. 6B). This indicates that Vpx is able to suppress IFN-α and IFN-β production in MVA-infected DCs.
FIG 6.
Vpx expression suppresses IFN-α and IFN-β production in MVA-vpx-infected DCs. Human DCs were infected with the poxviruses or VSV-M2 (positive control) at an MOI of 1. Cell-free supernatant was collected after 12 h and analyzed by enzyme-linked immunosorbent assay (ELISA) kits to determine concentrations of human IFN-α (Mabtech, Biozol, Eching, Germany) (A) and human IFN-β (PBL Assay Science, Acris, Herford, Germany) (B). Average values obtained from two experiments are given with standard deviations. (A) P values between MVA infections are indicated and are 0.029 compared to WR and 0.034 to MVA-vpx-249 or MVA-vpx-253 infection. (B) P values between MVA infections are indicated and are 0.020 compared to WR, 0.026 to MVA-vpx-249, and 0.024 to MVA-vpx-253 infection. P values were calculated with GraphPad Prism 7.04 software as one-way analysis of variance (ANOVA).
DISCUSSION
Infection of human DCs with poxviruses is abortive. The replication-competent VACV WR shows limited DNA replication, whereas that of MVA halts prior to DNA synthesis (12). MVA only achieves early but not late gene expression (10–12). Here, we show that this block could be released by expression of the SIV-PBj Vpx protein. SAMHD1 reduces the dNTP levels in human DCs, and Vpx expression by MVA causes the protein degradation of SAMHD1, which coincides with MVA-vpx DNA replication and partial restoration of late protein synthesis. Late protein synthesis might enhance the induction of immune responses in humans. However, a drawback might originate from the inhibition of IFN expression caused by MVA-vpx infection, which might be unfavorable for the induction of an adaptive immune response.
MVA infection of human DCs, in contrast to VACV WR infection, induces IFN-α production, since MVA lacks genes that are able to suppress IFN-α production. Upon cell entry, cytosolic poxviral DNA is sensed by cyclic GMP-AMP synthase (cGAS), which synthesizes 2′,3′-cGAMP and activates the stimulator of interferon genes (STING) and thereby IFN responses. Recently, poxins have been described as 2′,3′-cGAMP-degrading enzymes (28). The poxin gene B2R and the soluble IFN receptor are inactivated in MVA, which is supposed to be responsible for IFN expression upon MVA infection (26, 28), whereas VACV WR infection blocks type I IFN expression (26). Coexpression of Vpx by infection of human DCs with MVA-vpx allowed late gene expression, but blocked IFN-α and IFN-β production. The late gene product F17 has been described as an inhibitor of IFN induction (29). The F17 protein binds and sequesters Raptor and Rictor and thereby the regulators of mammalian target of rapamycin complex 1 and 2 (mTORC1 and mTORC2) (29). Upon infection of a cell with poxviruses, STING recruits tank-binding kinase 1 (TBK1), which phosphorylates IFN response factor 3 (IRF3), and IRF3 induces IFN-stimulated gene (ISG) expression. The poxvirus sensor cGAS produces cGAMP, which activates STING. Without F17 expression (here DC infection with MVA), cGAS accumulates and STING is activated, which leads to IFN-stimulated gene expression. In the presence of F17 protein (here DC infection with WR or MVA-vpx) the mammalian target of rapamycin (mTOR) is dysregulated, cGAS protein becomes degraded, and the host cell is unable to induce IFN-stimulated gene expression (29). Thereby, Vpx might indirectly suppress type I IFN production during poxviral infections.
On the other hand, vpx could directly influence IFN expression. Royle et al. proposed the possibility that divergent paths of plasmacytoid DC (pDC) differentiation driven by HIV-1 and HIV-2 caused the lower pathogenicity of HIV-2 infections (30). HIV-2 infection of peripheral blood mononuclear cells (PBMC) preferentially induced an antigen-presenting cell (APC) phenotype in pDC, which might result in more efficient T cell-mediated immunity and an immune-mediated control of HIV-2 infections. HIV-2, but not HIV-1, inhibited IFN-α production in response to CpG-A (25). This suppressive effect of Vpx on IFN-α production might also apply to MVA.
In summary, Vpx expression by MVA increased viral late gene expression in human DCs, which might enhance vector immunogenicity, although fewer type I IFNs were produced. It will be interesting to analyze if late gene expression could override the lack of IFN, and humanized mice might be a useful model for this type of analysis (31). Additional knockout of F17L might be considered to increase the immunogenicity of MVA-vpx. In general, however, the introduction of foreign genes into poxvirus genomes should be done with care, because, as shown here, they might change the phenotype of the vector. MVA-vpx gives new insights into virus-host cell interaction, late gene functions, and vector safety considerations.
MATERIALS AND METHODS
Cell culture.
All cells used in this study were cultured at 37°C under 5% CO2. DF-1 (CRL-12203), HeLa (ATCC CCL-2), MDBK (CCL-22), HEK 293T (CRL-1573), and A549 (CCL-185) cells were grown in Dulbecco’s modified Eagle medium (DMEM; Lonza, Verviers, Belgium). MDCK (PTA-7910) cells were cultured in Eagle’s minimal essential medium (EMEM; Biochrom, Berlin, Germany). All media were supplemented with 10% fetal bovine serum (FBS, vol/vol; PAA, Pasching, Austria) and 5% l-glutamine (200 mM; Lonza, Verviers, Belgium).
Plasmid construction and generation of recombinant MVAs.
The MVA 13.5L promoter (32) followed by the SIV-PBj1.9 vpx gene were generated by DNA synthesis (Invitrogen, Life Technologies, Regensburg, Germany) with flanking BamHI and PstI restriction sites. This fragment was cloned into the MVA recombination plasmid pIII-H5-lacZ2-A, replacing the endogenous pmH5 promoter with the immediate early 13.5L promoter (21). This plasmid (pIII-vpx253) allows the integration of foreign DNA into the deletion site III of MVA. The second plasmid was constructed by cloning the vpx gene into the blunted BamHI site of pIII-H5-lacZ2-A, thereby generating the plasmid pIII-vpx249, which placed the expression of vpx under the transcriptional control of the synthetic vaccinia virus early/late promoter mH5.
The two recombinant MVA-vpx viruses were generated following standard procedures using the K1L selection method (33, 34). Briefly, MVA-infected BHK cells were transfected with pIII-vpx253 or pIII-vpx249 plasmid DNA, following clonal isolation of plaques on RK-13 cells. Positive clones were further propagated on DF-1 cells and checked by PCR analysis of genomic viral DNA for the presence of the transgene and absence of the K1L gene. MVA-vpx stock viruses were grown on DF-1 cells, purified by ultracentrifugation through sucrose cushions, reconstituted to vaccine stocks in Tris-buffered saline (pH 7.4), and stored at −80 C.
Primers and expected DNA fragments for PCR analysis were as follows: K1L gene (K1L int-1, 5′-TGATGACAAGGGAAACACCGC; K1L int-2, 5′-GTCGACGTCATATAGTCGAGC [290 bp]), deletion III (Del III fw, 5′-GTACCGGCATCTCTAGCAGT; Del III rev, 5′-TGACGAGCTTCCGAGTTCC; 1,856 bp for recombinant MVA, 700 bp for MVA wild type [wt], and 4,177 bp for VACV WR), deletion IV (Del IV for, 5′-CCTGGACATTTAGTTTGAGTGTTCC; Del IV rev, 5′-CCGTCTTTTCTGGTGTAATATTCTAG; 750 bp for MVA and 4,518 bp for VACV WR), and C7L gene (C7L for, 5′-ATGGGTATACAGCACGAATTC; C7L rev, 5′-CATGGACTCATAATCTCTATAC; 447 bp).
Determination of titers of virus stocks.
Titers of virus stock solutions were determined from serial 10-fold dilutions of the virus stock. Serial dilutions of the virus stocks were plated on confluent BHK-21 monolayers grown in 6-well plates and incubated for 48 h. Virus-infected cells were detected by immunostaining. Cells were fixed in acetone-methanol (1:1) for 5 min. After washing, cells were incubated in phosphate-buffered saline (PBS) and 3% fetal calf serum (FCS) for 2 h with polyclonal rabbit anti-vaccinia virus antibody (catalog no. BP1076; Origene Technologies, Herford, Germany). After the cells were washed with PBS, horseradish peroxidase (HRP)-conjugated polyclonal goat anti-rabbit antibody PBS-3% FCS (catalog no. 111-035-045; Dianova, Hamburg, Germany) was added, and the mixture was incubated for 45 min. After washing with PBS, antibody-labeled cells were developed using True Blue substrate (0.4 ml/well; VWR, Darmstadt, Germany) for 10 min. Cells were washed with water, foci of stained cells were counted, and virus titers were calculated (in IU/ml).
Quantitative real-time PCR.
Vaccinia virus DNA replication was analyzed by quantitative real-time PCR (qPCR) with a TaqMan probe. This allowed the amount of viral DNA to be quantified. DNA was amplified with the two primers (rpo OVP F1 and rpo OVP R1) flanking the rpo18 gene, which is highly conserved among the poxviruses. Quantification was performed with a calibration line established with dilutions of a solution of 108 copies of the rpo18 gene. The PCR analysis was performed with a LightCycler 1.2 instrument (Roche, Mannheim, Germany).
Primers were as follows: rpo OVP F1. 5′-CTGTAGTTATAAACGTTCCGTGTG; rpo OVP R1, 5′-TTATCATACGCATTACCATTTCGA; rpo OVP TM1 (TaqMan probe), 6FAM-ATCGCTAAATGATACAGTACCCGAATCTCTACT-BBQ (TIB Molbiol Syntheselabor GmbH, Berlin, Germany).
Template DNA was isolated from infected cells (MOI = 3) 24 h after infection using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany). For the PCRs, 37.5 μg of this template DNA was used.
Generation of dendritic cells from human primary peripheral blood CD14+ cells.
Residual buffy coats of healthy donors were obtained from the German Red Cross Blood donor Services Baden-Wuertemberg-Hessen (Frankfurt, Germany). Human peripheral blood-derived monocytes were isolated from buffy coats by Ficoll-Histopaque sedimentation (Sigma-Aldrich, Darmstadt, Germany). CD14+ cells were subsequently isolated using a human CD14 MicroBeads kit (Milteny Biotec, Bergisch Gladbach, Germany) and a magnetically activated cell-sorting (MACS) system (Milteny Biotec). The cells were plated at a density of 4 × 106 cells/well into 6-well tissue culture plates (Sarstedt, Nümbrecht, Germany). Cells were differentiated into DCs by incubation for 6 days in RPMI medium containing 10% FCS, 100 U penicillin, 0.1 mg/ml streptomycin, 2 mM glutamine, 1% 1 M HEPES buffer, 0.2% 25 mM β-mercaptoethanol, 5 ng/ml GM-CSF, and 10 ng/ml IL-4, and used for infections with poxviruses.
Interferon alpha and beta ELISA.
DCs were plated in 24-well plates at a density of 1 × 106 cells/well and infected with the poxviruses at an MOI of 1. Supernatant was collected after 12 h of infection. Analyses were carried out with enzyme-linked immunosorbent assay (ELISA) kits to determine the concentrations of human IFN-α (Mabtech, Biozol, Eching, Germany) and human IFN-β (PBL Assay Science, Acris, Herford, Germany). The vesicular stomatitis virus M2 strain was used as a strong inductor of type I IFN and was kindly provided by Zoe Waibler (26). Infection was done at an MOI of 1.
Western blot analysis.
The Western blot was performed with a Bio-Rad semidry blotter. Proteins separated by SDS-PAGE were blotted onto polyvinylidene difluoride (PVDF) membranes with 50 mM sodium borate (pH 9.0), 20% methanol, and 0.1% SDS at 100 mA per membrane for 75 min. Afterwards, membranes were blocked with Roti-Block, and proteins were detected with a custom made anti-B5 polyclonal antibody (Eurogentec, Cologne, Germany), an anti-rabbit HRP-coupled secondary antibody, and antibodies against SAMHD1 (catalog no. 12586-1-AP; Proteintech Group, Manchester, UK), Vpx (no. 2710; NIH AIDS Reagent Program), and β-actin (catalog no. A5441; Sigma, Munich, Germany), followed by the ECL detection system (Amersham, Freiburg, Germany).
Analysis of apoptosis induction.
Human dendritic cells were plated in 6-well plates as confluent layers and were infected with the different vaccinia viruses at an MOI of 3 or treated with 1 μM staurosporine in dimethyl sulfoxide (DMSO). Mock-infected cells were treated with the same volume of DMSO for a control. After 16 h, cells were washed with PBS and fixed in fluorescence-activated cell sorter (FACS) tubes with 250 μl Cytofix-Cytoperm (BD Biosciences, Heidelberg, Germany) for 30 min at 4°C. Following the fixation/permeabilization, cells were washed twice in Perm/Wash solution (BD Biosciences, Heidelberg, Germany). Cells were stained for active caspase 3 by incubation with 20 μl phycoerythrin (PE)-labeled anti-active caspase 3 antibody (catalog no. 550821; BD Biosciences, Heidelberg, Germany) and 50 μl Perm/Wash solution for 30 min at room temperature, washed with Perm/Wash solution, and analyzed with a BD LSR II flow cytometer (BD Biosciences, Heidelberg, Germany).
ACKNOWLEDGMENTS
We thank Maren Krause, Lisa Henß, and Heike Baumann for excellent technical assistance.
REFERENCES
- 1.Sutter G, Moss B. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A 89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HCT, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LPS, Stoye JP, Crow YJ, Taylor IA, Webb M. 2011. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 6.Kim B, Nguyen LA, Daddacha W, Hollenbaugh JA. 2012. Tight interplay among SAMHD1 protein level, cellular dNTP levels, and HIV-1 proviral DNA synthesis kinetics in human primary monocyte-derived macrophages. J Biol Chem 287:21570–21574. doi: 10.1074/jbc.C112.374843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergamaschi A, Ayinde D, David A, Le Rouzic E, Morel M, Collin G, Descamps D, Damond F, Brun-Vezinet F, Nisole S, Margottin-Goguet F, Pancino G, Transy C. 2009. The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection. J Virol 83:4854–4860. doi: 10.1128/JVI.00187-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goujon C, Arfi V, Pertel T, Luban J, Lienard J, Rigal D, Darlix J-L, Cimarelli A. 2008. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J Virol 82:12335–12345. doi: 10.1128/JVI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollenbaugh JA, Gee P, Baker J, Daly MB, Amie SM, Tate J, Kasai N, Kanemura Y, Kim DH, Ward BM, Koyanagi Y, Kim B. 2013. Host factor SAMHD1 restricts DNA viruses in non-dividing myeloid cells. PLoS Pathog 9:e1003481. doi: 10.1371/journal.ppat.1003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drillien R, Spehner D, Bohbot A, Hanau D. 2000. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology 268:471–481. doi: 10.1006/viro.2000.0203. [DOI] [PubMed] [Google Scholar]
- 11.Kastenmuller W, Drexler I, Ludwig H, Erfle V, Peschel C, Bernhard H, Sutter G. 2006. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology 350:276–288. doi: 10.1016/j.virol.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Chahroudi A, Garber DA, Reeves P, Liu L, Kalman D, Feinberg MB. 2006. Differences and similarities in viral life cycle progression and host cell physiology after infection of human dendritic cells with modified vaccinia virus Ankara and vaccinia virus. J Virol 80:8469–8481. doi: 10.1128/JVI.02749-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamburuthugoda VK, Chugh P, Kim B. 2006. Modification of human immunodeficiency virus type 1 reverse transcriptase to target cells with elevated cellular dNTP concentrations. J Biol Chem 281:13388–13395. doi: 10.1074/jbc.M600291200. [DOI] [PubMed] [Google Scholar]
- 14.Traut TW. 1994. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 140:1–22. doi: 10.1007/bf00928361. [DOI] [PubMed] [Google Scholar]
- 15.Rehwinkel J, Maelfait J, Bridgeman A, Rigby R, Hayward B, Liberatore RA, Bieniasz PD, Towers GJ, Moita LF, Crow YJ, Bonthron DT, Reis e Sousa C. 2013. SAMHD1-dependent retroviral control and escape in mice. EMBO J 32:2454–2462. doi: 10.1038/emboj.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drexler I, Staib C, Kastenmuller W, Stevanovic S, Schmidt B, Lemonnier FA, Rammensee HG, Busch DH, Bernhard H, Erfle V, Sutter G. 2003. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci U S A 100:217–222. doi: 10.1073/pnas.262668999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasteiger G, Kastenmuller W, Ljapoci R, Sutter G, Drexler I. 2007. Cross-priming of cytotoxic T cells dictates antigen requisites for modified vaccinia virus Ankara vector vaccines. J Virol 81:11925–11936. doi: 10.1128/JVI.00903-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schüle S, Kloke B-P, Kaiser JK, Heidmeier S, Panitz S, Wolfrum N, Cichutek K, Schweizer M. 2009. Restriction of HIV-1 replication in monocytes is abolished by Vpx of SIVsmmPBj. PLoS One 4:e7098. doi: 10.1371/journal.pone.0007098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer H, Sutter G, Mayr A. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol 72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 20.Wyatt LS, Shors ST, Murphy BR, Moss B. 1996. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine 14:1451–1458. doi: 10.1016/S0264-410X(96)00072-2. [DOI] [PubMed] [Google Scholar]
- 21.Baur K, Brinkmann K, Schweneker M, Pätzold J, Meisinger-Henschel C, Hermann J, Steigerwald R, Chaplin P, Suter M, Hausmann J. 2010. Immediate-early expression of a recombinant antigen by modified vaccinia virus Ankara breaks the immunodominance of strong vector-specific B8R antigen in acute and memory CD8 T-cell responses. J Virol 84:8743–8752. doi: 10.1128/JVI.00604-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backes S, Sperling KM, Zwilling J, Gasteiger G, Ludwig H, Kremmer E, Schwantes A, Staib C, Sutter G. 2010. Viral host-range factor C7 or K1 is essential for modified vaccinia virus Ankara late gene expression in human and murine cells, irrespective of their capacity to inhibit protein kinase R-mediated phosphorylation of eukaryotic translation initiation factor 2α. J Gen Virol 91:470–482. doi: 10.1099/vir.0.015347-0. [DOI] [PubMed] [Google Scholar]
- 23.Nitsche A, Stern D, Ellerbrok H, Pauli G. 2006. Detection of infectious poxvirus particles. Emerg Infect Dis 12:1139–1141. doi: 10.3201/eid1207.060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veyer DL, Carrara G, Maluquer de Motes C, Smith GL. 2017. Vaccinia virus evasion of regulated cell death. Immunol Lett 186:68–80. doi: 10.1016/j.imlet.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Royo S, Sainz B, Hernandez-Jimenez E, Reyburn H, Lopez-Collazo E, Guerra S. 2014. Differential induction of apoptosis, interferon signaling, and phagocytosis in macrophages infected with a panel of attenuated and nonattenuated poxviruses. J Virol 88:5511–5523. doi: 10.1128/JVI.00468-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waibler Z, Anzaghe M, Frenz T, Schwantes A, Pöhlmann C, Ludwig H, Palomo-Otero M, Alcamí A, Sutter G, Kalinke U. 2009. Vaccinia virus-mediated inhibition of type I interferon responses is a multifactorial process involving the soluble type I interferon receptor B18 and intracellular components. J Virol 83:1563–1571. doi: 10.1128/JVI.01617-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng X, Ratner L. 2014. HIV-2 Vpx protein interacts with interferon regulatory factor 5 (IRF5) and inhibits its function. J Biol Chem 289:9146–9157. doi: 10.1074/jbc.M113.534321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaglesham JB, Pan Y, Kupper TS, Kranzusch PJ. 2019. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature 566:259–263. doi: 10.1038/s41586-019-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meade N, Furey C, Li H, Verma R, Chai Q, Rollins MG, DiGiuseppe S, Naghavi MH, Walsh D. 2018. Poxviruses evade cytosolic sensing through disruption of an mTORC1-mTORC2 regulatory circuit. Cell 174:1143–1157.e17. doi: 10.1016/j.cell.2018.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royle CM, Graham DR, Sharma S, Fuchs D, Boasso A. 2014. HIV-1 and HIV-2 differentially mature plasmacytoid dendritic cells into IFN-producing cells or APCs. Ji 193:3538–3548. doi: 10.4049/jimmunol.1400860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akkina R. 2013. Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol 25:403–409. doi: 10.1016/j.coi.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wennier ST, Brinkmann K, Steinhäußer C, Mayländer N, Mnich C, Wielert U, Dirmeier U, Hausmann J, Chaplin P, Steigerwald R. 2013. A novel naturally occurring tandem promoter in modified vaccinia virus Ankara drives very early gene expression and potent immune responses. PLoS One 8:e73511. doi: 10.1371/journal.pone.0073511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staib C, Drexler I, Sutter G. 2004. Construction and isolation of recombinant MVA. Methods Mol Biol 269:77–100. doi: 10.1385/1-59259-789-0:077. [DOI] [PubMed] [Google Scholar]
- 34.Staib C, Drexler I, Ohlmann M, Wintersperger S, Erfle V, Sutter G. 2000. Transient host range selection for genetic engineering of modified vaccinia virus Ankara. Biotechniques 28:1137–1142. doi: 10.2144/00286st04. [DOI] [PubMed] [Google Scholar]