Abstract
Aims
We investigated the prevalence of severe asthma, its comorbidities, and especially the use of oral corticosteroid (OCS) therapy in patients with severe asthma.
Methods
Pooled data from 3 961 429 patients insured (with statutory health insurance) during the year 2015 were analysed. Prevalence rates of severe asthma and its OCS-associated comorbidities in patients on high-dosage (HD) inhaled corticosteroid (ICS) in combination with a long-acting β agonist (LABA) therapy were compared with those of patients who were also treated with OCSs.
Results
The asthma prevalence was 7.3%, of which 8.7% (0.6% absolute) were treated with HD-ICS/LABAs. Of these, 33.6% received additional OCSs with calculated dosages between 0.9 and 9.1 mg·day−1. More than 80% of patients on HD-ICS/LABAs had at least one comorbidity. Disorders of the heart (67.5%), metabolism/ nutrition (51.4%), psychiatric disorders (36.0%), skeletal muscle/connective tissue and bone disorders (20.3%), and eye disorders (20.0%) were predominant. The prevalence of these disorders increased for patients also receiving OCS therapy, depending on the length of treatment. Mean therapy costs ranged from €4266 per patient without OCS therapy to €11 253 per patient on long-term OCS treatment. The largest share of costs was attributable to inpatient care.
Conclusion
The analyses show that OCSs are frequently prescribed in patients receiving HD-ICS/LABAs because of severe asthma and are they are frequently associated with adverse effects commonly reported with steroid usage. These data support a necessary change in severe asthma treatment, which is reflected in current treatment guidelines.
Short abstract
The prevalence of severe asthma in Germany is substantial. OCS therapy is frequent and associated with adverse effects. The data support a need for change in severe asthma treatment, which is already reflected in recent treatment guidelines. http://bit.ly/2z102iV
Introduction
More than 330 million people suffer from bronchial asthma worldwide [1]. Adequate therapy enables many patients to achieve good asthma control. However, some patients with asthma remain inadequately controlled despite regular high-dosage (HD) administration of an inhaled corticosteroid (ICS) in combination with a second controller (usually a long-acting β2 sympathomimetic (LABA)). These patients are classified with severe asthma according to the Global Initiative for Asthma (GINA) recommendation [2–4]. In contrast, the European Respiratory Society/American Thoracic Society Task Force on Severe Asthma considers that the definition of severe asthma is recovered for patients with severe refractory asthma [3]. Patients with severe asthma often suffer from persistent symptoms like cough, shortness of breath and tightness in the chest, acute exacerbations and substantially impaired health-related quality of life. Few data exist about the exact prevalence of severe asthma. Estimations suggest that approximately 5–10% of all asthma patients are severe [5]; however, a more detailed analysis has reported a lower prevalence (3.6% of patients in the Netherlands) [6].
In daily routine care, many patients with severe, inadequately controlled asthma are dependent on oral corticosteroid (OCS) therapy. Because of their effectiveness for acute conditions, steroids continue to be frequently used not only for the management of acute exacerbations (OCS bursts at usually high dosages), but also as an anti-inflammatory maintenance therapy (OCS long-term therapy). At the same time, however, there is growing awareness that repeated use, especially in long-term therapy, is often accompanied by major adverse effects.
The most common OCS-induced comorbidities are osteoporosis, lipid metabolism disorders, psychiatric disorders, cardiovascular disorders (especially hypertension), diabetes mellitus, and cataracts [7–12]. It has also been postulated that short-term treatment with OCS (e.g. in the context of an OCS burst) can trigger long-term adverse effects and secondary injury [11, 12]. So far, however, there have been a lack of data analyses investigating this for the German healthcare landscape to determine both the burden of disease for these patients and the partly high direct and indirect costs associated with OCS therapy.
In this context, the objective of the current work was to compare adult patient groups with severe asthma and differing OCS treatment regimens, and to evaluate the cost and safety aspects of short- and long-term therapy with OCS by routine data from the statutory health insurance (SHI) in Germany.
Methods
Data source
The basis for this analysis was the InGef research database [13]. It contains data from approximately 75 health insurance providers (as of January 2017), with anonymised data on resource consumption at the individual patient level, and approximately 4 million patients representing 4.8% of the German population and 5.6% of the German SHI-insured. They match the German population structure in terms of age and sex (Federal Statistical Office; as on December 31, 2013). Of these, 80% were followed-up for 6 years.
The analysis period for the present work was the year 2015. Only those insures who were continuously monitored in 2015 were included. Data from people who died in 2015 were also included if these data covered the previous period accordingly and were complete, yielding a total sample size of 3 961 429.
Definitions of care
Based on the available routine data, the following definitions were used: HD-ICS/LABA patients were adult patients with an International Statistical Classification of Diseases and Related Health Problems (ICD)-10-coded diagnosis of bronchial asthma and a HD-ICS prescription (according to GINA [2]) in combination with a LABA. OCS patients were defined as those persons who received at least one OCS prescription per year.
Duration of the OCS therapy was used as the central differentiation parameter. The number of days of an OCS treatment was defined by the prescribed package size (assumption: one tablet per day). If a patient did not redeem a further OCS prescription within 7 days after consumption of the last OCS prescription, the therapy was considered terminated. On the basis of OCS therapy duration, the following six subgroups were formed: 1) “without OCS prescription”; 2) “short-term infrequent” (one OCS prescription in 2015 not exceeding 20 days); 3) “short-term frequent” (more than one OCS prescription in 2015 not exceeding 20 days each), also referred to in the text as recurrent OCS bursts; 4) “long-term >20 to ≤90” (at least one OCS long-term treatment with a respective duration of more than 20 to 90 days); 5) “long-term >90 to ≤180” (at least one OCS long-term treatment with a respective duration of more than 90 to 180 days); and 6) “long-term >180” (at least one OCS long-term treatment with a respective duration of more than 180 days).
Comorbidities and adverse effects were recorded on an outpatient basis (general practitioner (GP) or specialist diagnoses) or on an inpatient basis (discharge diagnoses) using the ICD-10 codes (three digits). For all patients receiving OCSs, potential OCS-induced comorbidities based on the ICD-10 codes are described. Direct costs were defined as inpatient and outpatient costs, as well as costs for medication, remedies and aids. Indirect costs were defined as those that are paid by the SHI which include sickness benefit payments. In addition, days off work were recorded, and sick leave days were included, if they had an OCS adverse event-related ICD-10-GM diagnosis code.
Patient selection
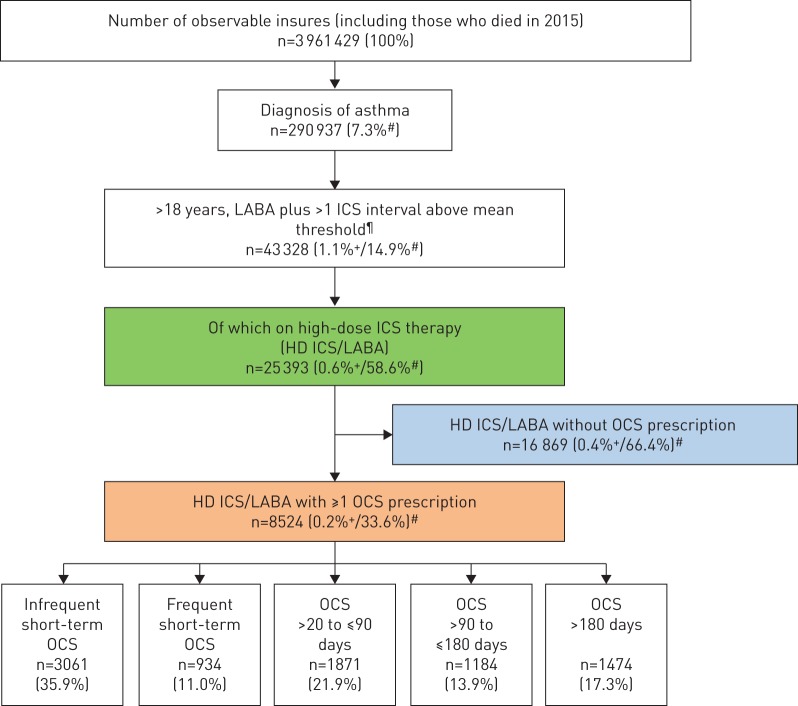
In a first step, the observable population from 2015 was restricted to adults with asthma (ICD-10 codes J45.0, J45.1, J45.8, J45.9; J46). Next, a gradual selection by pharmacotherapy for asthma (using Anatomical Therapeutic Chemical (ATC) codes) took place [14]. This was initially done by restriction to patients with HD-ICSs plus LABAs. To this end, all patients were excluded who had not received a prescription of LABAs (ATC: R03AC12, R03AK06, R03AC13, R03AK07, R03AK08, R03AK09, R03AK10, R03AK11, R03CC12, R03AC14, R03CC13, R03CC63). Subsequently, the patients treated with at least one ICS interval with a mean dosage above the threshold for a HD-ICS according to the GINA criteria [2] were identified (ATC: R03BA01, R03BA02, R03BA08, R03BA05, R03BA07). In the last step, the medication selection was narrowed down to the patients with at least one OCS prescription in a year (ATC: H02AB02, H02AB04, H02AB06, H02AB07, H02AB08, H02AB14). Subsequently, the patients with at least one OCS prescription were grouped by the length of treatment duration (figure 1).
FIGURE 1.
Selection steps of the oral corticosteroid (OCS) population (data year 2015). LABA: long-acting β2-agonist; ICS: inhaled corticosteroid; HD: high-dose. #: relative to patients in the box just above; ¶: patients with at least one ICS interval with a mean dosage above the upper threshold according to GINA [2]; +: relative to total sample.
Statistical analysis
The data were evaluated descriptively [15, 16]. Since 89% of the OCS patients received prednisolone (ATC code H02AB06) as the active pharmaceutical ingredient (API), the quantitative consumption of other systemic corticosteroids was identified and analysed through prednisolone equivalents.
Results
Prevalence
For the present analysis, from the total cohort (n=3 961 429), 290 937 patients were diagnosed with asthma (7.3%), of whom 25 393 adult patients were identified as having HD-ICS/LABA therapy prescribed (figure 1). This corresponds to 8.7% of the patients with asthma having a diagnosis of severe asthma according to the GINA classification.
Of these patients (HD-ICS/LABA), 33.6% also received at least one OCS prescription (n=8524) which is 2.9% of the whole asthma population. Here, 3061 patients received a single short-term OCS prescription. For 5463 patients, which corresponds to 64.1% of asthma patients with HD-ICS/LABA treatment, recurrent OCS bursts (OCS short-term frequent) or maintenance therapy with OCS (corresponding to GINA level 5, defined here as therapy duration >20 days) was prescribed.
Dosage and quantity of OCS therapy
The total number of OCS prescriptions (89% prednisolone) in the sample was 19 669 (table 1). Of these, 15.6% (3062/19 669) were accounted for by a single OCS burst (mean API quantity 313 mg). All other patients needed more frequent OCS intake (three prescriptions; 1826 mg of the API). A total of 38% of all OCS prescriptions were in the group of patients requiring the most intensive long-term treatment (≥180 days per treatment phase; 5.1 prescriptions; 3314 mg of the API). Average daily dosages increased from 0.9 mg to 9.1 mg according to increased intensity of the therapy group, as expected.
TABLE 1.
Prescription and cumulative oral corticosteroid (OCS) dose for short- and long-term treatments (in prednisolone equivalents, 2015)
| Patients | Female | Age years median (interquartile range) | Total prescriptions per year | Prescriptions per patient and year group mean | Cumulative OCS dose mg per patient per year | Cumulative OCS dose mg per patient per day | |
| Infrequent short-term treatment | 3061 (35.91%) | 56.9% | 59 (48–72) | 3062 | 1.00 | 313 | 0.9 mg |
| Frequent short-term to long-term >180 days | 5463 (64.09%) | 60.5% | 59 (49–71) | 16 607 | 3.04 | 1826 | 5.0 mg |
| Frequent short-term | 934 (10.96%) | 63.0% | 61 (51–72) | 2261 | 2.42 | 771 | 2.1 mg |
| Long-term >20 to ≤90 days | 1871 (21.95%) | 60.5% | 62 (52–72) | 3957 | 2.11 | 1203 | 3.3 mg |
| Long-term >90 to ≤180 days | 1184 (13.89%) | 57.5% | 65 (56–75) | 2870 | 2.42 | 1790 | 4.9 mg |
| Long-term >180 days | 1474 (17.29%) | 57.3% | 69 (59–76) | 7519 | 5.10 | 3314 | 9.1 mg |
| OCS patients total | 8524 (100%) | 59.8% | 61 (49–72) | 19 669 | 2.31 | 1282 | 3.5 mg |
Comorbidities
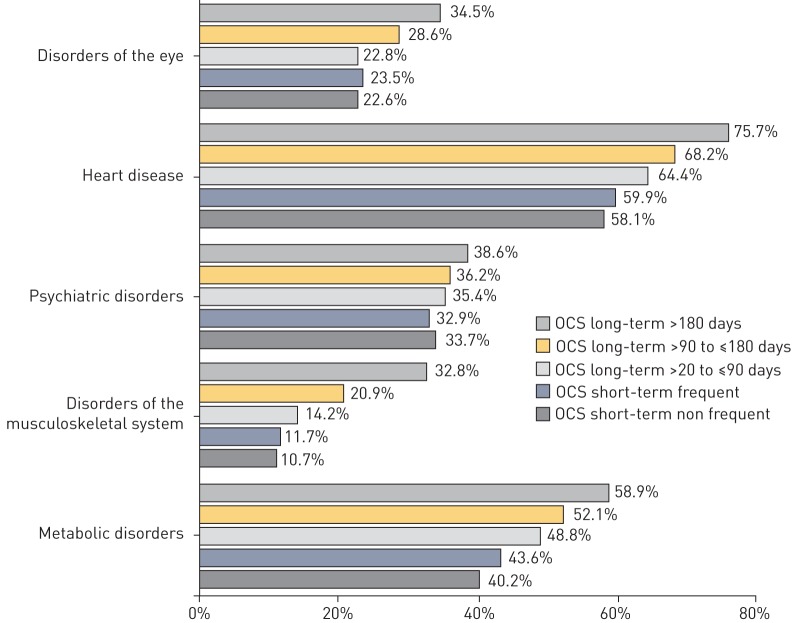
For 81.9% (13 814/16 869) of the patients with severe asthma but without OCS prescription, and for 88.0% (7503/8524) of the patients with additional OCS therapy, at least one of the OCS-associated comorbidities examined was coded. The five most frequent comorbidities observed during regular OCS therapy were heart disorders (67.5%), metabolic and nutritional disorders (51.4%), psychiatric disorders (36.0%), skeletal muscle, connective tissue and bone disorders (20.3%) and eye disorders (20.0%). The frequency of accompanying diagnoses per patient increased with increasing therapy intensity (figure 2 and table 2). The number of all OCS-associated comorbidities relative to group size increased from 1.87 to 2.76 with increasing treatment intensity (mean of 2.24).
FIGURE 2.
Top-5 oral corticosteroid (OCS)-induced comorbidities/adverse events.
TABLE 2.
Oral corticosteroid (OCS)-associated comorbidities/adverse effects by OCS therapy (duration)
| OCS-associated comorbidity/adverse effect | No prescription (n=16 869) | Infrequent short-term treatment (n=3061) | Frequent short-term to long-term treatment >180 days | OCS patients total (n=8524) | ||||
| Total (n=5463) | Frequent short-term (n=934) | Long-term >20 to ≤90 (n=1871) | Long-term >90 to ≤180 (n=1184) | Long-term >180 (n=1474) | ||||
| Eye disorders | 3521 (20.87%) | 693 (22.64%) | 1492 (27.31%) | 219 (23.45%) | 426 (22.77%) | 338 (28.55%) | 509 (34.53%) | 2185 (25.63%) |
| Endocrine disorders | 704 (4.17%) | 121 (3.95%) | 240 (4.39%) | 34 (3.64%) | 72 (3.85%) | 48 (4.05%) | 86 (5.83%) | 361(4.24%) |
| Disorders of the skin and subcutaneous tissue | 421 (2.50%) | 88 (2.87%) | 151 (2.76%) | 28 (3.00%) | 50 (2.67%) | 37 (3.12%) | 36 (2.44%) | 239 (2.80%) |
| Blood and lymphatic disorders | 183 (1.08%) | 30 (0.98%) | 125 (2.29%) | 18 (1.93%) | 36 (1.92%) | 23 (1.94%) | 48 (3.26%) | 155 (1.82%) |
| Gastrointestinal disorders | 315 (1.87%) | 50 (1.63%) | 158 (2.89%) | 25 (2.68%) | 48 (2.57%) | 34 (2.87%) | 51 (3.46%) | 208 (2.44%) |
| Immune system disorders | 2784 (16.50%) | 596 (19.47%) | 934 (17.10%) | 173 (18.52%) | 351 (18.76%) | 193 (16.30%) | 217 (14.72%) | 1530 (17.95%) |
| Nervous system disorders | 322 (1.91%) | 46 (1.50%) | 118 (2.16%) | 8 (0.86%) | 40 (2.14%) | 31 (2.62%) | 39 (2.65%) | 164 (1.92%) |
| Cardiac disorders | 9510 (56.38%) | 1778 (58.09%) | 3687 (67.49%) | 559 (59.85%) | 1205 (64.40%) | 807 (68.16%) | 1116 (75.71%) | 5465 (64.11%) |
| Psychiatric disorders | 4954 (29.37%) | 1030 (33.65%) | 1966 (35.99%) | 307 (32.87%) | 662 (35.38%) | 428 (36.15%) | 569 (38.60%) | 2996 (35.15%) |
| Weakening of the immune defence with increased risk of infection | 455 (2.70%) | 105 (3.43%) | 190 (3.48%) | 25 (2.68%) | 77 (4.12%) | 40 (3.38%) | 48 (3.26%) | 295 (3.46%) |
| Musculoskeletal and connective tissue disorders | 1600 (9.48%) | 328 (1072%) | 1106 (20.25%) | 109 (11.67%) | 266 (14.22%) | 247 (20.86%) | 484 (32.84%) | 1434 (16.82%) |
| Metabolism and nutrition disorders | 6850 (40.61%) | 1229 (40.15%) | 2805 (51.35%) | 407 (43.58%) | 913 (48.80%) | 617 (52.11%) | 868 (58.89%) | 4034 (47.33%) |
| Patients with at least one OCS-associated comorbidity/adverse effect | 13 814 (81.89%) | 2574 (84.09%) | 4929 (90.23%) | 807 (86.40%) | 1654 (88.40%) | 1083 (91.47%) | 1385 (93.96%) | 7503 (88.02%) |
| Number of all OCS-associated comorbidities/adverse effects relative to group size | 1.87 | 1.99 | 2.37 | 2.05 | 2.22 | 2.40 | 2.76 | 2.24 |
Direct health-related costs
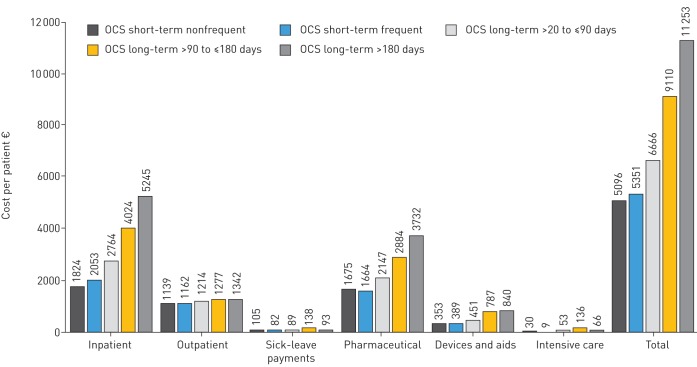
Overall, the annual total cost for all patients on OCS therapy was €60 442 134 (n=8524), whereas the cost for the significantly larger group (n=16 869) of patients without OCS therapy was €72 million (table 3). The mean total annual cost per patient and year was €4266 for a patient without an OCS prescription. This increased continuously with the duration and intensity of therapy from €5096 per patient on infrequent short-term OCS treatment to €11 253 per patient on long-term treatment for >180 days in a treatment phase (figure 3).
TABLE 3.
Statutory health insurance (SHI) costs associated with oral corticosteroid (OCS) therapy for asthma in the sample (2015) rounded to full €
| (% of total costs) total | Cost per patient mean±sd | Extrapolation to SHI | Extrapolation to the German population | |
| Inpatient costs | ||||
| No OCSs | 24 275 598 | 1439±4503 | 433 420 485 | 497 577 478 |
| OCS total | (41.6) 25 169 611 | 2953±8130 | 449 382 345 | 515 902 089 |
| Infrequent short-term | 5 584 650 | 1824±6208 | 99 709 247 | 114 468 691 |
| Frequent short-term | 1 917 646 | 2053±4676 | 34 237 959 | 39 306 027 |
| Long-term >20 to ≤90 days | 5 171 143 | 2764±8572 | 92 326 439 | 105 993 045 |
| Long-term >90 to ≤180 days | 4 764 609 | 4024±8234 | 85 068 115 | 97 660 308 |
| Long-term >180 days | 7 731 563 | 5245±11 465 | 138 040 585 | 158 474 019 |
| Outpatient costs | ||||
| No OCSs | 16 372 180 | 971±835 | 292 311 574 | 335 580 945 |
| OCS total | (17.1) 10 335 019 | 1212±955 | 184 523 120 | 211 837 123 |
| Infrequent short-term | 3 486 655 | 1139±899 | 62 251 306 | 71 466 045 |
| Frequent short-term | 1 085 762 | 1162±874 | 19 385 367 | 22 254 882 |
| Long-term >20 to ≤90 days | 2 271 890 | 1214±962 | 40 562 686 | 46 566 971 |
| Long-term >90 to ≤180 days | 1 512 427 | 1277±1096 | 27 003 116 | 31 000 248 |
| Long-term >180 days | 1 978 286 | 1342±971 | 35 320 645 | 40 548 977 |
| Medication costs | ||||
| No OCSs | 24 788 284 | 1469±3549 | 442 574 076 | 508 086 027 |
| OCS total | (32.4) 19 612 646 | 2301±6884 | 350 167 375 | 402 000 840 |
| Infrequent short-term | 5 126 605 | 1675±3550 | 91 531 236 | 105 080 132 |
| Frequent short-term | 1 554 272 | 1664±2576 | 27 750 222 | 31 857 943 |
| Long-term >20 to ≤90 days | 4 016 750 | 2147±5261 | 71 715 702 | 82 331 407 |
| Long-term >90 to ≤80 days | 3 414 204 | 2884±7136 | 60 957 754 | 69 981 016 |
| Long-term >180 days | 5 500 816 | 3732±12 821 | 98 212 460 | 112 750 343 |
| Costs for remedies and aids | ||||
| No OCSs | 5 211 548 | 309±1147 | 93 047 822 | 106 821 210 |
| OCS total | (7.4) 4 459 583 | 523±2009 | 79 622 124 | 91 408 175 |
| Infrequent short-term | 1 081 684 | 353±957 | 19 312 558 | 22 171 296 |
| Frequent short-term | 363 416 | 389±1200 | 6 488 495 | 7 448 954 |
| Long-term >20 to ≤90 days | 844 355 | 451±1816 | 15 075 249 | 17 306 760 |
| Long-term >90 to ≤180 days | 931 294 | 787±3751 | 16 627 480 | 19 088 760 |
| Long-term >180 days | 1 238 834 | 840±2192 | 22 118 342 | 25 392 406 |
| Sickness benefits | ||||
| No OCSs | 1 323 779 | 78±803 | 23 634 960 | 27 133 521 |
| OCS total | (1.4) 865 275 | 102±925 | 15 448 767 | 17 735 568 |
| Infrequent short-term | 319 927 | 105±1034 | 5 712 024 | 6 557 545 |
| Frequent short-term | 76 770 | 82±656 | 1 370 667 | 1 573 560 |
| Long-term >20 to ≤90 days | 167 263 | 89±822 | 2 986 344 | 3 428 398 |
| Long-term >90 to ≤180 days | 163 908 | 138±1091 | 2 926 434 | 3 359 619 |
| Long-term >180 days | 137 408 | 93±803 | 2 453 297 | 2 816 446 |
| Total costs | ||||
| No OCSs | 71 971 388 | 4266±6690 | 1 284 988 917 | 1 475 199 180 |
| OCS total | (100) 60 442 134 | 7091±12 115 | 1 079 143 731 | 1 238 883 795 |
| Infrequent short-term | 15 599 520 | 5096±8206 | 278 516 370 | 319 743 708 |
| Frequent short-term | 4 997 866 | 5351±6670 | 89 232 710 | 102 441 367 |
| Long-term >20 to ≤90 days | 12 471 401 | 6666±11 897 | 222 666 422 | 255 626 581 |
| Long-term >90 to ≤180 days | 10 786 442 | 9110±12 785 | 192 582 900 | 221 089 950 |
| Long-term >180 days | 16 586 906 | 11 253±18 418 | 296 145 329 | 339 982 190 |
FIGURE 3.
Statutory health insurance costs per patient associated with oral corticosteroid (OCS) therapy for asthma in the sample (2015) rounded to full €.
The greatest share of the €7091 costs per patient was accounted for by inpatient care (€2953; 41.6%), followed by pharmacotherapy (€2301; 32.4%) and outpatient care (€1212; 24.2%). While average inpatient costs, medication costs, and cost of remedies and aids per patient increased depending on the intensity and duration of OCS therapy, outpatient costs and sickness benefit payments remained stable (figure 3).
Indirect health-related costs
The percentage of patients on sick leave because of common OCS-associated comorbidities was 3.2% in both patient groups (without OCSs and with an OCS prescription; table 4). Patients on maintenance therapy >180 days per treatment phase were on sick leave for almost 1 week longer than patients with infrequent short-term treatment (43.0 versus 36.5 days). Overall, however, in association with the larger group size, the length of sick leave attributable to patients without OCS therapy was highest (18 245 days), accounting for 65.4% of the total group.
TABLE 4.
Oral corticosteroid (OCS)-associated sick leave in asthma patients on high-dosage inhaled corticosteroids in combination with a long-acting β-agonist (LABA) and OCS therapy in the sample (2015)
| Total | Patients with at least one sick leave | Total days of sick leave in the subgroup | Sick leave days per patient | Mean number of days per sick leave# | |
| No OCS | 16 869 | 537 (3.2%) | 18 245 | 1.1 | 34.0±10.9 |
| All OCS patients | 8524 | 270 (3.2%) | 9641 | 1.1 | 35.7±11.5 |
| Infrequent short-term | 3061 | 122 (4.0%) | 4458 | 1.5 | 36.5±13.4 |
| Frequent short-term | 934 | 34 (3.6%) | 998 | 1.1 | 29.4±11.9 |
| Long-term >20 to ≤90 days | 1871 | 54 (2.9%) | 1476 | 0.8 | 27.3±7.8 |
| Long-term >90 to ≤180 days | 1184 | 32 (2.7%) | 1505 | 1.3 | 47.0±13.9 |
| Long-term >180 days | 1474 | 28 (1.9%) | 1204 | 0.8 | 43.0±8.3 |
| All HD-ICS/LABA patients | 25 393 | 870 (3.4%) | 27 886 | 1.1 | 34.6±11.1 |
HD: high-dosage; ICS: inhaled corticosteroids. #: in patients with at least one sick leave in 2015; sick leave was included if the patient was coded with an OCS adverse event-related International Classification of Diseases (10th Revision, German Modification) code as the diagnosis.
For all patients (regardless of actual sick leave), the insurance providers paid a total of €1 323 779, or €78 per patient per year, in sickness benefits for all patients without OCSs, and €865 275 (€102 per patient) for patients with OCSs. The group of patients with OCSs long-term >90 to ≤180 received the greatest per capita sickness benefit payments, which amounted to €138 per patient. The actual costs for sickness benefits individually incurred by patients on sick leave were many times greater and increased with the duration and intensity of OCS therapy.
Discussion
The present study describes the prevalence of asthma in a representative sample from Germany in terms of age and sex, based on SHI routine data. In this sample of adult patients, the asthma prevalence was 7.3%, which is greater than the estimated 5% reported earlier for the overall German population [17]. Of these asthma patients, 8.7% (0.6% absolute) were treated with HD-ICS/LABAs, defined as steps 4/5 according to the GINA criteria [2], consistent with the reported global prevalence of severe asthma of approximately 5–10% of asthma patients [5, 6]. The frequency of patients on HD-ICS/LABAs who required additional OCSs was 2.9% and is comparable to data from the Netherlands that reported a frequency of 3.6% [5]. A possible explanation for the slightly lower prevalence in the present analysis may be a result of exclusive consideration of prescription data; since additional clinical data on current asthma control were not available within SHI, both prescription data and clinical data were considered in the analysis of the Dutch population. In 2015 82.9% of the asthma patients in Germany who had maintenance therapy with HD-ICS/LABAs and needed OCSs at least once, received an intensive therapy with OCSs (either frequent short-term or long-term).
The present analysis demonstrates that, in Germany, OCS therapy is an established and frequently applied treatment option for patients with severe, inadequately controlled asthma. While OCSs may be appropriate and a relatively well-tolerated treatment option for acute exacerbations (i.e. OCS bursts at usually high dosages), these data revealed the frequent use of comparatively high dosages during long-term treatment. Patients who received long-term OCS for more than 180 days in a year received an average of 9.1 mg of prednisolone equivalent per day and were substantially above the national and international guideline recommendations that recommend OCS maintenance therapy in step 5 of the therapy regimen only as secondary and intermittent treatment and at dosages of less than 7.5 mg of prednisolone equivalent per day [2, 18, 19]. In addition, OCSs at a rather high dosage (3.3 mg·day−1) was also administered widely (i.e. for all patients with therapy duration >20 days), which is of concern, as frequent OCS-associated adverse effects have been reported with usage from a daily dosage >2.5 mg [20].
Comorbidities in patients with asthma are frequent, increase with the duration and intensity of OCS therapy, and mainly affect certain organ systems. Here, a distinction must be made between frequently accompanying but not causally linked comorbidities and direct/indirect, OCS-induced adverse effects [21]. Comparable frequencies were reported from the analysis of a healthcare database in the United Kingdom, which described at least one potential steroid-induced comorbidity in 93% and more than three in 53% of all patients with severe asthma [9]. The dosages used, amounting to 1960 mg per year, were essentially comparable with the data from our analysis (1826 mg), as was the profile of most frequently observed comorbidities, despite slightly different definitions. A further analysis of prescription data from the United States described an increase in comorbidities in asthmatic patients with intensive OCS therapy (at least 30 days with OCS intake per year) compared with asthma patients without OCS therapy, resulting in increased frequencies of osteoporosis, cataract/glaucoma, diabetes mellitus and hypertension. The frequencies of the comorbidities associated with OCS maintenance therapy described in the present analysis are comparable with the United States data clustered into categories of skeletal muscle, connective tissue and bone diseases, ocular disorders, metabolic and nutritional disorders, and cardiac conditions [10]. Dosage dependencies have been described by Amelink [7] both for psychiatric conditions and for diabetes. The increase in comorbidities we observed for patients receiving short-term OCS therapy also confirms data analysed from earlier studies reporting an increase in OCS-associated comorbidities with short-term OCS use for less than 30 days [12] and relatively low-dosage OCS maintenance therapy below the so-called Cushing threshold [9].
Asthma, and especially severe asthma, are a significant burden on payers. The cost analyses available for Germany, however, are predominantly outdated and do not relate to the current state of knowledge, modern treatment strategies, and cost and reimbursement structure [22, 23]. In addition, they rarely differentiate by asthma severity, and they take different perspectives (e.g. SHI, pension insurance), hampering comparability. For example, the direct total costs of patients with atopic asthma associated with seasonal allergic rhinitis are estimated by Schramm et al. [24] to be €569 per adult in mild asthma and up to €2048 for severe asthma. If the indirect costs are included, the amount increases to up to €9286 per year and patient. Kirsch et al. [23] determined total asthma costs per case and year of between €445 and €2543 by means of a systematic literature search.
We found that mean annual direct costs per patient and year increased from €5096, for patients with HD-ICS/LABAs and a one-time short-term OCS prescription, to €8208 for the group of patients with multiple OCS bursts of therapy or maintenance-therapy OCSs. Long-term treatment with OCSs for more than 180 days per year more than doubled the cost compared with the patient group with a one-time, short-term OCS prescription (€5096 versus €11 253). The latter amount roughly corresponds to the costs for severe asthma of €11 703 per patient and year determined in a meta-analysis for Europe, the United States and Canada, but is greater than the average costs calculated by Barry et al. [25] for England (€5137). According to the literature from Germany, medication costs account for the largest part of per patient costs, followed by hospitalisations [22, 24, 26]. In our current analysis, medication costs and hospitalisation were also the main cost drivers, reflecting a high OCS burden in association with the severity of the asthmatic disorder and comorbidities.
As a matter of principle, only billable data were registered as routine SHI data. These are collected at various interfaces in the healthcare system (e.g. physicians, pharmacists, hospitals), and inconsistencies and errors may occur, for example regarding confirmation of the asthma diagnosis [27]. This also relates to the potential other reasons for OCS use than asthma, including musculoskeletal and connective tissue disorders. Furthermore, eosinophilic granulomatosis with polyangiitis was not ruled out but, according to our analysis, only affected less than 0.5% of the population under investigation. Moreover, the total macroeconomic costs cannot be reflected conclusively, as only costs for a maximum of 6 weeks per year off work are covered by the SHI, while longer sickness absence, rehabilitation and early and partial retirement were covered by the pension fund. This may have led to distortions and loss of accuracy in further calculations (such as of the indirect costs) but more likely resulted in an underestimation of cost, rather than an overestimation. Distortions can also occur during the selection of billing codes (e.g. ICD-10 three-digit codes for comorbidities/adverse effects). For example, the percentage of OCS-induced conditions such as cataracts or osteopenia cannot be precisely estimated, since the groups of “eye disorders” or “skeletal muscle, connective tissue or bone disorders” can also include other diagnoses.
In conclusion, we have provided novel prevalence information that demonstrates that, despite maintenance therapy with HD-ICS/LABAs, severe asthma is inadequately controlled and requires the use of OCS maintenance therapy. It indicates that OCS therapy is applied at relatively high dosages in everyday care and is associated with many adverse effects, commonly reported with steroid usage. These data thus support a necessary change in the therapy of severe asthma, which is already reflected in national and international guidelines with the inclusion of biologics for respective patients.
Acknowledgments
The authors thank Sebastian Braun, Christian Jacob, Kim-Sarah Krinke and Dominic Meise of Xcenda GmbH (Hannover, Germany), and Wolfgang Greiner of the University of Bielefeld (Bielefeld, Germany) for contributions to the study design. Writing and editing support, including preparation of the draft manuscript under the direction and guidance of the authors, incorporating author feedback and manuscript submission, was provided by Gesine van Mark of IPPMed GmbH (Cloppenburg, Germany) and Michael A. Nissen of AstraZeneca (Gaithersburg, MD, USA). This support was funded by AstraZeneca.
Footnotes
Support statement: Funding for this study was provided by AstraZeneca. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: C. Taube reports that AstraZeneca supported the present study. He also reports research support from Novartis and Boehringer Ingelheim, and lecturing and consulting fees from AstraZeneca, TEVA, GSK and Chiesi, outside the submitted work.
Conflict of interest: P. Bramlage reports consultancy for AstraZeneca during the conduct of the study.
Conflict of interest: A. Hofer is an employee of AstraZeneca.
Conflict of interest: D. Anderson is an employee of AstraZeneca.
References
- 1.Global Asthma Network Global Asthma Report 2018. www.globalasthmareport.org/ Date last updated: 2018. Date last accessed: March 11, 2019.
- 2.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention 2018. www.ginasthma.org Date last updated: 2018. Date last accessed: March 11, 2019.
- 3.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 4.van Buul AR, Taube C. Treatment of severe asthma: entering the era of targeted therapy. Expert Opin Biol Ther 2015; 15: 1713–1725. [DOI] [PubMed] [Google Scholar]
- 5.Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. [DOI] [PubMed] [Google Scholar]
- 6.O'Byrne PM, Naji N, Gauvreau GM. Severe asthma: future treatments. Clin Exp Allergy 2012; 42: 706–711. [DOI] [PubMed] [Google Scholar]
- 7.Amelink M, Hashimoto S, Spinhoven P, et al. Anxiety, depression and personality traits in severe, prednisone-dependent asthma. Respir Med 2014; 108: 438–444. [DOI] [PubMed] [Google Scholar]
- 8.Price D, Trudo F, Jie JLZ et al. , Oral corticosteroids increase risks of onset of diabetes mellitus and osteoporosis in a UK patient population. Poster presented at the CHEST 2017 Annual Meeting, October 28 to November 1, 2017, Toronto, Ontario, Canada, 2017. [Google Scholar]
- 9.Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016; 71: 339–346. [DOI] [PubMed] [Google Scholar]
- 10.Zazzali JL, Broder MS, Omachi TA, et al. Risk of corticosteroid-related adverse events in asthma patients with high oral corticosteroid use. Allergy Asthma Proc 2015; 36: 268–274. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan PW, Ghushchyan VH, Globe G, et al. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol 2018; 141: 110–116 e117. [DOI] [PubMed] [Google Scholar]
- 12.Waljee AK, Rogers MA, Lin P, et al. Short-term use of oral corticosteroids and related harms among adults in the United States: population-based cohort study. BMJ 2017; 357: j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersohn F, Walker J. Characteristics and external validity of the German Health Risk Institute (HRI) Database. Pharmacoepidemiol Drug Saf 2016; 25: 106–109. [DOI] [PubMed] [Google Scholar]
- 14.WIdO Anatomisch-therapeutisch-chemische-Klassifikation mit Tagesdosen – Amtliche Fassung des ATC-Index mit DDD-Angaben für Deutschland im Jahr 2018. www.wido.de/amtl_atc-code.html Date last updated: 2019. Date last accessed: March 11, 2019.
- 15.Arbeitsgruppe Erhebung und Nutzung von Sekundärdaten der Deutschen Gesellschaft für Sozialmedizin und Prävention, Deutschen Gesellschaft für Epidemiologie GPS – good practice in secondary data analysis: revision after fundamental reworking. http://dgepi.de/assets/Leitlinien-und-Empfehlungen/ea8d1effb2/Practice-in-Secondary-Data-Analysis.pdf Date last updated: 2018. Date last accessed: March 11, 2019. [DOI] [PubMed]
- 16.Swart E, Bitzer EM, Gothe H, et al. A consensus German reporting standard for secondary data analyses, version 2 [STROSA-STandardisierte BerichtsROutine für SekundardatenAnalysen]. Gesundheitswesen 2016; 78: e145–e160. [DOI] [PubMed] [Google Scholar]
- 17.Nowak D, Heinrich J, Jorres R, et al. Prevalence of respiratory symptoms, bronchial hyperresponsiveness and atopy among adults: West and East Germany. Eur Respir J 1996; 9: 2541–2552. [DOI] [PubMed] [Google Scholar]
- 18.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) Nationale VersorgungsLeitlinie Asthma – Langfassung, 3. Auflage. Version 1, 2018. www.asthma.versorgungsleitlinien.de Date last updated: 2018. Date last accessed: March 11, 2019.
- 19.Buhl R. S2k-Leitlinie zur Diagnostik und Therapie von Patienten mit Asthma (AWMF-Registernummer: 020-009), 2017. www.awmf.org/uploads/tx_szleitlinien/020-009l_S2k_Asthma_Diagnostik_Therapie_2017-11_1.pdf Date last updated: 2017. Date last accessed: March 11, 2019.
- 20.Zeiger RS, Schatz M, Li Q, et al. Burden of chronic oral corticosteroid use by adults with persistent asthma. J Allergy Clin Immunol Pract 2017; 5: 1050–1060 e1059. [DOI] [PubMed] [Google Scholar]
- 21.Kankaanranta H, Kauppi P, Tuomisto LE, et al. Emerging comorbidities in adult asthma: risks, clinical associations and mechanisms. Mediators Inflamm 2016; 2016: 3690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aumann I, Prenzler A, Welte T, et al. [Epidemiology and costs of asthma in Germany - a systematic literature review]. Pneumologie 2014; 68: 557–567. [DOI] [PubMed] [Google Scholar]
- 23.Kirsch F, Teuner CM, Menn P, et al. [Costs of illness for asthma and COPD in adults in Germany]. Gesundheitswesen 2013; 75: 413–423. [DOI] [PubMed] [Google Scholar]
- 24.Schramm B, Ehlken B, Smala A, et al. Cost of illness of atopic asthma and seasonal allergic rhinitis in Germany: 1-yr retrospective study. Eur Respir J 2003; 21: 116–122. [DOI] [PubMed] [Google Scholar]
- 25.Barry LE, Sweeney J, O'Neill C, et al. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res 2017; 18: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puig-Junoy J, Pascual-Argente N. [Socioeconomic costs of asthma in the European Union, United States and Canada: a systematic review]. Rev Esp Salud Publica 2017; 91. [PMC free article] [PubMed] [Google Scholar]
- 27.Reinhold T, Andersohn F, Hessel F, et al. Die Nutzung von Routinedaten der gesetzlichen Krankenkassen (GKV) zur Beantwortung gesundheitsökonomischer Fragestellungen – eine Potenzialanalyse. Gesundh ökon Qual manag 2011; 16: 153–159. [Google Scholar]