Abstract
This letter reports on a novel cost-efficient and multifunctional barcode-like sensors array (BLSA) printed with a conductive bioinspired smart ink. The conductive ink (P@G ink), which can be further chemically engineered with different organic ligands, was generated via facile one-pot hydrothermal reduction of graphene oxide (GO) in dopamine (DA) as coreductan Usingvarious chemical derivatives of the P@G inks on a flexible substrate (e.g., Kapton), a highly integrated BLSA as well as smart nose/tongue mimic array were generated for simultaneous sensing and distinguishing of complex physical and chemical stimuli, including temperature, light, air pressure, relative humidity, and volatile organic compounds (VOCs). Due to these very attractive features, the reported P@G ink-based BLSA would have the potential for unique opportunities regarding “all-in-one”—yet cost-effective—disposable electronics and sensors.
Keywords: bioinspired ink, electronic nose/tongue, multifunctional sensor, pattern recognition, barcode
Since common real applications are usually presented in/as mixture of stimuli,1 it is particularly appealing and important to unveil multifunctional (or multiparametric) sensing systems that allow simultaneous sensing and differentiation of different chemical stimuli (e.g., humidity and volatile organic compounds, VOCs) and physical stimuli (e.g., temperature and pressure).2 In recent years, many examples of multifunctional sensing techniques have been proven in multifarious aspects, such as wearable electronics,3 humanoid robotics,4 precision agriculture,5 and health monitoring.6 Field effect transistor (FET)/chemiresistor-based electronics integrated with the capacity of functional nanomaterials (e.g., carbon nanotubes, metal nanoparticles, etc.) have been fabricated as both solid-state and flexible and/or stretchable sensors for multifunctional sensing.7−11 Although great progress has been made, sophisticated complexity (e.g., designs and/or techniques) is often required and considerable costs are incurred in the fabrication process of most electronic sensors, posing certain barriers to their widespread application.12 It therefore remains a long-standing scientific and technological challenge to promote high-performance multifunctional electronics with low-cost materials and/or fabrication, easy operating principles, facile interpreted outputs, good reproducibility, and simple/rapid scalable implementation.
To lessen the complexity and costs that accompany the routine procedures of electronic device fabrication, emerging printed/written electronics and epidermal electronics (electronic tattoos) utilizing functional inks now provide researchers with numerous options,13−15 opening the way to rapid and sustainable low-cost fabrication of systems for many different applications including flexible sensors, photodetectors, displays, logic circuits, portable batteries, and radiofrequency identification (RFID) tags.16−19 In general, silver-based nanomaterials, carbon-based nanomaterials, and their derivatives are frequently used as functional inks in these applications.20,21 It is highly desirable that ink technologies would incorporate other functional materials for sprouting novel smart inks with multiple concurrent functions. In nature, there are hugely well-adapted structures and materials with unique properties and/or functionalities. For instance, the cuttlefish (a type of mollusc) releases a diffuse cloud of ink from its glands as an escape mechanism against predators. This ink is a mixed electronic–protonic material, in part made up of melanin (an indole-based natural pigment).22 The mussel (another mollusc) has extraordinarily secure adhesion to diverse substrates, even to wet surfaces, due to adhesive foot proteins, which is also shared with the catecholic nature of melanin-like cuttlefish ink.23 In learning from nature, melanin and melanin-related biopolymers (e.g., polydopamine) as functional materials have now been involved in many excellent biochemical and biomedical applications.24 Enlightened by this approach, developing and mining bioinspired materials with tailored properties/functions gives us the potential to find unprecedented concepts for the development of new electronic applications.
In this study, we present fundamentally fascinating writing electronics for multifunctional sensing via the combination of a conductive bioinspired smart ink and a creative design of a barcode-like sensor array (BLSA). The smart ink can be readily prepared in batches, easily engineered with tailor-made interfaces, and universally written on suitable low-cost substrates (e.g., Kapton) to build flexible BLSAs, which can act as distinguishing signal indicators of many different stimuli (i.e., temperature, light exposure, relative humidity, air pressure, and VOCs). The present study should greatly underpin scalable and cost-effective development of wearable and portable electronic devices for the future.
Conductive ink is chosen for our proposed barcode-like sensor array (BLSA) due to its cost-effectiveness and accessibility for large-scale production. Moreover, it can be readily dispersed in green chemical solvents, possesses good tailoring performances, and coats different substrates well in a binder-free format. Thus, the development of BLSA begins with the fabrication of an appropriate smart ink. In this respect, we have employed a simple one-pot hydrothermal reduction of graphene oxide (GO) in dopamine (DA) as coreductant to fabricate a bioinspired smart ink (P@G ink), as referred to in our recent work,2 which can be easily dispersed in ethanol. As a control, reduced GO (rGO) and polydopamine (PDA) were prepared under the same condition as P@G ink. Transmission electron microscopy (TEM) was used to characterize the morphology of GO, rGO, PDA, and P@G ink (Figure S1). The colloidal viscosity property of P@G ink was further examined by a rheometer. The P@G ink (4 mg mL–1) showed a viscosity of 2.16–0.01 Pa·s at the range of 0.01–500 s–1 shear rate (Figure S2). We verified that P@G ink is excellent for coating frequently used substrates (e.g., Kapton, a commercially available polyimide film) without any need for additional surface treatment (Figure 1a). Moreover, rapid drying is achieved with ethanol as the eco-friendly solvent, and the binder-free style of P@G ink is also of significant advantage over other reported inks regarding the issue of high-speed, green-chemical, and large-scale fabrication of flexible resistors18,19,21 (Figure 1b). With the assistance of commercially available copper tapes as the alignment, P@G ink can be written onto Kapton pieces to unveil controlled variable-width barcode-like stripes (Figure 1c). More interestingly, thanks to its unveiled biosafety,2 P@G ink can be written on fingernails (Figure 1d) or skin (Figure S3) as a conductive tattoo, which is waterproof (Figure S3) and wearable (>3 days). The waterproof and wearable capabilities of P@G ink tattoos can result from the strong affinity of the mussel-inspired PDA matrix with the rGO.24 Apart from the smart coating/writing properties of P@G ink discussed above, the integration of conductivity also offers the P@G ink-written substrates as electronics with sensory characteristics. As a proof-of-concept design, fingernail writings with P@G ink are prepared and linked with wires (Figure 1d) to act as a wearable sensory electronics to different stimuli, such as breath (Figure 1e) and UV exposure (Figure 1f), owing to the thermoconductivity and photoconductivity of rGO as a component.25,26
Figure 1.
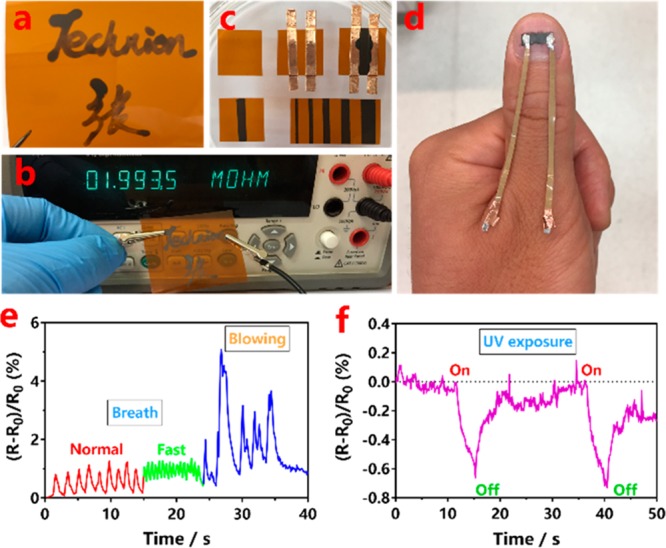
(a,b) Images showing conductive texts written on transparent Kapton film. (c) Writing Kapton film with controlled variable-width ink stripes. (d) Image showing that P@G ink can be written on a fingernail as a conductive tattoo and a wearable resistor. (e) The resistive responses of P@G ink-based wearable fingernail sensory electronics toward breath and (f) UV exposure.
We must bear in mind that P@G ink-written electronics can respond differently to many stimuli. Since many common stimuli are among the complexity met in real life, the main concern lies in the effective classification and identification of each stimulus. To address this issue, array-based “chemical nose/tongue” sensing approaches, analogous to mammalian olfaction and tasting,27 would work by discriminating a distinct pattern of responses generated by the analytes, leading to a fingerprint for the classification and identification of each analyte. The array-based nose/tongue-mimic strategy depends on the interactions between multiple reporter elements and the analytes of interest.28,29 Our P@G ink can be developed as multiple reporters, because the catechol in its PDA matrix is reactive and can be easily oxidized into quinone under basic conditions. As shown in Figure S4, the resultant quinone can react with thiol-containing chemicals by a pathway of Michael addition, and amine-containing chemicals by means of a Schiff base reaction/Michael addition in a simple and facile mix-and-react manner at room temperature.24
Five organic ligands including 2 thiol-containing small molecules (T1, T2), 2 amine-containing small molecules (N1, N2), and one amine-containing polymer (P) were chosen for engineering the surface of P@G ink (Table S1). The surface morphology of the dried inks was characterized by using scanning electron microscope (SEM) (Figure S5). The results reveal that the different ligands can significantly influence the surface structure of dried inks, and the resultant surfaces have distinct water contact angles (CA) (Figure S6). It has been reported that the performances of nanoparticle-based electronic sensors can be tuned by modification of ligands with different types or chain lengths with regard to temperature, volatile organic compounds (VOCs), etc.12 Thus, chemical ligand capped P@G inks were tailored to contain adequate chemical diversity for the development of a nose/tongue-mimic array regarding both physical (e.g., temperature) and chemical stimuli (e.g., VOCs). As demonstrated in Figure 1c, P@G ink was written with precise alignment on many substrates. Predictably, different chemically modified P@G inks written on a single piece of a substrate in barcode style would spark a novel planar sensor array, representing an integrated strategy to train its sensory responses to multiple stimuli. Thus, an innovative barcode-like sensors array (BLSA) was developed by P@G ink and 5 ligand-modified P@G inks (T1-P@G, T2-P@G, N1-P@G, N2-P@G, and P-P@G) written on a Kapton film (Figure 2).
Figure 2.
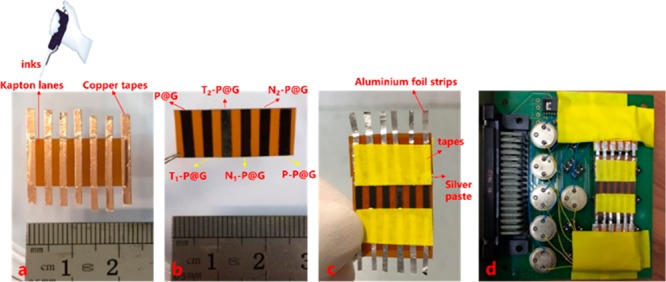
(a–c) Images giving examples of the design and fabrication of a 6-lane BLSA by different inks integrated on a Kapton film. (d) BLSA fixed onto an electronic board for testing.
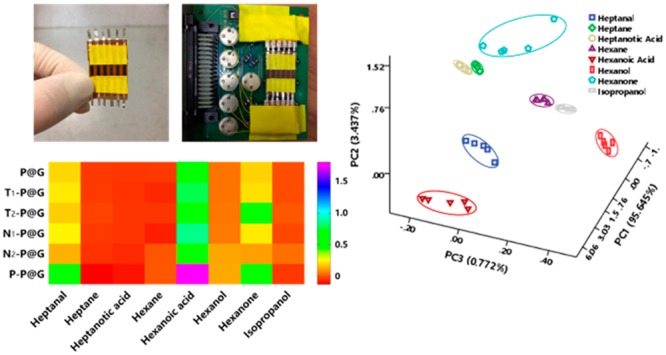
The multifunctional sensing abilities of BLSA to physical and chemical stimuli (temperature, light exposure, air pressure, RH, and VOCs) were systematically investigated (Figure 3 and Figures S7–S11). Full details of the experiments and data analysis are set out in the Supporting Information. Figure 3 shows that each type of stimulus has its own highly individual color-change barcode pattern of resistance responses using chemically modified P@G inks as the sensor array. Note that the selected chemical ligands play an important role; for instance, P–P@G ink sensor has stronger responses to all the indicated stimuli compared with the other chemically modified P@G ink sensors, which may be attributed to the polymer ligand (polyethylenimine) used having a higher molecular weight and volume for better tuning of the responsiveness of these P@G inks. Hence, P@G inks modified with distinct chemical ligands and linked to the design of BLSA, as a new type of pattern recognition element, can effortlessly generate substantial stimulus-relevant pattern responses for further sensing applications.
Figure 3.
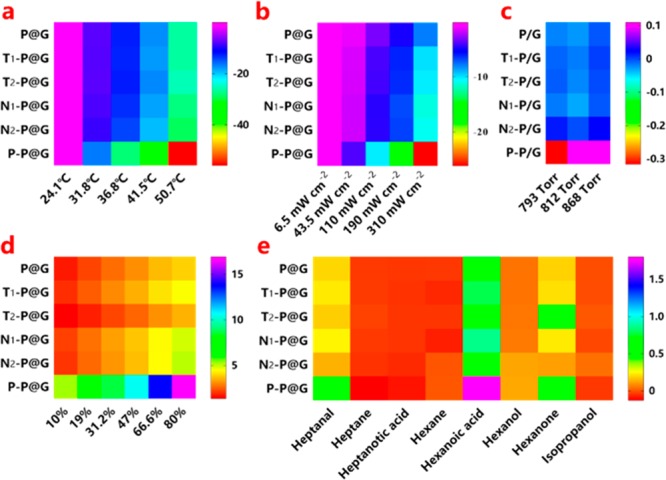
Heat maps represent the responses of BLSA to temperature (a), light exposure (b), air pressure (c), relative humidity (d), and volatile organic compounds (e).
To differentiate the resulting patterns of BLSA for each stimulus, principal component analysis (PCA, a powerful computational technique)30 was used (Figures S7–S12). Five replicates of resistance responses were recorded for each stimulus with different degrees against BLSA, and then dealt with PCA to yield 3 canonical factors, which denoted linear combinations of the resistivity-response matrix (6 barcode-like ink sensors × the number of analytes × 5 replicates). The 2 most significant factors were then used to create a 2D plot (Figures S7d, S8d, S9c, S10d, S11b, and S12c), in which each point represents the response pattern for an individual stimulus sample against BLSA. From the results, each stimulus with varied degrees is clearly distinguished in this BLSA pattern recognition system, confirming the feasibility of BLSA as a novel and smart chemical nose/tongue. Since the second discriminant factor (PC2) is <40%, it is possible to simply apply the first discriminant factor (PC1) for quantitatively characterizing the varying extents of each stimulus (Figures S7e, S8e, S10e, and S12d).
Considerably different responses were ranges can be found between the studied stimuli using BLSA (Figures S7a–S11a). In this case, it is envisaged that this BLSA would distinguish between multiple stimuli by PCA. For this propose, we considered the pattern responses of all the stimuli together. Figure 4 shows that the canonical resistance–response patterns from all stimuli (temperature, air pressure, VOCs, RH, and light exposure) could be effectively clustered into distinct groups in the independent region of the 2D PCA plot, verifying that the BLSA ability to distinguish between complex stimuli, permitting the simultaneous sensing of a range of stimuli. The power of our BLSA comes from the as-prepared hybrid material, affording superior synergy between the bioinspired PDA and the rGO, as discussed above. Our proposed BLSA-based pattern recognition platform would be referentially evolved to monitor biochemical analytes relating to different responsive biochemical ligands (e.g., DNA) via PDA’s versatile reactivity, and further in evaluating their molecular interactions. It is reported that medical device companies encounter many challenges in matching today’s technology desires while balancing strong industry cost pressures. Every feature and every benefit need to be balanced against cost to make for a successful value-based solution.31 In response to this issue, the highly integrated design and easy-to-write nature of BLSA on flexible and commercially available Kapton provide enormous potential for the development of “all-in-one”—yet low-cost—disposable electronics.
Figure 4.
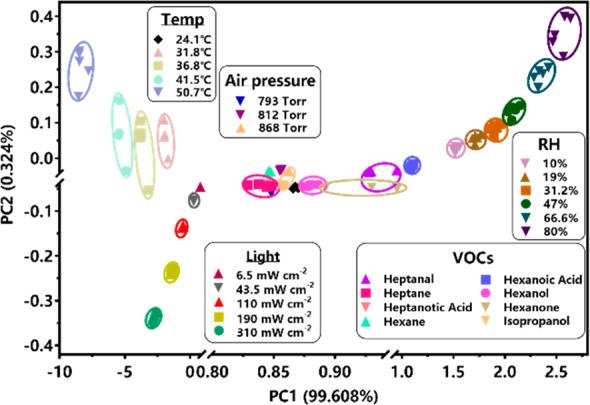
Canonical score plot of BLSA’s responses to temperature, light exposure, air pressure, relative humidity (RH), and volatile organic compounds (VOCs).
Since there is some overlap between the resistance–response patterns from the VOCs that were tested in the 2D PCA plots using BLSA, we used a three-dimensional (3D) PCA plot to fully elucidate the differences in the VOC-related resistance–response patterns (Figure 5). Consequently, the 8 VOCs tested (which included aldehyde, alkane, acid, ketone, and alcohol listed in Table S2) were clearly differentiated using BLSA. VOCs are produced in the human body as products of metabolic processes, and metabolomics analysis of VOCs (i.e., volatolomics) emitted from biological fluids (breath, skin, blood, urine, etc.) can be clinically valuable as diagnostic indicators, this approach being a noninvasive means of identifying different pathologies, e.g. cancer, diabetes mellitus, wound infection, etc.32,33 From these results, it is expected that the our BLSA would be a useful tool for these purposes in healthcare applications.
Figure 5.
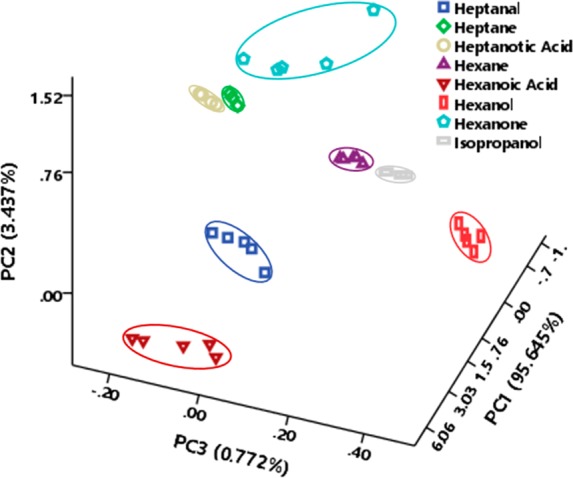
3D canonical score plot for the response pattern of BLSA as obtained from PCA against 10 ppm VOCs.
To summarize, we have brought the first design of barcode-like sensor array (BLSA), written with a conductive bioinspired smart ink to be of use in multifunctional sensory applications. This smart ink (P@G ink) results from the integration of a melanin-related biopolymer (PDA) and graphene oxide (GO) in a one-pot routine reaction. A range of chemically engineered P@G inks can be written on a flexible substrate (e.g., Kapton) to configure a novel and highly integrated BLSA. BLSA is a low-cost yet powerful method of responding to multiple chemical and physical stimuli, and has the ability to effectively differentiate between complex stimuli during their simultaneous sensing, especially in the way that it can discriminate VOCs. Because of these intriguing features, we expect that the novel P@G ink-based BLSA electronics will lead to many versatile and meaningful sensing applications, and inspire further research on bioinspired smart electronics.
Acknowledgments
This research was supported from the Phase-II Grand Challenges Explorations award of the Bill and Melinda Gates Foundation (OPP1109493) and Horizon 2020 ICT Pro-grant under the SNIFFPHONE (644031). M.Z. also thanks the support from the National Natural Science Foundation of China (21775044, 21675053, 21635003), Key Project of the Shanghai Science and Technology Committee (19ZR1473300, 19411971700, 18DZ1112700), and the Fundamental Research Funds for the Central Universities.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssensors.9b01561.
Chemicals, instrumentation, prepared of tested compounds, evaluation, and data analysis (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wu W.; Haick H. Materials and wearable devices for autonomous monitoring of physiological markers. Adv. Mater. 2018, 30, 1705024. 10.1002/adma.201705024. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Sun J. J.; Khatib M.; Lin Z. Y.; Chen Z. H.; Saliba W.; Gharaa A.; Horev Y. D.; Kloper V.; Milyutin Y.; Huynh T. P.; Brandon S.; Shi G.; Haick H. Time-space-resolved origami hierarchical electronics for ultrasensitive detection of physical and chemical stimuli. Nat. Commun. 2019, 10, 1120. 10.1038/s41467-019-09070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariya M.; Nyein H. Y. Y.; Javey A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. 10.1038/s41928-018-0043-y. [DOI] [Google Scholar]
- Park J.; Kim M.; Lee Y.; Lee H. S.; Ko H. Fingertip skin–inspired microstructured ferroelectric skins discriminate static/dynamic pressure and temperature stimuli. Sci. Adv. 2015, 1, e1500661 10.1126/sciadv.1500661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futagawa M.; Iwasaki T.; Murata H.; Ishida M.; Sawada K. A miniature integrated multimodal sensor for measuring pH, EC and temperature for precision agriculture. Sensors 2012, 12, 8338–8354. 10.3390/s120608338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G.; Tee B. C. K.; Mei J.; Appleton A. L.; Kim D. H.; Wang H.; Bao Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. 10.1038/ncomms2832. [DOI] [PubMed] [Google Scholar]
- Yang S.; Chen Y. C.; Nicolini L.; Pasupathy P.; Sacks J.; Su B.; Yang R.; Sanchez D.; Chang Y. F.; Wang P.; Schnyer D.; Neikirk D.; Lu N. Cut-and-paste” manufacture of multiparametric epidermal sensor systems. Adv. Mater. 2015, 27, 6423–6430. 10.1002/adma.201502386. [DOI] [PubMed] [Google Scholar]
- Jin H.; Huynh T. P.; Haick H. Self-healable sensors based nanoparticles for detecting physiological markers via skin and breath: toward disease prevention via wearable devices. Nano Lett. 2016, 16, 4194–4202. 10.1021/acs.nanolett.6b01066. [DOI] [PubMed] [Google Scholar]
- Lipomi D. J.; Vosgueritchian M.; Tee B. C. K.; Hellstrom S. L.; Lee J. A.; Fox C. H.; Bao Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. 10.1038/nnano.2011.184. [DOI] [PubMed] [Google Scholar]
- Shehada N.; Cancilla J. C.; Torrecilla J. S.; Pariente E. S.; Brönstrup G.; Christiansen S.; Johnson D. W.; Leja M.; Davies M. P. A.; Liran O.; Peled N.; Haick H. Silicon nanowire sensors enable diagnosis of patients via exhaled breath. ACS Nano 2016, 10, 7047–7057. 10.1021/acsnano.6b03127. [DOI] [PubMed] [Google Scholar]
- Peng G.; Tisch U.; Adams O.; Hakim M.; Shehada N.; Broza Y. Y.; Billan S.; Abdah-Bortnyak R.; Kuten A.; Haick H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- Segev-Bar M.; Bachar N.; Wolf Y.; Ukrainsky B.; Sarraf L.; Haick H. Multi-parametric sensing platforms based on nanoparticles. Adv. Mater. Technol. 2017, 2, 1600206. 10.1002/admt.201600206. [DOI] [Google Scholar]
- Zhu Z.; Guo S. Z.; Hirdler T.; Eide C.; Fan X.; Tolar J.; McAlpine M. C. 3D printed functional and biological materials on moving freeform surfaces. Adv. Mater. 2018, 30, 1707495. 10.1002/adma.201707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A.; Ahn B. Y.; Adams J. J.; Duoss E. B.; Bernhard J. T. J.; Lewis A. Pen-on-paper flexible electronics. Adv. Mater. 2011, 23, 3426–3430. 10.1002/adma.201101328. [DOI] [PubMed] [Google Scholar]
- Kim D. H.; Lu N.; Ma R.; Kim Y. S.; Kim R. H.; Wang S.; Wu J.; Won S. M.; Tao H.; Islam A.; Yu K. J.; Kim T.; Chowdhury R.; Ying M.; Xu L.; Li M.; Chung H. J.; Keum H.; McCormick M.; Liu P.; Zhang Y. W.; Omenetto F. G.; Huang Y.; Coleman T.; Rogers J. A. Epidermal electronics. Science 2011, 333, 838–843. 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- Yeo W. H.; Kim Y. S.; Lee J.; Ameen A.; Shi L.; Li M.; Wang S.; Ma R.; Jin S. H.; Kang Z.; Huang Y.; Rogers J. A. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 2013, 25, 2773–2778. 10.1002/adma.201204426. [DOI] [PubMed] [Google Scholar]
- Chen K.; Gao W.; Emaminejad S.; Kiriya D.; Ota H.; Nyein H. Y. Y.; Takei K.; Javey A. Printed carbon nanotube electronics and sensor systems. Adv. Mater. 2016, 28, 4397–4414. 10.1002/adma.201504958. [DOI] [PubMed] [Google Scholar]
- Hu G.; Kang J.; Ng L. W. T.; Zhu X.; Howe R. C. T.; Jones C. G.; Hersam M. C.; Hasan T. Functional inks and printing of two-dimensional materials. Chem. Soc. Rev. 2018, 47, 3265–3300. 10.1039/C8CS00084K. [DOI] [PubMed] [Google Scholar]
- Bonaccorso F.; Bartolotta A.; Coleman J. N.; Backes C. 2D-crystal-based functional inks. Adv. Mater. 2016, 28, 6136–6166. 10.1002/adma.201506410. [DOI] [PubMed] [Google Scholar]
- Bashouti Y. M.; Resch S.; Ristein J.; Mačković M.; Spiecker E.; Waldvogel R. S.; Christiansen S. Functionalization of silver nanowires surface using Ag-C bonds in a sequential reductive method. ACS Appl. Mater. Interfaces 2015, 7, 21657–21661. 10.1021/acsami.5b06830. [DOI] [PubMed] [Google Scholar]
- Cheng C.; Zhang J.; Li S.; Xia Y.; Nie C.; Shi Z.; Cuellar-Camacho J. L.; Ma N.; Haag R. A water-processable and bioactive multivalent graphene nanoink for highly flexible bioelectronic films and nanofbers. Adv. Mater. 2018, 30, 1705452. 10.1002/adma.201705452. [DOI] [PubMed] [Google Scholar]
- Derby C. D. Cephalopod ink: production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700–2730. 10.3390/md12052700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications—cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. 10.3390/ijms18071561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Ai K.; Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- Park W.; Hu J.; Jauregui L. A.; Ruan X.; Chen Y. P. Electrical and thermal conductivities of reduced graphene oxide/polystyrene composites. Appl. Phys. Lett. 2014, 104, 113101. 10.1063/1.4869026. [DOI] [Google Scholar]
- Liang H.; Ren W.; Su J.; Cai C. Photoconductivity of reduced graphene oxide and graphene oxide composite films. Thin Solid Films 2012, 521, 163–167. 10.1016/j.tsf.2011.12.086. [DOI] [Google Scholar]
- Persaud K.; Dodd G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. 10.1038/299352a0. [DOI] [PubMed] [Google Scholar]
- Hu W.; Wan L.; Jian Y.; Ren C.; Jin K.; Su X.; Bai X.; Haick H.; Yao M.; Wu W. Electronic Noses: From Advanced Materials to Sensors Aided with Data Processing. Adv. Mater. Technol. 2018, 4, 1800488. 10.1002/admt.201800488. [DOI] [Google Scholar]
- Xue S. F.; Chen Z. H.; Han X. Y.; Lin Z. Y.; Wang Q. X.; Zhang M.; Shi G. DNA encountering terbium(III): A smart “chemical nose/tongue” for large-scale time-gated luminescent and lifetime-based sensing. Anal. Chem. 2018, 90, 3443–3451. 10.1021/acs.analchem.7b05167. [DOI] [PubMed] [Google Scholar]
- Jurs P. C.; Bakken G. A.; McClelland H. E. Computational methods for the analysis of chemical sensor array data from volatile analytes. Chem. Rev. 2000, 100, 2649–2678. 10.1021/cr9800964. [DOI] [PubMed] [Google Scholar]
- https://www.mpo-mag.com/contents/view_online-exclusives/2018-06-29/electronics-integration-into-disposable-and-single-use-medical-devices/.
- Cozzolino R.; Magistris L. D.; Saggese P.; Stocchero M.; Martignetti A.; Stasio M. D.; Malorni A.; Marotta R.; Boscaino F.; Malorni L. Use of solid-phase microextraction coupled to gas chromatography–mass spectrometry for determination of urinary volatile organic compounds in autistic children compared with healthy controls. Anal. Bioanal. Chem. 2014, 406, 4649–4662. 10.1007/s00216-014-7855-z. [DOI] [PubMed] [Google Scholar]
- Broza Y. Y.; Vishinkin R.; Barash O.; Nakhleh M. K.; Haick H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. 10.1039/C8CS00317C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


