Abstract
The shape of the craniofacial skeleton is constantly changing through ontogeny, and reflects a balance between developmental patterning and mechanical-load induced remodeling. Muscles are a major contributor to producing the mechanical environment that is crucial for “normal” skull development. Here we use an F5 hybrid population of Lake Malawi cichlids to characterize the strength and types of associations between craniofacial bones and muscles. We focus on four bones/bone complexes, with different developmental origins, alongside four muscles with distinct functions. We used micro-computed tomography to extract 3D information on bones and muscles. 3D geometric morphometrics and volumetric measurements were used to characterize bone and muscle shape, respectively. Linear regressions were performed to test for associations between bone shape and muscle volume. We identified three types of associations between muscles and bones: weak, strong direct (i.e., muscles insert directly onto bone), and strong indirect (i.e., bone is influenced by muscles without a direct connection). In addition, we show that whereas the shape of some bones are relatively robust to muscle-induced mechanical stimulus, others appear to be highly sensitive to muscular input. Our results imply that the roles for muscular input on skeletal shape extend beyond specific points of origin or insertion, and hold significant potential to influence broader patterns of craniofacial geometry. Thus, changes in the loading environment, either as a normal course of ontogeny or if an organism is exposed to a novel environment, may have predicable effects on skeletal shape via near and far-ranging effects of muscular loading.
Keywords: Muscle, bone, craniofacial, cichlid, geometric morphometrics
Introduction
Different sources of variation have the capacity to influence the phenotype across distinct developmental periods, often with differing outcomes. For example, perturbations that occur during early development can result in large deviations from more ‘normal’ variation, while variation arising at later stages via remodeling often produces more subtle, but functionally relevant shifts in the phenotype (Klingenberg, 2003; Lazić, Carretero, Crnobrnja-Isailović, & Kaliontzopoulou, 2015). Documenting how, and when, phenotypic variation arises is an active area of research that is critical for both an evolutionary and clinical understanding of complex traits.
The craniofacial skeleton is a multifaceted structure produced via a hierarchical developmental program that involves multiple molecular and cellular processes, including (but not limited to) neural crest migration, cellular condensation, cartilage growth, bone deposition, and muscle-bone interactions (Hallgrímsson et al., 2009; Hallgrimsson, Mio, Marcucio, & Spritz, 2014). These disparate processes combine to influence craniofacial geometry but untangling which mechanisms determine which component of craniofacial shape is difficult due to the overlapping nature of their expression. Mutagenesis screens can be useful, but these are typically limited to the earliest role for a gene, with later sources of variation being masked by developmental pleiotropy. Genome-wide association studies (GWAS) can also be used to evaluate the genetic underpinnings of craniofacial variation, however they typically account for a relatively small component of the overall variation (Robinson, Wray, & Visscher, 2014). Further, neither mutant screens nor GWAS can reveal roles for epigenetic process (sensu Waddington (Waddington, 1942)), such as environmental factors, which may account for much of the remaining variation that is “missing” from association studies (Bossdorf, Richards, & Pigliucci, 2008; Caballero, Tenesa, & Keightley, 2015).
The environment is a critical epigenetic regulator of muscle and bone development. Muscle forces are required for normal bone development and lack of this mechanical environment causes bone to exhibit abnormalities such as joint fusion, stunted bone growth, and limited bone process formation (Felsenthal & Zelzer, 2017). Studies from mouse muscle knockouts and chick jaw paralysis models, whereby the embryo develops without a mechanical environment, demonstrate how the lack of a mechanical environment severely restricts the normal growth of bones (Brunt, Norton, Bright, Rayfield, & Hammond, 2015; Rot-Nikcevic, Downing, Hall, & Kablar, 2007). Molecular pathways that influence both muscle and bone growth may provide mechanisms to better understand the epigenetic landscape of craniofacial development. Indeed, there is accumulating evidence to suggest that the molecular and developmental origins of muscles and bones are interlinked and able to respond dynamically to mechanical stimulus via the Wnt and Hippo pathways (Chen, Liu, You, & Simmons, 2010; Yu & Guan, 2013). Similarly, their developmental origins appear to overlap. For example, chondroblasts and myoblasts lie in close proximity and differentiate together during early development (Schilling & Kimmel, 1997). Further, mesodermally derived somites dictate the path of neural crest cells that will ultimately form the anterior portion of the cranial skeleton (Theveneau & Mayor, 2012), while the more posterior bones of the skull are mesodermally derived (Kague et al., 2012). Finally, diet studies, whereby individuals are fed hard or soft foods, have demonstrated the ability of the entire craniofacial skeleton to remodel in response to different mechanical stimuli (Anderson, Renaud, & Rayfield, 2014; Spassov, Toro-Ibacache, Krautwald, Brinkmeier, & Kupczik, 2017).
Here we examine one such mechanism that influences craniofacial patterning, muscle-bone interactions, and while this mechanism can act at both larval (e.g., Hu & Albertson 2017) and adult stages (e.g., Witten & Huysseune 2009), we explore the later adult phase once muscles and bones are fully formed. Specifically, we seek to determine how the mechanical environment facilitated by muscle force statistically predicts craniofacial skeletal variation. We use a fish model to answer this question as they exhibit an indeterminate pattern of growth, meaning, although they are reproductively mature, their bones and muscles are continually growing and capable of responding to mechanical stimuli via remodeling. While the influence of muscle force on local bone remodeling and density have been widely studied (e.g., Felsenthal & Zelzer, 2017; Rabey et al., 2015; Santana, 2018; Zelzer, Blitz, Killian, & Thomopoulos, 2014), this study contributes to the growing literature on muscle-bone interactions (e.g., Fabre, Perry, Hartstone-rose, & Elien, 2018) and, in particular, emphasizes the impact muscles have on effecting global facial geometry.
Muscle-induced centers of stress and strain should be heterogeneously distributed across the skull producing a series of potential relationships between muscle volume and bone shape (Figure 1). We use muscle volume as a proxy for mechanical input, as in Folland & Williams (2007). While volume is considered less predictive compared to physiological cross sectional area (PCSA), which combines muscle fiber, density, and mass data (Lieber & Ward, 2011), PCSA would require destructive sampling of all our individuals which is beyond the scope of this study. Our overarching hypothesis is that a significant proportion of skeletal shape variation is due to the mechanical environment established by the surrounding musculature. To test this hypothesis we regressed measures of bone shape onto muscle volume. With these analyses, we tested several specific predictions: (1) that points of muscle insertion should be centers of greater stress and strain relative to sites of origination (Maas & Sandercock, 2010), (2) that some bones may be more or less robust to the mechanical environment than others (Ehrlich & Lanyon, 2002), and (3) that that mechanical stimulation of bone development via muscle may act both locally, at a process, and globally, over the entire craniofacial skeleton (Chen et al., 2010; Schulte et al., 2013; Yucesoy, 2010). The null hypothesis is that bone shapes are not associated with muscle volume.
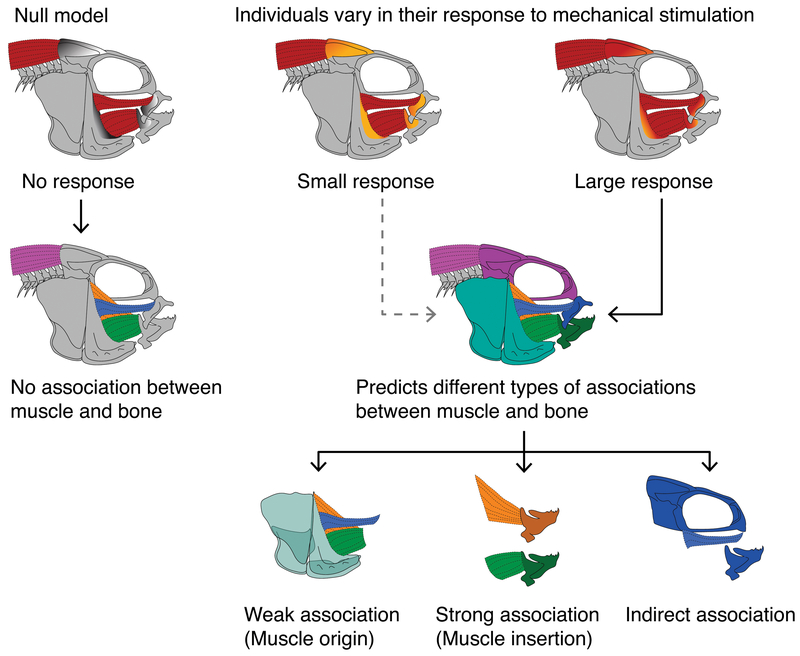
Figure 1.
Predictions for the types of associations that we may observe between muscles and bones, and how they arise. Our null model suggests that bone does not have the capacity to respond to mechanical stimulus, based on muscle volume, such that no associations will exist between bone shape and muscle volume. Our alternative model suggests that, based on the degree of mechanical stimulus or the extent to which bone can respond, a range of different types of associations between muscle and bone will be detected. These may be (1) weak, (2) strong direct, or (3) strong indirect associations (see main text for details).
To test these predictions we examined associations among several craniofacial bones and muscles in a hybrid population of east African cichlids. We selected the mandible, maxilla, suspensorium, and neurocranium as these bones/bone complexes are involved in multiple ecologically relevant tasks (e.g., feeding, respiration, housing the brain and sensory organs). Variation among individuals may reflect intrinsic differences in the development of these bones, or differences in how they function, including trade-offs between different tasks, and the mechanical environment they are subjected to. Additionally, evolutionary constraints and evolvability (the ability to generate adaptive phenotypic variation) should operate differentially on these bones, with the feeding apparatus (mandible, maxilla) likely exhibiting the greatest variability (Hu, Parsons, & Albertson, 2014; Pigliucci, 2008). We also selected four muscles: the epaxials, and three of the adductor mandibulae muscles, the A1, A2, and A3. These muscles are involved in feeding, respiring, and/or powering the axial muscles (Camp, Roberts, & Brainerd, 2015), and either insert or originate on the bones included in our study, providing strong potential for load-induced bone remodeling.
East African cichlid fish are an excellent model for such studies, as they represent an exceptionally species rich and morphologically diverse radiation of teleost fishes. In particular, they exhibit unparalleled diversity in their craniofacial muscle and bone morphology, which reflects their occupation of many different trophic niches in both benthic and pelagic environments (Albertson, Markert, Danley, & Kocher, 1999; Streelman & Danley, 2003). We exploit the relatively recent divergence of this clade to produce an F5 hybrid population between two parental species that exhibit adaptations for either a mechanically demanding or undemanding diet. Importantly, these hybrids segregate various aspects of bone and soft-tissue morphology (Concannon & Albertson, 2015; M. R. Conith et al., 2018; Parsons et al., 2016; Parsons, Wang, Anderson, & Albertson, 2015; Parsons, Son, & Albertson, 2011), which allow us to ascertain the types and degrees of associations between our muscles and bones of interest.
Methods
Hybrid Population
Our hybrid population stems from two wild caught African cichlids from Lake Malawi: Labeotropheus fuelleborni (LF) and Tropheops ‘red cheek’ (TRC). LF and TRC belong to the mbuna clade (Malinsky et al., 2017) of Lake Malawi cichlids, which typically reside close to the rocky shorelines. Both feed by scraping algae from rocks, although their feeding behavior and craniofacial morphology differ. LF exhibit a steep skull profile with wide, short, and highly robust oral jaws. This species is considered a highly specialized algae scraper that feeds by cropping algae from the substrate. To accommodate this task they have evolved a series of hard and soft tissue adaptations to generate and resist higher biting forces (M. R. Conith et al., 2018; Cooper, Wernle, Mann, & Albertson, 2011). While TRC also pluck algae from rocks, they used a very different mode of food collection, mainly a twisting and jerking motion to free algae from substrate. Further, the genus Tropheops exhibits more variation in foraging mode that includes nipping, sifting and suction feeding (Albertson, 2008; Konings, 2007). Relative to LF, TRC possess shallow craniofacial profiles and longer, narrow oral jaws.
Hybrids segregate many of these craniofacial traits and exhibit a spectrum of craniofacial morphologies from more LF-like to more TRC-like, and even transgressive phenotypes (Parsons, Son, et al., 2011). We used 140 F5 hybrids for this study. All hybrids were fed the same diet of algae and egg yolk flakes, so differences among hybrids should result from intrinsic factors controlling muscle volume and bone shape. We also included 4 LF and 4 TRC individuals to examine variation in muscle volume. Parentals were not included in the morphological analysis component of the study.
X-ray Scanning Information
We used X-ray micro computed tomography (μCT) to acquire muscular volume and skeletal shape from our hybrid population. To gain skeletal images for 3D modeling we scanned all specimens at 25-35 micron resolution at 95kV and 90μA. To gain muscle volume data we submerged all individuals in 2.5% Lugol iodine solution for 24 hours. The iodine is absorbed into the muscle tissues and acts as a contrast agent given its radio-opaque properties (Gignac et al., 2016). We scanned all iodine-stained specimens at 20-25 micron resolution at 115kV and 105μA with a 0.1mm copper filter. All μCT models were acquired using an X-Tek HMXST 225 μCT scanner (Nikon Corporation). We segmented out the bones and muscles using Mimics v.19 (Materialise NV), and then exported the 3D models to Geomagic 2014 v.1.0 (3D Systems) to remove noise and calculate muscle volumes.
Specimen storage in ethanol, and iodine staining protocols can cause shrinkage of soft tissues, including muscles, which may result in smaller overall volume measures. We took a number of steps to alleviate the effects of shrinkage on our muscle volume estimations. Because these fish are relatively small, e.g., ~7cm standard length, we were able to use short staining times (24 hours) and lower stain concentrations (2.5%). This is important, as longer staining times and higher stain concentrations can cause greater tissue shrinkage (Vickerton, Jarvis, & Jeffery, 2013). We also scanned specimens within a year of their original fixation to limit shrinkage from alcohol storage, and completed iodine staining and scanning within two months to reduce differences attributed to variation in storage periods (Hedrick et al., 2018). Finally, all specimens were subjected to similar storage and staining protocols, helping to ensure that any tissue deformation that has occurred was uniform across specimens. We therefore do not consider specimen storage or our staining protocols to be a significant confounding factor in our analysis.
Morphological Analysis
We extracted shape information from four different regions of the skull that consist of either single bone or a collection of multiple bones. These regions comprised the neurocranium, suspensorium, mandible, and maxilla (see Figure 2 for more detailed anatomical information). Skeletal terminology follows Barel, Witte, & Van Oijen (1976) and Albertson & Kocher (2001). We used 3D geometric morphometrics to characterize the shape of these four regions by placing a series of landmarks (LMs) at key functional positions (i.e., processes, muscle insertion points, sutures etc.) on the bones (Figure 3; Table S1). We placed 11 LMs on the mandible, 9 on the maxilla, 10 on the suspensorium, and 14 on the neurocranium using Landmark Editor (Wiley, 2006). We used general least squares Procrustes superimposition (GPA) to remove the effects of size, translation, and rotation on the landmarks from the four skeletal regions (Rohlf, 1998). We performed a Procrustes ANOVA between centroid size and skeletal shape and found a significant effect of allometry (r2 = 0.061; F = 8.96; p = 0.01). We removed the allometric component of the shape variation in subsequent analyses by performing a regression of shape on geometric centroid size to generate a landmark data set based on residuals (Adams & Otárola-Castillo, 2013). We then performed a principal component (PC) analysis on these residual landmarks to quantify variation in shape. We exported the first three PC axes that represented between 20-40% of the total shape variation for each skeletal region, and saved these PC scores for use during the muscle-bone correlation analysis. The percent variation explained for each bone or bone complex is similar to those values reported for other morphometric analyses of hybrid cichlid populations (e.g., Parsons et al., 2015). We also plotted the first three PC axes for each skeletal region to provide a morphospace to visualize the major differences in shape variation among individuals.
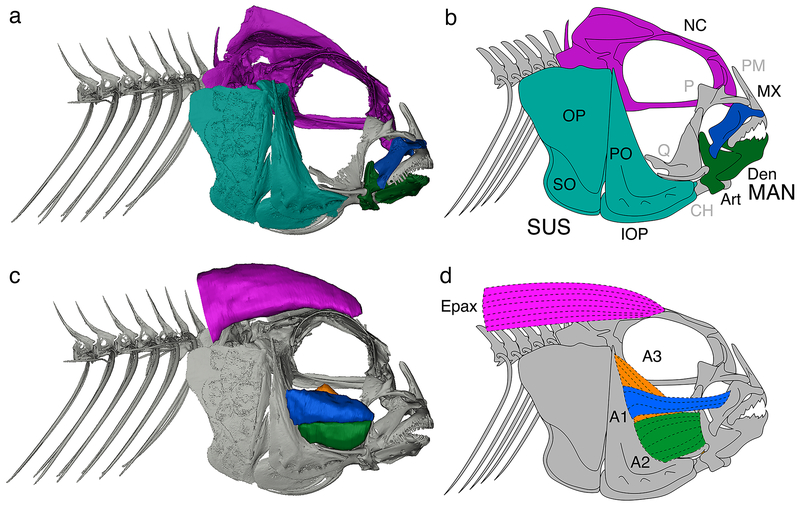
Figure 2.
Bones and muscles assessed in this study. Skeletal CT scan (a) and associated line drawing outlining bones/bone complexes (b). Muscles overlaid on skeletal CT scan (c) and associated line drawing outlining muscles of interest (d). Bone color code: purple, neurocranium; blue, maxilla; green, mandible; turquoise, suspensorium. Muscle color code: purple, epaxials (Epax); blue, A1; green, A2; orange, A3. Abbreviations: NC, neurocranium; SUS, suspensorium; MAN, mandible; Art, articular; CH, ceratohyal; Den, Dentary; IOP, interopercle; MX, maxilla; OP, opercle; P, palatine; PM, pre-maxilla; PO, preopercle; Q, quadrate; SO, subopercle.
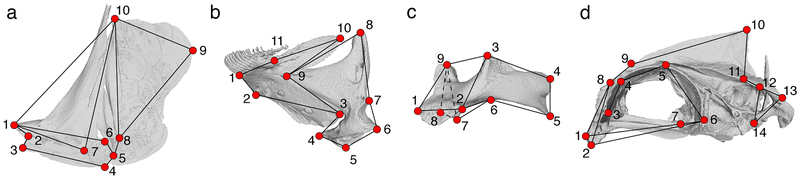
Figure 3.
Landmark positions used for each of the four bones present in this study. Wireframes are projected over each CT scan. The same wireframe scheme is repeated in the morphospaces in Figure 4 to demonstrate the primary axes of shape variation. Full landmark descriptions can be found in Table S1.
We attempted to mitigate and quantify landmark error in two ways; firstly a single author (AJC) performed all landmarking to eliminate inter-observer error. Secondly, we calculated intra-observer landmark error for each individual landmark using the method described by Singleton (2002). We landmarked a single specimen 15 times in order to create an error dataset. We then performed GPA on the 15 replicated samples and extracted the coordinates of the consensus shape. Following Singleton (2002), we calculated the distance between the coordinates of each landmark in the replicated sample and the coordinates of the consensus shape and took a mean of that distance. We then calculated the distance between the coordinates of the consensus centroid and the consensus landmark coordinates. We calculated a ratio of the mean distance between the mean distances from the consensus landmarks to the trial landmarks and the distance from the centroid to the consensus landmarks in order to quantify the error (Singleton, 2002). Finally, we multiplied this by 100 percent to find the percent error. Mean landmarking error rates for each bone or bone complex are deemed acceptable if they are less than 2% (Singleton, 2002). While Singleton’s (2002) method quantifies error for each landmark individually, it is calculated based on a ratio involving the distance from each landmark to the consensus centroid, and so landmarks closer to the centroid will have higher error (Cramon-Taubadel, Frazier, & Lahr, 2007). We observe a similar pattern in our dataset. For this reason, and because GPA mathematically distributes variation across all landmarks, we focus on global error for each bone or bone complex by reporting the average error across all landmarks.
We extracted volume information from four different muscles on the cichlid skull; epaxials and three components of the Adductor mandibulae complex – A1, A2, A3. We selected these muscles as they are large, well characterized muscles involved in functionally demanding activities (adducting the jaw, swimming) and are in close proximity to our bones of interest (Liem & Osse, 1975). To correct for differences in skull size among individuals, which may result in larger muscle volumes as a product of the larger overall head size, we regressed muscle volume by centroid size, and used muscle volume residuals in subsequent analyses. Finally, we performed linear regressions between each of our four skeletal regions and the each of the four muscle volume residuals. A single author (DTL) performed all muscle segmentation to eliminate inter-observer error. To assess intra-observer repeatability of our muscle volume measures we randomly selected ten individuals and segmented out each of the muscles of interest (A1, A2, A3, epaxial) and calculated the technical error of measurement (TEM). TEM is a commonly used estimate of measurement precision (Perini, de Oliveira, Ornelia, & de Oliveira, 2005), and is calculated following equation 1:
| (1) |
Where D is the difference between the first and second volume measurement for a given muscle and N is the number of individuals included in the sample. We then calculated relative TEM (rTEM) to express the error rate as a percentage (equation 2).
| (2) |
Where TEM is the absolute TEM from equation 1, and is the grand mean of the arithmetic means from both measurement replications across all individuals for a muscle. rTEM values <5% are typically considered as an acceptable error rate (Weinberg, Scott, Neiswanger, & Marazita, 2005).
Results
Morphometric Analyses
We find that the primary axes of variation for each bone or bone complex broadly mimics the major morphological axes present across the rock dwelling cichlids in Lake Malawi (Cooper et al., 2010). In particular, shape variation in the F5 is broadly arrayed along axes that distinguish species that forage via suction versus biting strategies, and represent morphological tradeoffs between speed and power during jaw movements. As such, patterns of variation have distinct biomechanical correlates that should serve to influence the function of the musculoskeletal complex (Figure 4). For instance, the first PC axis for the mandible (21% of the variation) represents change in the length of the retroarticular process (RA), where the interopercle ligament inserts, as well as the ascending arm of the articular (AA), on which the second subdivision of the adductor mandibulae (A2) inserts. PC2 (12% of the variation) reflects mandible width, while PC3 (11% of the variation) captures variation in dentary depth and RA size. PC1 for the maxilla (14% of the variation) reflects a number of correlated changes including maxilla length, intermaxillary process width, and position of the maxillary fenestra. PC2 (13% of the variation) describes the shape of the maxillary wing and head, while PC3 (12% of the variation) reflects the depth of the central part of the maxilla, which is where the tendon of the A1 muscle inserts. PC1 for the neurocranium (19% of the variation) represents overall length, depth of the structure, as well as the angle of the vomerine process. PC2 (10% of the variation) defines the anterior-posterior position of the supraoccipital crest (SOC), and the width of the neurocranium. PC3 (8% of the variation) reflects the height of the SOC and the position of the posttemporal–supracleithral (PTSC) joint. Suspensorium PC1 (18% of the variation) reflects changes in anterior-posterior length, particularly of the preopercle (PO). PC2 (15% of the variation) reflects PO and interopercle (IOP) depth, while PC3 (10% of the variation) describes dorsal-ventral depth of the anterior portion of the IOP (where the IOP ligament attaches), as well as the shape of the articulation surface between the IOP and subopercle (SO).
Figure 4.
Patterns of morphospace occupation in the neurocranium, suspensorium, mandible and maxilla. Morphological wireframes reflect most positive or most negative location on the shape space.
Average landmark error ranged between 1.24% (neurocranium) and 1.94% (maxilla) with a mean percent error across all bones of 1.71% (Table S2). This mean error is very small and unlikely to have significantly impacted our analyses.
Muscle volumes
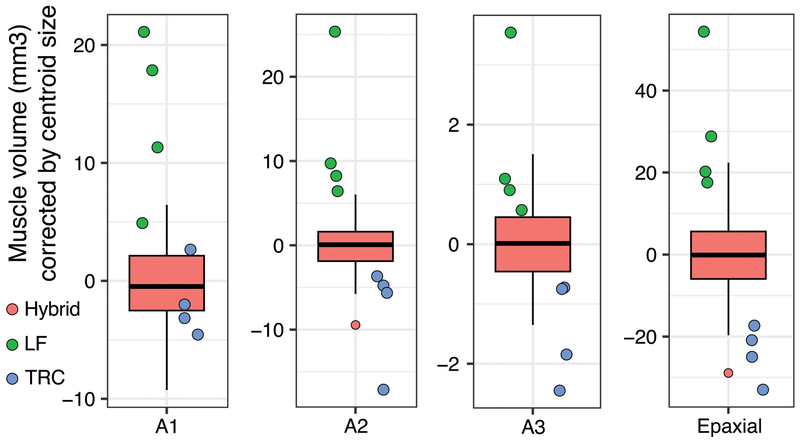
We note a high degree of variation among F5 muscle volumes. The epaxial muscles exhibit the greatest variation among individuals (SD=6.67mm3), while the adductor mandibulae muscles exhibit less (A1, SD=2.71mm3; A2, SD=2.41mm3; A3, SD=0.60mm3). When we examine the distribution of corrected muscle volumes including the parental species, we find that LF muscles are consistently larger than those in TRC. Moreover, for A2, A3, and epaxial muscle volumes, hybrids are intermediate relative to either LF or TRC, while hybrid A1 muscle volumes are skewed toward the TRC distribution (Figure 5). This pattern is consistent with dominance of TRC alleles for A1 muscle volume, while A2, A3, and epaxial muscle volumes exhibit additive modes of inheritance. We find relatively low levels of intra-observer error in our muscle volume measurements (Table S3). Error rates range from 3.87% in the epaxial muscle, to 4.97% in the A2. These error rates are below the 5% error rate typically considered as acceptable (Weinberg et al., 2005).
Figure 5.
Variation in muscle volume among hybrid and parental cichlid populations. Size corrected muscle volumes are represented for each of the four craniofacial muscles we evaluated. Hybrids, red box and whisker plots; LF, green points; TRC, blue points.
Correlations
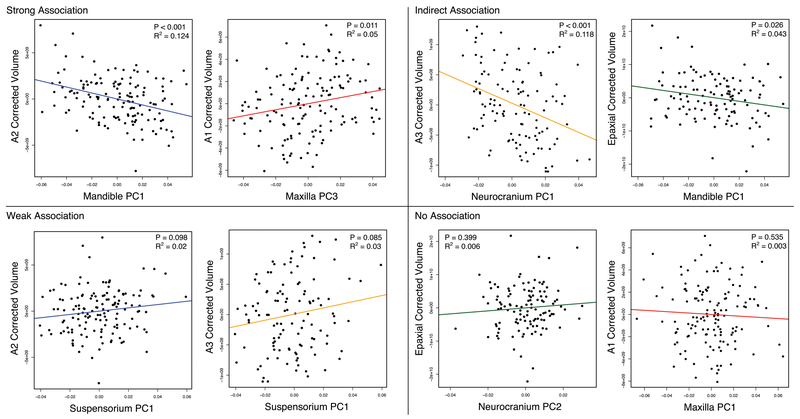
We find a suite of correlations between bone shape and muscle volume across all bone-muscle complexes (Table 1). Although there is variation in the number and strength of the correlations (represented by the r2 statistic), all bones exhibit some type of association with at least one muscle. Thus, our analyses provide no support for the null hypothesis of no association. We highlight a number of notable associations in Figure 6, and present the full complement of correlations in Table 1. In particular, we illustrate instances of association or non-association based on the categories outlined in Figure 1.
Table 1.
Results from the linear regression analysis between the different muscles and bones. Strongly significant associations (α>0.05) are shown in boldface, weaker associations (α>0.10) are shown in italics. P-values are shown at top, and r2-values are shown at bottom as percent variance explained.
| Bones | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-values | Mandible | Maxilla | Suspensorium | Neurocranium | ||||||||
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| A1 | >0.001 | 0.382 | 0.716 | 0.535 | 0.494 | 0.010 | 0.092 | 0.173 | 0.030 | 0.002 | 0.401 | >0.001 |
| A2 | >0.001 | 0.505 | 0.437 | 0.109 | 0.534 | 0.161 | 0.098 | 0.230 | 0.031 | 0.002 | 0.360 | >0.001 |
| A3 | 0.023 | 0.150 | 0.279 | 0.653 | 0.807 | 0.573 | 0.085 | 0.484 | 0.091 | >0.001 | 0.417 | 0.664 |
| Epaxials | 0.026 | 0.137 | 0.152 | 0.042 | 0.828 | 0.242 | 0.034 | 0.015 | 0.272 | 0.006 | 0.399 | 0.012 |
| r2-values | ||||||||||||
| A1 | 12.72% | 0.55% | 0.10% | 0.28% | 0.34% | 4.66% | 2.05% | 1.34% | 3.35% | 6.71% | 0.51% | 13.69% |
| A2 | 12.40% | 0.32% | 0.44% | 1.85% | 0.28% | 1.42% | 1.98% | 1.04% | 3.34% | 6.70% | 0.61% | 11.58% |
| A3 | 4.47% | 1.83% | 1.03% | 0.18% | 0.05% | 0.28% | 2.60% | 0.43% | 2.51% | 11.80% | 0.58% | 0.17% |
| Epaxials | 4.30% | 1.93% | 1.79% | 3.58% | 0.04% | 1.20% | 3.89% | 5.11% | 1.06% | 6.51% | 0.63% | 5.36% |
Figure 6.
Subset of linear regressions between different bones and muscles. Four quadrants reflect the different types of associations that can exist between muscle and bones.
We detect weak associations (0.05>p<0.1; 2.0>r2<2.6) between the suspensorium and all of the adductor musculature (A1-A3). Specifically, suspensoirium length (PC1) is weakly associated with adductor musculature. This association may arise due to those muscles originating from the suspensorium, transducing relatively diffuse mechanical feedback across this structure. We also observe relatively strong direct associations (p<0.05; r2>2.6) between a number of muscles and bones. Mandible PC1 (e.g., lengths of the AA and RA) exhibits a strong association with the A2 and A3 muscles, which insert onto the AA and the medial side of the mandible, respectively. In addition, maxilla PC3 (medial depth) is correlated with the A1 muscle, which inserts onto this region of the maxilla. Finally, neurocranium length and depth (PC1), as well as SOC height and PTSC joint position (PC3) were associated with epaxial volume.
A surprising trend in this dataset was evidence for strong indirect associations (p<0.05; r2>2.6) between bone and muscle, and we observe this across all bones in our sample (Table 1). These associations may arise due to the establishment of a “global” mechanical environment by muscles that can influence bone shape without a direct connection to them (Maas & Sandercock, 2010; Yucesoy, 2010). For example, the epaxials, which insert onto the neurocranium, were associated with mandible process morphology (i.e., PC1), suspensorium length (i.e., PC1) as well as PO and IOP depth (PC2), and maxilla length (PC1). The adductor complex (A1-A3) was highly associated with neurocranium length and depth (PC1), as well as SOC height and PTSC joint position (PC3). When considering the complete dataset, we find it notable that without any direction attachment, muscle volume could account for up to 14% of the variation in bone shape based on the r2 statistic (Table 1).
Discussion
Muscles are an important source of variation in craniofacial geometry
Soft-tissue traits are a relatively understudied source of variation in influencing the shapes of hard tissues (Fabre et al., 2018). Skeletal morphology is often used to answer certain questions related to the function, diet or even ecology of organisms, but the surrounding musculature that powers these bony structures is typically not assessed to the same degree. However, new technologies such as contrast-enhanced CT scanning has permitted the collection of large amounts of muscular data to better understand how the skull operates as a functional complex, rather than a collection of individual units. Here we combine skeletal and muscular data to assess the degree of form-function associations across various bones and muscles that are involved in ecological relevant tasks such as locomotion or feeding.
We find multiple associations between particular aspects of bone shape and muscle volume. In general, we observe variation across functionally relevant aspects of craniofacial morphology, independent of dietary differences as all fish were reared in a common environment. These whole-scale changes mimic the major axes of morphological diversification among various cichlid linages (Cooper et al., 2010). That a hybrid population, which is segregating various craniofacial alleles, exhibits similar patterns of variation as natural populations suggests a conservation of developmental trajectories and mechanisms (Powder, Milch, Asselin, & Albertson, 2015). Our work on muscle-bone associations suggests further that variation in muscle size may contribute to stereotypical patterns of cichlid skeletal variation.
All bones examined were correlated with at least one muscle, underscoring the ubiquity of muscular input in shaping the cichlid skull. We break down the types of associations into three major categories: weak associations, strong direct associations, and strong indirect associations. Variation in the degree and type of associations observed implies there are differences in how robust some bones are to mechanical input. While our analysis was not exhaustive, it was comprehensive insofar as examining the core muscle complex involved in jaw adduction (i.e., A1-A3), as well as the major muscle group involved in head-lifting (i.e., the epaxials), a critical action during suction feeding. Further, we analyzed the shape of most bones involved in cichlid prey collection (i.e., however, the pharyngeal jaws, which are involved in prey processing, were not analyzed here).
Some regions of the cichlid skull appear highly sensitive to muscular input
The neurocranium and mandible appear to be particularly sensitive to muscle load-induced bone remodeling, which is to say they both displayed strong direct and indirect associations with the muscles we tested (Table 1; Figure 6; Figure 7). That the mandible is a hotspot of remodeling may not be especially surprising, given the developmental, functional, and evolutionary attributes of this structure. For instance, the cichlid mandible appears to develop as a morphologically integrated structure (e.g., Albertson & Kocher, 2006; Albertson, Streelman, Kocher, & Yelick, 2005; Parsons, Cooper, & Albertson, 2011), which may allow it to respond independently to both mechanical stimulus and natural selection relative to nearby cranial bones. The mechanical assertion is evidenced by recent feeding trial experiments whereby cichlid populations were separated into two groups and provided either a hard or soft diet (Parsons et al., 2016; Parsons, Taylor, Powder, & Albertson, 2014). The investigators identified global changes in shape occurring across the mandible, but among the strongest and most consistent changes were localized to sites of muscle and ligament attachment, specifically the AA and RA, respectively (Parsons et al., 2016). This demonstrates that the environment can be a significant source of variation, and that bone remodeling can be adaptive, as the primary centers of bone deposition in those cichlids exposed to a hard food diet produced an increase in mechanical advantage to facilitate the consumption of a mechanically demanding diet. The evolutionary assertion is generally supported by the observation that mandibular shape is one of the most conspicuous aspects of cichlid morphological diversity (Cooper et al., 2010). This is thought to be due, at least in part, to the evolution of a set of highly specialized pharyngeal jaws in cichlids, which are functionally decoupled from the oral jaws (Hulsey, García de León, & Rodiles-Hernández, 2006). This permits the oral jaws to focus on capturing or manipulating a food item, while the pharyngeal jaws are responsible for food processing. This decoupling between the oral and pharyngeal jaws suggests that the mandible is under fewer functional constraints, which may result in a greater capacity for muscle-induced variability and evolutionary change (Hulsey et al., 2006; Liem & Osse, 1975).
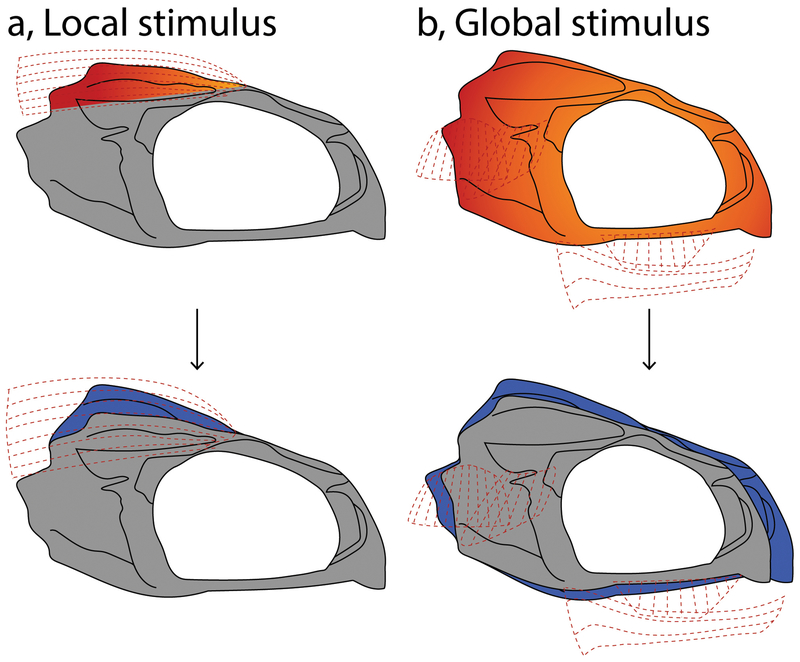
Figure 7.
Hypothetical schematic for how competing mechanical environments produced by different muscles can influence either local (a) or global remodeling (b), using the neurocranium as an example. Sunburst colors reflect regions under mechanical stress, hotter colors reflect regions of greater force, and cooler colors reflect regions under lower force. Blue regions on the bone reflect sites of new bone deposition as a result of mechanical stimulus. Under the scenario of local change, load-induced bone remodeling is limited to the site of muscle insertion (a). A global response occurs when the entire element remodels in response to a more general and ubiquitous mechanical environment (b).
The observation that the shape of the neurocranium is highly associated with muscle volumes is more unexpected. Unlike the mandible, the neurocranium is a multi-functional structure responsible for housing the brain and other sensory structures; it also articulates with the oral jaws, pharyngeal jaws, suspensorium, pectoral girdle and spine. Given these competing roles, we expected the neurocranium to be more constrained with respect to muscle-induced variability. In contrast, we find that multiple aspects of neurocranium shape are strongly correlated with muscle volume. This observation underscores the important roles that the neurocranium plays during feeding, including dissipating forces generated by the oral jaws (Cooper et al., 2011). Morphological diversity in skull shape is also high among cichlids (Cooper et al., 2010), and predictive of foraging niche (Ford et al., 2016), which suggests selection is able to strike a balance between multiple functional demands when coordinating an adaptive response (Hulsey, Mims, & Streelman, 2007). For example, we found an association between the shape of the PTSC joint on the neurocranium (PC3), and epaxial muscle volume (Figure S4). While these structures are not in direct contact with one another, they both participate in the action of suction feeding; the epaxial muscles participate by driving head lifting (Camp et al., 2015), while the PTSC joint acts via its articulation with the pectoral girdle, on which much of the musculature involved in lower jaw depression originates (Liem & Osse, 1975). Thus, changes in this complex will alter suction feeding performance, and this association illustrates how discrete aspects of cichlid head anatomy vary in a coordinated manner along biomechanically relevant axes.
For both the mandible and neurocranium, it is possible that behavioral plasticity coupled with muscle input is key to enhanced evolutionary potential along discrete eco-morphological axes. In the case of the neurocranium, this has occurred despite competing functional demands. This would suggest that within the cranium, muscle-induced remodeling is limited to regions not directly associated with brain or eye function, a hypothesis that could be tested through a more rigorous analysis of the neurocranium.
Some regions of the cichlid skull appear robust to muscular input
An equally notable outcome of this study is the observation that some bones appear to be more robust to the muscle-induced mechanical environment. While we do observe overall geometric change in the maxilla, with levels of variation that are comparable to those in other elements, few of the major axes of shape variation were associated with muscle volume at the local or global level. The maxilla exhibited one direct association that appears to have a functional consequence; the depth of the maxillary shank, which is the site of A1 insertion, was associated with A1 muscle volume. Hence, a deeper maxillary shank can facilitate a larger site for the muscle to attach to. We posit that the dearth of maxilla-muscle associations result from aspects of maxilla shape being primarily governed by ligaments (e.g., intermaxillary ligament (M. R. Conith et al., 2018), palatomaxillary ligament (Figure S1)). The geometry of the maxilla is defined by the size and shape of four large processes, each of which articulates or otherwise interacts with a different bone or set of bones (Figure S1–S3) (Barel et al., 1976). Laterally, the shank process articulates with the mandible via a thick ligament. Medially, there are two wings that collectively form the saddle, which rests on top of the dentigerous arm of the premaxilla. The rostral most process is the premaxillad wing, which connects to the contralateral side of the head via the intermaxillary ligament. The caudal most process is the palantinad wing, which articulates with the pterygoid process of the palantine bone. Centrally, the dorsal wing interacts with the lacrimal bone laterally, and the A1 medially. This complex geometry underscores the complex functional roles played by the maxilla during jaw movement. For example, the maxilla acts as an important lever-arm during jaw protrusion, which is enabled by this complex series of linkages (Westneat, 1990). Thus, the shape of the maxilla must be functionally integrated with many surrounding bones, which means that the remodeling of this element may be more dependent upon ligamentous (i.e., bone-to-bone) rather than tendinous (i.e., muscle-to-bone) input (Figure S1–S2).
The suspensorium represents another bone complex with only weak associations with muscle volume. Functionally, the suspensorium represents an interesting test case for how the strength of muscle-induced mechanical feedback may influence the strength of the association. The adductor complex originates on the suspensorium, and muscle origination sites are posited to experience lower levels of mechanical loading compared to sites of insertion (Maas & Sandercock, 2010). This may indicate that, although the suspensorium is in direct contact with the adductor complex, the presumptively lower loading regime produces less overall feedback between the muscles and bones, reducing the amount of load-induced remodeling. Notably, the suspensorium does exhibit two strong indirect correlations with the epaxial muscles, specifically with overall suspensorium length (PC1), as well as PO and IOP depth (PC2). This may result from the functional integration between these structures. Specifically, the epaxials power suction feeding via head lifting (Camp et al., 2015), while the suspensorium influences this action in a number of ways. For example, the size of the suspensorium relates to the overall size of the buccal cavity, which has a large effect on suction feeding (Wainwright et al., 2007). Additionally, the suspensorium forms a critical component of the opercle four-bar linkage system (Westneat, 1990), and the dimensions of this bony complex influence the kinetics of these systems. Thus, both the epaxials and suspensorium represent core muscular and skeletal input for suction feeding, and coordinated changes in these two structures may be the result of feedback within this functional system.
Conclusions
The craniofacial skeleton is encased in soft tissues and the interaction between these traits can produce mechanical feedback that may promote or depress the deposition of bone. Therefore, soft tissue traits such as muscles can have a profound effect on bone shape. Here, we study the degree of association between the shape of four craniofacial bones or bone complexes and four craniofacial muscles involved in feeding within a hybrid population of cichlids. We find that the association between muscles and bones can be categorized in three ways: either weak, strong direct (i.e., muscle inserts onto the bone), or strong indirect (i.e., bone resides in a mechanical environment produced by a nearby muscle). Up to 14% of the variation in skeletal shape can be predicted by muscle volume. While this might not seem like a strikingly large percentage, when considered in the context of craniofacial genetics it becomes more notable. Specifically, dozens of craniofacial QTL have been identified in cichlids to date (e.g., Albertson, Streelman, & Kocher, 2003b; Parsons et al., 2015), and with few exceptions each QTL on average explains less than 14% of the variation in shape. Thus, muscle volume can explain as much, if not more, of the variation in bone shape compared to craniofacial QTL, thereby making a significant contribution to the totality of variation in these bones. A logical next step will be to perform QTL mapping in this population for both the bone and muscle traits described here. An expectation that arises from data presented here is that the genotype-phenotype map for each will overlap, thereby implying key roles for genetic pleiotropy in regulating bone and muscle shape. This is not to say that the relationship will be unidirectional, with muscle size-determining genes influencing bone shape. There is accumulating evidence to suggest that certain molecules, expressed at the interface between bone and connective tissue, can facilitate a feedback loop between these two tissues thereby promoting growth of each (Felsenthal & Zelzer, 2017).
Finally, we describe associations that manifest themselves at two levels of organization, both locally (i.e., at a process), and globally (i.e., across unconnected bones). This suggests that the influence of muscle-input on bone shape extends beyond points of direct contact, and that the mechanical environment established by muscles can have far more generalized impact on skeletal geometry. This has several important implications. For one, dietary shifts over life history can be facilitated by changes in the mechanical environment leading to different allometric relationships between organismic size and skeletal shape (Hellig, Kerschbaumer, Sefc, & Koblmüller, 2010). In addition, changes in muscle and skeletal shape can allow populations to exploit novel or transient food sources (Anderson et al., 2014; A. J. Conith, Meagher, & Dumont, 2018). Finally, differences among bones in their association with muscle suggest that different craniofacial bones are more or less robust to muscle-induced mechanical stimulation (Maas & Sandercock, 2010). Taken together, we find that muscles are an important determinant of skeletal geometry and potent source of craniofacial variation.
Supplementary Material
Acknowledgements
We thank Greg Lin at Harvard for micro-CT access, Daniel Pulaski for assistance with segmentation software, and members of the Albertson lab for constructive comments on key elements of this project.
Funding
National Institutes of Health #R01DE026446
References
- Adams DC, & Otárola-Castillo E (2013). Geomorph: an R Package for the Collection and Analysis of Geometric Morphometric Shape Data. Methods in Ecology and Evolution, 4(4), 393–399. 10.1111/2041-210X.12035 [DOI] [Google Scholar]
- Albertson RC (2008). Morphological Divergence Predicts Habitat Partitioning in a Lake Malawi Cichlid Species Complex. Copeia, 2008(3), 689–698. 10.1643/CG-07-217 [DOI] [Google Scholar]
- Albertson RC, & Kocher TD (2001). Assesing morphological differences in an adaptative trait: A landmark-based morphometric approach. Journal of Experimental Zoology, 289(289), 385–403. [DOI] [PubMed] [Google Scholar]
- Albertson RC, & Kocher TD (2006). Genetic and developmental basis of cichlid trophic diversity. Heredity, 97(3), 211–221. 10.1038/sj.hdy.6800864 [DOI] [PubMed] [Google Scholar]
- Albertson RC, Markert JA, Danley PD, & Kocher TD (1999). Phylogeny of a rapidly evolving clade: the cichlid fishes of Lake Malawi, East Africa. Proceedings of the National Academy of Sciences, 96(9), 5107–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson RC, Streelman JT, & Kocher TD (2003). Genetic basis of adaptive shape differences in the cichlid head. Journal of Heredity, 94(4), 291–301. 10.1093/jhered/esg071 [DOI] [PubMed] [Google Scholar]
- Albertson RC, Streelman JT, Kocher TD, & Yelick PC (2005). Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proceedings of the National Academy of Sciences of the United States of America, 102, 16287–16292. 10.1073/pnas.0506649102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PSL, Renaud S, & Rayfield EJ (2014). Adaptive plasticity in the mouse mandible. BMC Evolutionary Biology, 14(1), 85 10.1186/1471-2148-14-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel CDN, Witte F, & Van Oijen MJP (1976). The shape of the skeletal elements in the head of a generalized Haplochromis species: H. elegans trewavas 1933 (Pisces, Cichlidae). Netherlands Journal of Zoology, 26(2), 163–265. 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- Bossdorf O, Richards CL, & Pigliucci M (2008). Epigenetics for ecologists. Ecology Letters, 11(2), 106–115. 10.1111/j.1461-0248.2007.01130.x [DOI] [PubMed] [Google Scholar]
- Brunt LH, Norton JL, Bright JA, Rayfield EJ, & Hammond CL (2015). Finite element modelling predicts changes in joint shape and cell behaviour due to loss of muscle strain in jaw development. Journal of Biomechanics, 48(12), 3112–3122. 10.1016/j.jbiomech.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tenesa A, & Keightley PD (2015). The nature of genetic variation for complex traits revealed by GWAS and regional heritability mapping analyses. Genetics, 201(4), 1601–1613. 10.1534/genetics.115.177220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp AL, Roberts TJ, & Brainerd EL (2015). Swimming muscles power suction feeding in largemouth bass. Proceedings of the National Academy of Sciences, 112(28), 8690–8695. 10.1073/pnas.1508055112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Liu C, You L, & Simmons CA (2010). Boning up on Wolff’s Law: Mechanical regulation of the cells that make and maintain bone. Journal of Biomechanics, 43(1), 108–118. 10.1016/j.jbiomech.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Concannon MR, & Albertson RC (2015). The genetic and developmental basis of an exaggerated craniofacial trait in East African cichlids. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 324(8), 662–670. 10.1002/jez.b.22641 [DOI] [PubMed] [Google Scholar]
- Conith AJ, Meagher MA, & Dumont ER (2018). The Influence of Climatic Variability on Morphological Integration, Evolutionary Rates, and Disparity in the Carnivora. The American Naturalist, 191(6), 000–000. 10.1086/697376 [DOI] [PubMed] [Google Scholar]
- Conith MR, Hu Y, Conith AJ, Maginnis MA, Webb JF, & Albertson RC (2018). Genetic and developmental origins of a unique foraging adaptation in a Lake Malawi cichlid genus. Proceedings of the National Academy of Sciences, 115(27), 7063–7068. 10.1073/pnas.1719798115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WJ, Parsons K, McIntyre A, Kern B, McGee-Moore A, & Albertson RC (2010). Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift-lakes. PloS One, 5(3), e9551 10.1371/journal.pone.0009551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WJ, Wernle J, Mann K, & Albertson RC (2011). Functional and Genetic Integration in the Skulls of Lake Malawi Cichlids. Evolutionary Biology, 38(3), 316–334. 10.1007/s11692-011-9124-9 [DOI] [Google Scholar]
- Cramon-Taubadel N. von, Frazier BC, & Lahr MM (2007). The problem of assessing landmark error in geometric morphometrics: theory, methods, and modifications. American Journal of Physical Anthropology, 134(1), 24–35. 10.1002/ajpa [DOI] [PubMed] [Google Scholar]
- Ehrlich PJ, & Lanyon LE (2002). Mechanical strain and bone cell function: A review. Osteoporosis International, 13(9), 688–700. 10.1007/s001980200095 [DOI] [PubMed] [Google Scholar]
- Fabre A, Perry JMG, Hartstone-rose A, & Elien AUR (2018). Do Muscles Constrain Skull Shape Evolution in Strepsirrhines? The Anatomical Record, 301(2), 291–310. 10.1002/ar.23712 [DOI] [PubMed] [Google Scholar]
- Felsenthal N, & Zelzer E (2017). Mechanical regulation of musculoskeletal system development. Development, 144(23), 4271–4283. 10.1242/dev.151266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folland JP, & Williams AG (2007). The adaptations to strength training: Morphological and neurological contributions to increased strength. Sports Medicine, 37(2), 145–168. 10.2165/00007256-200737020-00004 [DOI] [PubMed] [Google Scholar]
- Ford AGP, Rüber L, Newton J, Dasmahapatra KK, Balarin JD, Bruun K, & Day JJ (2016). Niche divergence facilitated by fine-scale ecological partitioning in a recent cichlid fish adaptive radiation. Evolution, 70(12), 2718–2735. 10.1111/evo.13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gignac PM, Kley NJ, Clarke JA, Colbert MW, Morhardt AC, Cerio D, … Witmer LM (2016). Diffusible iodine-based contrast-enhanced computed tomography (diceCT): An emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. Journal of Anatomy, 228(6), 889–909. 10.1111/joa.12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, & Marcucio RS (2009). Deciphering the Palimpsest: Studying the Relationship Between Morphological Integration and Phenotypic Covariation. Evolutionary Biology. 10.1007/s11692-009-9076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrimsson B, Mio W, Marcucio RS, & Spritz R (2014). Let’s Face It—Complex Traits Are Just Not That Simple. PLoS Genetics, 10(11), 10–12. 10.1371/journal.pgen.1004724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick BP, Yohe L, Vander Linden A, Dávalos LM, Sears K, Sadier A, … Dumont E (2018). Assessing Soft-Tissue Shrinkage Estimates in Museum Specimens Imaged With Diffusible Iodine-Based Contrast-Enhanced Computed Tomography (diceCT). Microscopy and Microanalysis, 24(3), 284–291. 10.1017/S1431927618000399 [DOI] [PubMed] [Google Scholar]
- Hellig CJ, Kerschbaumer M, Sefc KM, & Koblmüller S (2010). Allometric shape change of the lower pharyngeal jaw correlates with a dietary shift to piscivory in a cichlid fish. Naturwissenschaften, 97(7), 663–672. 10.1007/s00114-010-0682-y [DOI] [PubMed] [Google Scholar]
- Hu Y, & Albertson RC (2017). Baby fish working out: An epigenetic source of adaptive variation in the cichlid jaw. Proceedings of the Royal Society B: Biological Sciences, 284(1860), 20171018 10.1098/rspb.2017.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Parsons K, & Albertson RC (2014). Evolvability of the Cichlid Jaw: New Tools Provide Insights into the Genetic Basis of Phenotypic Integration. Evolutionary Biology, 41(1), 145–153. 10.1007/s11692-013-9254-3 [DOI] [Google Scholar]
- Hulsey CD, García de León FJ, & Rodiles-Hernández R (2006). Micro- and macroevolutionary decoupling of cichlid jaws: a test of Liem’s key innovation hypothesis. Evolution, 60(10), 2096–2109. 10.1111/j.0014-3820.2006.tb01847.x [DOI] [PubMed] [Google Scholar]
- Hulsey CD, Mims MC, & Streelman JT (2007). Do constructional constraints influence cichlid craniofacial diversification? Proceedings of the Royal Society B: Biological Sciences, 274(1620), 1867–1875. 10.1098/rspb.2007.0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kague E, Gallagher M, Burke S, Parsons M, Franz-Odendaal T, & Fisher S (2012). Skeletogenic Fate of Zebrafish Cranial and Trunk Neural Crest. PLoS ONE, 7(11), 1–13. 10.1371/journal.pone.0047394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP (2003). Developmental perspective on developmental instability: theory, models and mechanisms In Developmental instability: causes and consequences (pp. 14–34). [Google Scholar]
- Konings A (2007). Malawi Cichlids in Their Natural Habitat (4th ed.). Cichlid Press. [Google Scholar]
- Lazić MM, Carretero MA, Crnobrnja-Isailović J, & Kaliontzopoulou A (2015). Effects of Environmental Disturbance on Phenotypic Variation: An Integrated Assessment of Canalization, Developmental Stability, Modularity, and Allometry in Lizard Head Shape. The American Naturalist, 185(1), 44–58. 10.1086/679011 [DOI] [PubMed] [Google Scholar]
- Lieber RL, & Ward SR (2011). Skeletal muscle design to meet functional demands. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1570), 1466–1476. 10.1098/rstb.2010.0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, & Osse JWM (1975). Biological Versatility, Evolution, and Food Resource Exploitation in African Cichlid Fishes. American Zoologist, 15(2), 427–454. [Google Scholar]
- Maas H, & Sandercock TG (2010). Force transmission between synergistic skeletal muscles through connective tissue linkages. Journal of Biomedicine and Biotechnology, 2010 10.1155/2010/575672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky M, Svardal H, Tyers AM, Miska EA, Genner MJ, Turner GF, & Durbin R (2017). Whole Genome Sequences Of Malawi Cichlids Reveal Multiple Radiations Interconnected By Gene Flow. BioRxiv, 143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons KJ, Concannon M, Navon D, Wang J, Ea I, Groveas K, … Albertson RC (2016). Foraging environment determines the genetic architecture and evolutionary potential of trophic morphology in cichlid fishes. Molecular Ecology, 25(24), 6012–6023. 10.1111/mec.13801 [DOI] [PubMed] [Google Scholar]
- Parsons KJ, Cooper WJ, & Albertson RC (2011). Modularity of the Oral Jaws Is Linked to Repeated Changes in the Craniofacial Shape of African Cichlids. International Journal of Evolutionary Biology, 2011, 1–10. 10.4061/2011/641501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons KJ, Son YH, & Albertson RC (2011). Hybridization Promotes Evolvability in African Cichlids: Connections Between Transgressive Segregation and Phenotypic Integration. Evolutionary Biology, 38(3), 306–315. 10.1007/s11692-011-9126-7 [DOI] [Google Scholar]
- Parsons KJ, Taylor AT, Powder KE, & Albertson RC (2014). Wnt signalling underlies the evolution of new phenotypes and craniofacial variability in Lake Malawi cichlids. Nature Communications, 5, 1–11. 10.1038/ncomms4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons KJ, Wang J, Anderson G, & Albertson RC (2015). Nested Levels of Adaptive Divergence: The Genetic Basis of Craniofacial Divergence and Ecological Sexual Dimorphism. G3, 5(8), 1613–1624. 10.1534/g3.115.018226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini T. a, de Oliveira GL, Ornelia JS, & de Oliveira FP (2005). Technical error of measurement in anthropometry. Revista Brasileira de Medicina Do Esporte, 11, 86–90. [Google Scholar]
- Pigliucci M (2008). Is evolvability evolvable? Nature Reviews. Genetics, 9, 75–82. 10.1038/nrg2278 [DOI] [PubMed] [Google Scholar]
- Powder KE, Milch K, Asselin G, & Albertson RC (2015). Constraint and diversification of developmental trajectories in cichlid facial morphologies. EvoDevo, 6(1). 10.1186/s13227-015-0020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabey KN, Green DJ, Taylor AB, Begun DR, Richmond BG, & McFarlin SC (2015). Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. Journal of Human Evolution, 78, 91–102. 10.1016/j.jhevol.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MR, Wray NR, & Visscher PM (2014). Explaining additional genetic variation in complex traits. Trends in Genetics, 30(4), 124–132. 10.1016/j.tig.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf FJ (1998). On applications of geometric morphometrics to studies of ontogeny and phylogeny. Systematic Biology, 47, 147–167. 10.1080/106351598261094 [DOI] [PubMed] [Google Scholar]
- Rot-Nikcevic I, Downing KJ, Hall BK, & Kablar B (2007). Development of the mouse mandibles and clavicles in the absence of skeletal myogenesis. Histology and Histopathology, 22(1–3), 51–60. Retrieved from https://digitum.um.es/jspui/handle/10201/27535 [DOI] [PubMed] [Google Scholar]
- Santana SE (2018). Comparative Anatomy of Bat Jaw Musculature via Diffusible Iodine-Based Contrast-Enhanced Computed Tomography. Anatomical Record, 301(2), 267–278. 10.1002/ar.23721 [DOI] [PubMed] [Google Scholar]
- Schilling TF, & Kimmel CB (1997). Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development, 124(15), 2945–2960. 10.1006/dbio.2001.0201 [DOI] [PubMed] [Google Scholar]
- Schulte FA, Ruffoni D, Lambers FM, Christen D, Webster DJ, Kuhn G, & Müller R (2013). Local Mechanical Stimuli Regulate Bone Formation and Resorption in Mice at the Tissue Level. PLoS ONE, 8(4). 10.1371/journal.pone.0062172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton M (2002). Patterns of cranial shape variation in the Papionini (Primates: Cercopithecinae). Journal of Human Evolution, 42(5), 547–578. 10.1006/jhev.2001.0539 [DOI] [PubMed] [Google Scholar]
- Spassov A, Toro-Ibacache V, Krautwald M, Brinkmeier H, & Kupczik K (2017). Congenital muscle dystrophy and diet consistency affect mouse skull shape differently. Journal of Anatomy, 231(5), 736–748. 10.1111/joa.12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streelman TJ, & Danley PD (2003). The stages of vertebrate evolutionary radiation. Trends in Ecology & Evolution, 18(3), 126–131. 10.1016/S0169-5347(02)00036-8 [DOI] [Google Scholar]
- Theveneau E, & Mayor R (2012). Neural crest delamination and migration: From epithelium-to-mesenchyme transition to collective cell migration. Developmental Biology, 366(1), 34–54. 10.1016/j.ydbio.2011.12.041 [DOI] [PubMed] [Google Scholar]
- Vickerton P, Jarvis J, & Jeffery N (2013). Concentration-dependent specimen shrinkage in iodine-enhanced microCT. Journal of Anatomy, 223(2), 185–193. 10.1111/joa.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH (1942). Canalization of development and the inheritance of acquired characters. Nature, 150(3811), 563–565. 10.1038/150563a0 [DOI] [Google Scholar]
- Wainwright P, Carroll AM, Collar DC, Day SW, Higham TE, & Holzman RA (2007). Suction feeding mechanics, performance, and diversity in fishes. Integrative and Comparative Biology, 47(1), 96–106. 10.1093/icb/icm032 [DOI] [PubMed] [Google Scholar]
- Weinberg SM, Scott NM, Neiswanger K, & Marazita ML (2005). Intraobserver error associated with measurements of the hand. American Journal of Human Biology, 17(3), 368–371. 10.1002/ajhb.20129 [DOI] [PubMed] [Google Scholar]
- Westneat MW (1990). Feeding mechanics of teleost fishes (Labridae; Perciformes): A test of four-bar linkage models. Journal of Morphology, 205(3), 269–295. 10.1002/jmor.1052050304 [DOI] [PubMed] [Google Scholar]
- Wiley DF (2006). Landmark Editor 3.0. Institute for Data Analysis and Visualization, University of California, Davis: Retrieved from http://graphics.idav.ucdavis.edu/research/EvoMorph [Google Scholar]
- Witten PE, & Huysseune A (2009). A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biological Reviews, 84(2), 315–346. 10.1111/j.1469-185X.2009.00077.x [DOI] [PubMed] [Google Scholar]
- Yu FX, & Guan KL (2013). The Hippo pathway: Regulators and regulations. Genes and Development, 27(4), 355–371. 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucesoy CA (2010). Epimuscular myofascial force transmission implies novel principles for muscular mechanics. Exercise and Sport Sciences Reviews, 38(3), 128–134. 10.1097/JES.0b013e3181e372ef [DOI] [PubMed] [Google Scholar]
- Zelzer E, Blitz E, Killian ML, & Thomopoulos S (2014). Tendon-to-bone attachment: From development to maturity. Birth Defects Research Part C - Embryo Today: Reviews, 102(1), 101–112. 10.1002/bdrc.21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.