Abstract
The gastrointestinal (GI) mucosa is central to HIV pathogenesis, and the integrin α4β7 promotes the homing of immune cells to this site. Data from SIV animal models suggest that α4β7 blockade provides prophylactic and therapeutic benefits. Here we show that pre-HIV infection levels of α4β7+ peripheral blood CD4+ T cells, independent of other T cell phenotypes and genital inflammation, were associated with increased rates of HIV acquisition in South African women. This association was stronger when infection was caused by HIV strains containing V2 Env motifs with a preference for α4β7 binding. A similar acquisition effect was observed in a Kenyan cohort, and in non-human primates (NHPs) following intravaginal SIV challenge. In addition, pre-HIV α4β7+ CD4+ T cells predicted higher set point viral load and a >2-fold increased rate of CD4 decline. These results were confirmed in SIV-infected NHPs. Increased frequencies of pre-HIV α4β7+ CD4+ T cells were also associated with higher post-infection expression of LPS binding protein, a microbial translocation marker, suggestive of more extensive gut damage. CD4+ T cells expressing α4β7 were rapidly depleted very early in HIV infection, particularly from the GI mucosa, and were not restored by early antiretroviral therapy (ART). This study provides a link between α4β7 expression and HIV clinical outcomes in humans, in line with observations made in NHPs. Given the availability of a clinically approved anti-α4β7 monoclonal antibody for treatment of inflammatory bowel disease, these data support further evaluation of targeting α4β7 integrin as a clinical intervention during HIV infection.
One sentence summary:
The role of α4β7+ CD4+ T cells in HIV pathogenesis
Introduction
Several lines of evidence suggest that the gastrointestinal (GI) mucosa and the associated lymphoid tissue play a critical role in HIV pathogenesis. Infections by HIV and SIV rapidly deplete CD4+ T cells from this site during the first weeks of infection(1-3), and the resulting, extensive damage to the homeostasis of gut tissue persists into chronic HIV infection. One consequence of the extensive gut damage is the translocation of gut-resident bacterial products into the blood, which has been proposed to be a major source of chronic immune activation that drives HIV pathogenesis(4).
The homing of immune cells to the inductive and effector sites of the large and small intestine is facilitated by the expression of the integrin α4β7 on these cells and its preferred ligand MAdCAM-1, which is constitutively expressed on the high endothelial venules of all GI tissues(5). Several lines of evidence suggest that α4β7-expressing CD4+ T cells are important in HIV pathogenesis. These include direct binding of α4β7 to some HIV strains(6-9), not as an HIV entry co-receptor per se (10-12), but rather as a molecule that may facilitate attachment of the virus to its optimal target cells. This role for α4β7 in facilitating localization and attachment of virus to cells may be particularly important during HIV transmission, when availability of target cells is a rate-limiting step for the virus. Many laboratories have shown that CD4+ T cells expressing α4β7 are preferentially infected both in vitro and ex vivo (6, 8, 12-14), including during acute SIV infection and in experiments using HIV clade C viruses(15), the predominant clade in South Africa. We have previously shown that α4β7 expression on HIV target cells in the female reproductive tract (FRT) was associated with other markers of optimal HIV target cells, including CCR5 expression(16).
The in vivo implications of modulating α4β7 have been highlighted by studies that have utilized a primatized anti-α4β7 monoclonal antibody (mAb) (derived from the Act1 clone). When administered just prior to and during acute intravenous SIVmac239 infection, Act1 mediated moderate reductions in plasma viral load but substantial reductions in gut pro-viral DNA(17). More dramatic results were obtained when Act1 was administered prior to low-dose repeat vaginal challenge with SIVmac251; a significant delay in SIV acquisition was observed(18). Animals that eventually acquired SIV showed markedly reduced damage to gut-associated lymphoid tissue (GALT), in addition to other lymphoid and mucosal tissues. When given in combination with antiretroviral therapy (ART), anti-α4β7 promoted potent therapeutic effects, leading to post-treatment virological control in 8/8 treated animals compared to 0/7 controls(19). These findings suggest that α4β7 expressing cells play a central role in SIV transmission and pathogenesis.
One important gap in the literature is the role of α4β7 at the time of HIV exposure in humans. Here we report evidence that frequencies of α4β7 expressing CD4+ T cells predict both increased risk of HIV acquisition and more rapid disease progression in a cohort of high-risk women from KwaZulu-Natal, South Africa. With a number of anti-α4β7 blockers in various stages of clinical development, these findings inform the potential translation of these drugs in the treatment of HIV disease.
Results
Participants
We compared levels of α4β7 integrin expression on CD4+ T cells in blood samples from individuals who later acquired HIV to controls that remained HIV uninfected for the duration of the CAPRISA 004 study. Cases were sampled at the last available pre-HIV infection visit, with a sampling median of 110 days (IQR 65, 182) pre-HIV infection. Controls (n = 106) were matched to cases (n = 59) at a 2:1 ratio on the basis of study arm, age (5-year window), and month of enrolment. No major differences between cases and controls were observed for a number of demographic, clinical, and behavioral variables (Table 1). Additional analyses were conducted in study cohorts from Kenya, Uganda, the RV254/SEARCH 010 cohort in Thailand (Table S1-3), and in non-human primates (NHPs).
Table 1.
Characteristics of the CAPRISA 004 study population
| Variable | Cases (n=59) Median (IQR) |
Controls (n=106) Median (IQR) |
P value |
|---|---|---|---|
| Age* | 23 (20, 25) | 22 (20, 28) | .864 |
| Urban site | 19/59 (32.2) | 40/106 (37.7) | .503 |
| TFV arm* | 23/59 (39.0) | 43/106 (40.6) | .87 |
| DMPA use | 50/59 (84.7) | 85/106 (80.2) | .532 |
| Vaginal discharge | 25/59 (42.4) | 35/106 (33.0) | .242 |
| Ulcers | 1/59 (1.7) | 6/106 (5.7) | .423 |
| HSV-2 sero-status (at trial entry) | 32/59 (54.2) | 53/106 (50.0) | .629 |
| Sex acts, past 30 days | 5 (2.5, 6.6) | 5 (3, 8) | .281 |
| Parity | 1 (1, 2) | 1 (1, 1) | .878 |
Part of the matching criteria
Pre-HIV frequencies of integrin β7Hi CD4+ T cells are associated with HIV acquisition
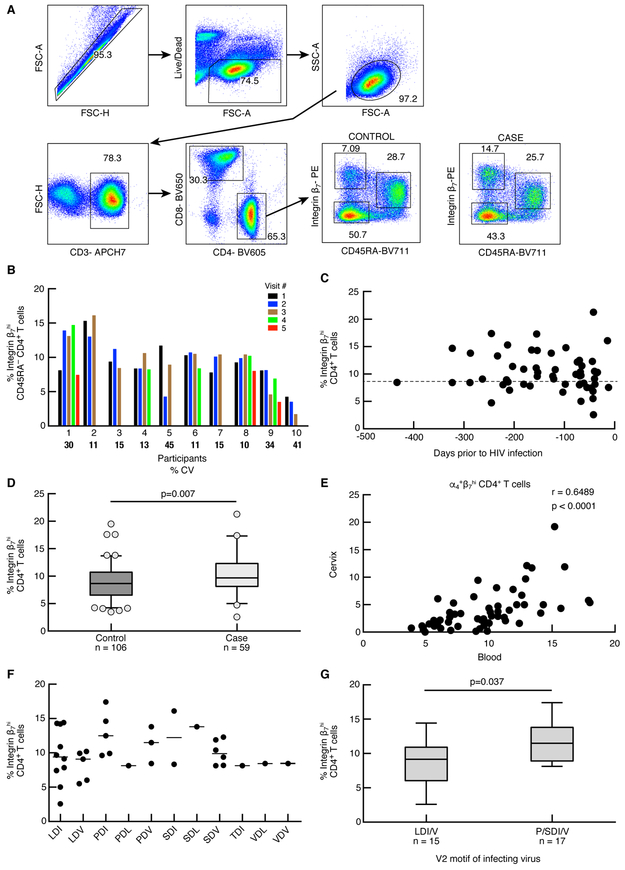
Previous studies have demonstrated that β7Hi cells in the blood are >99% α4β7+ (6, 20); therefore β7Hi CD45RA− gating was used to quantify α4β7 expression on CD4+ T cells (herein referred to as β7Hi cells). We identified three populations of CD4+ T cells based on the relative density of the integrin β7 and CD45RA expression that were consistently measured across the study population: β7Hi CD45RA−, β7Int CD45RA+ and β7Neg CD45RA− (Fig. 1A). Because it is difficult to sample cases immediately prior to HIV infection, we investigated the stability of β7Hi frequencies in blood samples over multiple HIV uninfected visits in a subset of participants (range 2-5 visits, Fig. 1B). The median co-efficient of variation (CV) was 15% (IQR 11-36), indicating relatively stable expression in most individuals. We then determined whether any association between HIV infection and higher frequencies of β7Hi CD4+ T cells might be explained by sampling carried out closer to the estimated time of HIV infection (Fig. 1C). Our analysis indicates that this was not the case; the frequencies of β7Hi CD4+ T cells were consistent among cases and controls regardless of sample timing, congruent with the stability data presented in Figure 1B.
Figure 1. Effect of pre-infection β7Hi CD45RA− CD4+ T cell frequency on HIV acquisition risk in CAPRISA 004 study.
A) Parent gating strategy for the analysis of frozen PBMCs from the CAPRISA 004 study. The staining profile of PBMC from a representative HIV-uninfected participant is shown. β7 gating is shown for one representative control and one case sample obtained at a HIV-uninfected time point. B) Stability of β7Hi CD4+ T cells overtime. Samples from 10 patients (x axis) were assayed from 3-5 HIV uninfected visits (colored bars) depending on sample availability. Median coefficient of variation (CV) between HIV uninfected time points for every individual was calculated. Individual CVs are indicated in the graph. C) Sampling time points for the pre-HIV β7Hi measurements. The number of days prior to HIV infection that the sample was obtained (x-axis) is plotted against β7Hi frequency (y-axis). The dashed line represents the median Integrin β7Hi expression by CD4+ T cells D) The frequency of β7Hi CD45RA−CD4+ T cells in cases (n=59) and controls (n=106). Conditional logistic regression analysis was used to measure the effect of pre-infection β7Hi levels on HIV acquisition. E) Spearman correlation between α4β7Hi CD4+ T cell frequency between cervix and blood in the Nairobi/Uganda study (n=54) F) Infecting viral V2 motifs and pre-HIV frequencies of β7Hi CD4+ T cells G) Pre-HIV frequencies of β7Hi CD4+ T cells in cases infected by viruses with V2 loops containing the P/SDI/V and LDI/V motifs (n=32). Differences between groups were analyzed using unpaired t-test.
In our primary endpoint analysis, the frequency of β7Hi cells was higher in samples from cases (median 9.7%, IQR 8.1-12.3%) than controls (median 8.7%, IQR 6.5-10.7%). In conditional logistic regression analyses, each percent of pre-HIV infection β7Hi CD4+ T cells correlated with 17% increased risk of HIV acquisition (OR 1.17, 95% 1.05 −1.32, p=0.007, Fig. 1D). In contrast, no significant associations were observed for either β7Int or β7Neg populations and HIV acquisition (p=0.242 and 0.882 respectively, Fig. S1).
We next carried out multivariable modeling to adjust for variables that may confound the HIV acquisition analysis. Integrin β7Hi cell frequency remained associated with HIV acquisition with a similar effect estimate after adjusting for study site, HSV-2 sero-status, abnormal vaginal discharge, number of sexual partners and sex acts/month, condom and depomedroxyprogesterone acetate (DMPA) usage (aOR 1.16, 95% 1.03-1.32, p=0.016, Table 2). We also adjusted the analysis for genital inflammation (defined as 5/9 pro-inflammatory cytokines in the upper quartile(21)), and found that frequencies of β7Hi cells remained a predictor of HIV outcome in a model that included all of the other covariates listed above (aOR 1.15, 95% 1.02-1.30, p=0.028). Additionally, frequencies of β7Hi cells remained a predictor of HIV outcome in a model that included both CD4+ T cell activation (aOR 1.18, 95% 1.05-1.33, p=0.005) and CD8+ T cell activation (aOR 1.18, 95% 1.05-1.32, p=0.007) defined by HLA-DR and CD38 co-expression. We carried out further phenotypic profiling to compare levels of CCR5, Ki67, CD38, and HLA-DR expression between the three main β7 subsets (Fig. S2). β7Hi cells were comparable to at least one of the other β7-associated subsets with respect to phenotypic markers that have been associated with HIV pathogenesis, further indicating that the observed effect is specific to β7Hi expression.
Table 2.
Multivariable analysis of HIV acquisition and integrin β7Hi CD4+ T cells using conditional logistic regression
| Variable | P value | aOR | 95.0% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Integrin β7Hi CD45RA− CD4+ T cells | .016 | 1.163 | 1.029 | 1.316 | |
| Urban study site | .091 | .349 | .103 | 1.181 | |
| HSV-2 seropositive at trial entry | .223 | 1.606 | .750 | 3.438 | |
| Abnormal vaginal discharge | .248 | 1.646 | .707 | 3.831 | |
| Number of new casual partners in the last 30 days | .669 | .916 | .614 | 1.367 | |
| Median # of sex acts / month | .100 | 1.110 | .980 | 1.258 | |
| Condom use | Never (ref) | .486 | 1 | - | - |
| Always | .134 | .399 | .120 | 1.327 | |
| Occasionally | .371 | .623 | .221 | 1.757 | |
| Most times | .740 | .824 | .262 | 2.587 | |
| DMPA use | .294 | 1.880 | .578 | 6.120 | |
The HIV acquisition results were validated in additional 41 participants (11 cases and 30 controls) from an independent cohort of female sex workers from Nairobi (Table S3), with a very similar odds ratio for HIV acquisition risk observed as in CAPRISA 004 (Table S4, OR 1.19, 95% CI 0.94-1.52, p=0.148). Combining the data from the two cohorts (n=206), we found that each percent increase in β7Hi expression correlated with an 18% increase in HIV risk (OR 1.18, 95% CI 1.06-1.31, p=0.002). These data demonstrate that α4β7 is a consistent predictor of HIV acquisition risk in two independent human cohorts.
We further analyzed pre-infection α4β7 expression on blood CD4+ T cells as a predictor of SIV acquisition in NHPs exposed to weekly intra-vaginal challenges with SIVmac251. These animals were rhesus macaques (RMs) that were in the control arm (irrelevant IgG) of a published study(18). After adjusting for age, parity, and menses, RMs with higher α4β7 levels acquired SIV more rapidly than RMs with lower α4β7 frequencies (aHR 1.20/% α4β7, 95% CI 0.99-1.44, p=0.057, Fig. S3). These results are congruent with the previous NHP observations (14, 22) and our human cohort data, confirming that α4β7 expression on CD4+ T cells is associated with both HIV and SIV infection risk.
Levels of α4β7 integrin expression by CD4+ T cells correlated in corresponding samples from blood and cervix
We next explored whether levels of α4β7+ CD4+ T cells in the blood reflected levels in the female reproductive tract (FRT), the main site of HIV exposure during heterosexual transmission. While cervical specimens were not available in CAPRISA 004, we evaluated whether α4+β7Hi cells in the blood correlated with α4+β7Hi cells from endocervical cytobrushes in women from Uganda and Kenya (Fig. 1E). Positive correlations were observed consistently between cohorts (combined r=0.65, p<0.0001, n=57). These data suggest that assessing systemic β7Hi cells is likely reflective of α4+β7Hi levels in the FRT.
Pre-HIV levels of α4β7 are associated with early HIV env sequences
Several reports have suggested that α4β7 can bind to the gp120 second variable loop (V2) of some strains of HIV Env directly(7, 9, 23, 24). Specifically, the P/SDI/V V2 motif has been associated with increased α4β7-dependent in vitro replication and is over-represented in the South African epidemic, particularly in KwaZulu-Natal(7, 15). With these observations in mind, we hypothesized that the frequency of β7Hi cells pre-HIV infection would correlate with the V2 sequences of early-transmitting viruses encoding P/SDI/V motif. Sequences of acute/early HIV envelopes were available for 32 CAPRISA 004 participants at a median of 5 weeks post-HIV infection (IQR 3, 7). Indeed, participants infected by viruses with V2 loops containing the P/SDI/V motif had higher levels of pre-HIV β7Hi cells than those infected by viruses with LDI/V motifs (median 11.5, IQR 8.9, 13.8; vs. median 9.1, IQR 6.0-10.9, p=0.0366, Fig. 1F and 1G). These data suggest that the risk of HIV acquisition mediated by α4β7 might be particularly pronounced when exposure involves viruses containing certain V2 motifs associated with enhanced α4β7 binding.
Pre-HIV infection β7Hi frequencies are associated with HIV disease progression
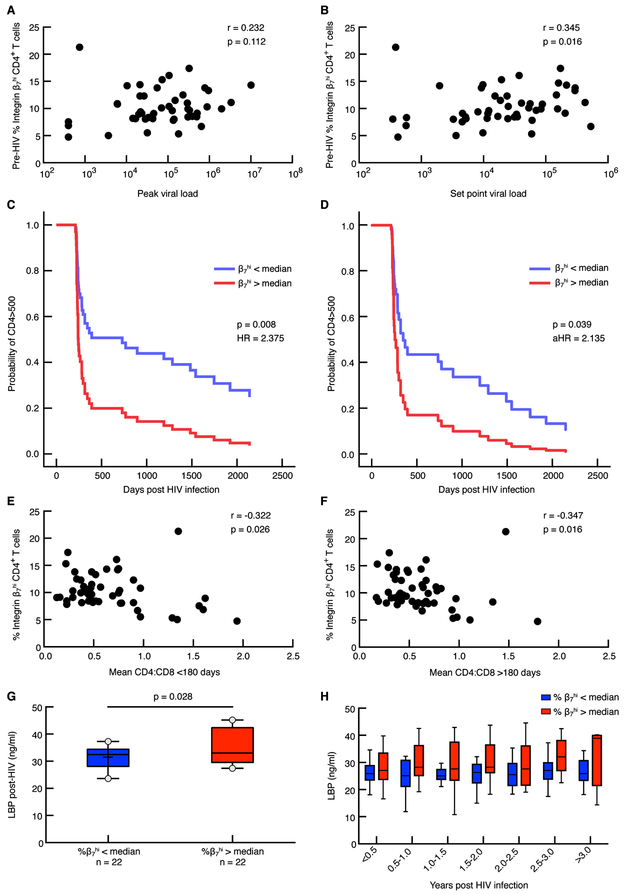
To determine if pre-HIV infection frequencies of β7Hi CD4+ T cells predicted the rate of HIV disease progression, we correlated the frequency of these cells with both peak and set-point viral load (VL), the rate of CD4+ T cell decline prior to ART initiation, and the median CD4:CD8 ratio post-HIV infection (Fig. 2). Modest correlations between β7Hi cell frequency and both peak (<180 days post-HIV) and set point VL (average of >180 day measurements) were observed (r=0.232, p=0.112 for peak; r=0.345, p=0.016 for set point, Fig. 2A and 2B); in contrast, no correlations were observed for β7Int or β7Neg cells (Fig. S4A and B).
Figure 2. Effect of pre-infection β7Hi CD45RA− CD4+ T cell frequency on disease progression in patients that became infected in CAPRISA 004/002 study.
A) Correlation between pre-infection β7Hi frequency and peak VL (n= 49) B) Correlation between pre-infection β7Hi frequency and set point viral load (n=49) C). The frequency of pre-infection β7Hi cells as a predictor of CD4+ T cells decline<500 cells/μl (n=48) analyzed using Cox regression models D) Pre-infection β7Hi cells as a predictor of CD4+ T cells decline<500 cells/μl in a multivariable Cox regression model correcting for age, study site, study arm, set point viral load, DMPA use and HSV-2 status at baseline E) Correlation between pre-infection β7Hi frequency and mean CD4:CD8 ratio < 180 days post infection (n=48) F) Correlation between pre-infection B7Hi frequency and mean CD4:CD8 ratio > 180 days post infection (n=48) G) Median post-infection plasma levels of LBP expression in cases with pre-infection β7Hi CD4+ T cell expression above (n=22) and below(n=22) median. H) Longitudinal plasma LBP levels at 6 month intervals in CAPRISA 002 cases. Linear mixed models were used to compare LBP levels over time. Spearman correlation was used to analyze associations between the two variables.
The frequency of β7Hi cells was a strong predictor of CD4+ T cell decline below 500 cells/μl; individuals with β7Hi cells above the median of β7Hi expression progressed to CD4<500 at more than twice the rate of those below the β7Hi median (HR 2.38, 95% CI 1.25-4.51, p=0.008, Fig. 2C). At day 500, approximately 80% of those above the β7Hi median had progressed, compared to approximately 50% of those below the β7Hi median. In multivariable Cox regression models (Table 3), inclusion of plasma VL as a covariate had an impact on the strength of association, suggesting that the β7Hi-associated rate of disease progression might be mediated in part by higher levels of HIV replication. However, in the full model, including VL and other important covariates (age, study site, study arm, DMPA use and HSV-2 status at baseline), pre-HIV levels of β7Hi cells remained a significant predictor of the rate of CD4+ T cell decline (aHR 2.14, 95% CI 1.04-4.39, p=0.039, Fig. 2D).
Table 3.
Multivariable analysis of CD4 decline and integrin β7Hi CD4+ T cells using Cox regression
| Variable | P value | aHR | 95.0% CI | |
|---|---|---|---|---|
| Lower | Lower | |||
| Integrin β7Hi CD45RA− CD4+ T cells | .039 | 2.135 | 1.039 | 4.389 |
| Age | .041 | 1.106 | 1.004 | 1.219 |
| Urban study site | .520 | .747 | .308 | 1.814 |
| Study arm | .159 | .598 | .293 | 1.223 |
| Log10 pVL (set point) | <.001 | 2.538 | 1.649 | 3.905 |
| DMPA use | .829 | 1.126 | .384 | 3.304 |
| HSV-2 seropositive at baseline | .952 | .975 | .429 | 2.217 |
Furthermore, we observed an inverse association between pre-HIV β7Hi cells and post-HIV CD4:CD8 ratio, another marker of disease progression(25) (r=−0.322, p=0.026 for measurements <180 days post-HIV, and r=−0.347 and p=0.016 for measurements >180 days post-HIV, Fig. 2E and 2F). Again, neither β7Int or β7Neg cell frequencies were associated with CD4+ T cell decline < 500 cells/μl or CD4:CD8 ratio (Fig. S4C-E). We did not find any associations between activation markers expressed on bulk pre-infection CD4+ T cells and disease progression (Fig. S5, Table S5).
In order to establish a link between human and NHP data we characterized α4β7+ CD4+ T cell frequencies in 14 NHPs prior to IV injection with SIVmac239. Nine animals had low levels (<30%) while the remaining 5 animals had higher levels >30%) of α4β7+ on CD4+ T cells; these patterns of expression were similar in both the blood and the gut tissue. RMs with higher α4β7 expression experienced higher set point VL than animals with lower α4β7 expression (median from week 3-16, 331,680 versus 82,230 copies/ml), which was statistically significant in a linear mixed model analysis (p=0.033, Fig S6A). As observed in humans, RMs with higher α4β7 expression had a faster rate of CD4 decline (p<0.001, Fig S6B) demonstrating consistency between different primate species.
We hypothesized that the association between β7Hi cells and the rate of HIV disease progression might be mediated by more efficient transit of virus-infected cells into the GALT, leading to more extensive gut damage and its associated pathogenic effects. To test this hypothesis, we measured plasma levels of microbial translocation markers prospectively following acute HIV until ART initiation. We found that levels of LPS binding protein (LBP) were elevated in CAPRISA 004 participants with β7Hi cell frequencies above the median at all visits post-HIV infection, whether compared as median values (Fig. 2G) or at 6-month intervals (beta 0.54, 95% CI: 0.06-1.03, p=0.028, Fig. 2H). To determine if this was simply a reflection of more rapid progression, we utilized linear mixed models adjusting for viral load and CD4:CD8 ratio, measured at the same time points as LBP. LBP remained associated with higher β7Hi levels in the adjusted models (beta 0.59, 95% CI: 0.01-1.16, p=0.045). Additionally, we quantified plasma levels of another two commonly used markers: intestinal fatty-acid binding protein (I-FABP) and sCD14. While we did not observe a statistically significant difference in I-FABP and sCD14 levels (Table S6) in relation to β7Hi levels, this could be due to recent reports that have suggested these markers may not be specific to microbial translocation and are influenced by other causes of GI disease(26-28) and monocyte activation(29), respectively.
Depletion of β7Hi CD4+ T cells in the blood and gastrointestinal mucosa during acute HIV infection
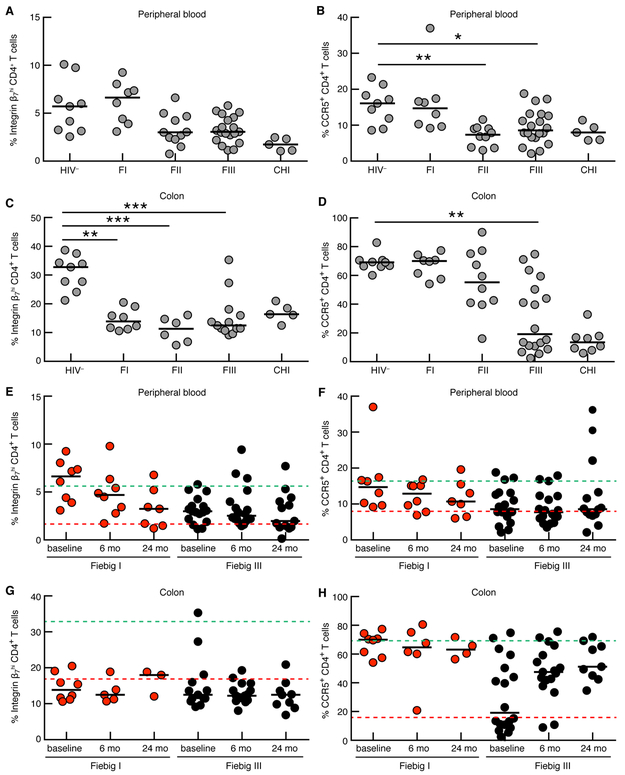
To determine the impact of acute HIV infection on the β7Hi CD4+ T cell population early in HIV infection in both the blood and the GI tract we compared frequencies of both CCR5+ and β7Hi cells at Fiebig (F) stages I, II, and III in participants enrolled in the RV254 early infection cohort to chronic HIV infected and uninfected participants (Fig. 3)(30). In the blood, β7Hi cells increased transiently in FI followed by a modest drop in those recruited from FII onwards (Fig. 3A). Similar kinetics were observed for blood CCR5 depletion, where levels of CCR5+ cells remained comparable to HIV uninfected levels in FI and significantly decreased during FII and FIII (Fig. 3B). In contrast in the GI tract, β7Hi depletion was evident in all Fiebig stages, with major depletion occurring from FI (Fig. 3C). This occurred more rapidly than CCR5 depletion, where the major loss of CCR5+ cells was only evident during the transition from FII to FIII (Fig. 3D). These data confirm that α4β7+ CD4+ T cells are depleted very early in HIV infection, particularly in the gut, providing a potential explanation as to why these cells predicted higher rates of HIV acquisition and disease progression in CAPRISA 004 study.
Figure 3. Depletion and post cART recovery of β7Hi and CCR5+ CD4+ T cells in peripheral blood and cells isolated from sigmoid colon during acute infection in RV254.
Frequencies of (A) β7Hi and (B) CCR5+ CD4+ T cells in the peripheral blood at different Fiebig (F) stage [HIV- (n=9), FI (n=8), FII (n=11), FIII(n=20) and chronic HIV infected (CHI, n=5)] C) Frequencies of β7Hi CD4+ T cells in the colon [HIV- (n=9), FI (n=8), FII (n=6), FIII(n=13) and CHI (n=5)] D) Frequencies of CCR5+ CD4+ T cells in the colon [HIV- (n=9), FI (n=8), FII (n=10), FIII(n=18) and CHI (n=8)]. Frequencies of (E) β7Hi and (F) CCR5+ CD4+ T cells in the peripheral blood following ART initiation in either FI [red data points, at baseline (n=8), 6m(n=8), 24m(n=7)] or FIII [black data points, at baseline (n=18), 6m(n=18) and 24m(n=14)]. (G) Frequencies of β7Hi CD4+ T cells in the colon following ART initiation in either FI [red data points, baseline (n=8), 6m(n=5), 24m(n=3)] or FIII [black data points, baseline (n=13), 6m(n=15) and 24m(n=9)] (H) CCR5+ CD4+ T cells in the colon following ART initiation in either FI [red data points, at baseline (n=8), 6m(n=6), 24m(n=4)] or FIII [black data points, at baseline (n=18), 6m(n=17) and 24m(n=9)]. The dashed lines represent the median of cells in healthy control participants (green, n=9) and in CHI patients (red, n=5). For A to D, Kruskal Wallis test was used followed by a Dunn’s multiple comparisons test to look for differences between HIV uninfected (HIV−) group and groups at different Fiebig stages. For E to H Friedman test was used to test for significant differences within each ART initiation group (FI and FIII). *P<0.05, **P< 0.01 and ***P<0.001.
Since ART was initiated upon diagnosis in RV254, we were not able to assess disease progression in this cohort. However, we determined the impact of ART on β7Hi and CCR5+ CD4+ T cell frequencies in both colon biopsies and blood over 2 years following very early HIV treatment at the time of acute diagnosis. In participants who initiated ART in either FI or FIII, two years of ART failed to restore blood β7Hi CD4+ T cells (Fig. 3E). Interestingly, although not statistically significant, a continuous loss of β7Hi CD4+ T cells in peripheral blood could be observed despite the fact that these participants initiated treatment during FI, suggesting that if anything these frequencies may continue to decline during treatment. Similarly, ART initiation failed to restore blood CCR5+ CD4+ T cells (Fig. 3F). However, the impact of ART on β7Hi and CCR5+ CD4+ T cells in the colon was strikingly different. The frequency of colonic β7Hi CD4+ T cells in patients who initiated ART in either FI or FIII were already reduced at initial baseline measurement and these levels showed no sign of recovery after 24 months of treatment (Fig 3G). In contrast, in participants who initiated ART in FI, colonic CCR5+ CD4+ T cells were not depleted and their frequency at 24 months of therapy was maintained at a level similar to healthy controls. Participants who initiated therapy in FIII showed an initial loss and a gradual, “near” recovery by month 24 (Fig. 3H). These data suggest that β7Hi CD4+ T cell depletion occurs very early during acute HIV infection, including in the GI tract compared to blood. It is also clear that ART is unable to restore the frequency of those CD4+ T cell frequencies in the GI tract, even when provided at the earliest time point, when gut damage is relatively minimal compared with chronic HIV.
Discussion
The integrin α4β7 plays an important role in promoting immune cell trafficking to the inductive and effector sites of the gastrointestinal tract, both of which are irreversibly damaged during acute HIV infection. The main aim of the present study was to evaluate the role of α4β7 integrin in humans at risk of HIV infection, an important step towards the translation of these promising pre-clinical studies. In line with our a priori hypotheses, expression of α4β7 on blood memory CD4+ T cells measured prior to HIV infection in South African women predicted both higher risk of HIV acquisition and a more rapid rate of HIV disease progression.
Although the association of α4β7 expression and HIV acquisition was relatively modest, results were consistent in independent cohorts in two different countries and in NHPs. Higher pre-infection α4β7 levels were previously associated with susceptibility to rectal SIV infection (14, 22). Additionally in a low-dose vaginal challenge, SIV infection was significantly delayed by blocking α4β7 using Act1(18). Consistencies in odds ratios for HIV acquisition risk between CAPRISA 004 and Kenyan FSW cohort demonstrate that α4β7 associates with HIV acquisition risk in women from different geographic locations. Reported findings demonstrate consistency in humans and show that results can be translated between human and non-human primates.
Interactions between α4β7 and HIV env may assist the virus in locating its ideal target cells(31). The findings herein show that higher frequency of α4β7 CD4+ T cells was associated with preferential infection by HIV-1 containing gp120 V2 motifs (P/SDV/I) that have been associated with higher α4β7 binding and are over-represented in clade C sequences from KwaZulu-Natal, South Africa, the region where the CAPRISA 004 study was conducted (7, 15). The α4β7-binding motif has been implicated as an important epitope for HIV antibody responses, including both bNAbs (32, 33) and those that correlated with protection against HIV infection in the RV144 vaccine study(34). The role of pre –infection a4b7 expression on HIV pathogenesis likely depends on the nature of the transmitting virus.
Levels of α4β7 expression had a strong impact on the rate of HIV disease progression; in particular, as measured by the rate of CD4+ T cell decline. As observed with HIV acquisition, the progression effect was highly similar between RMs and humans. In addition, the data from RV254 suggest that α4β7+ CD4+ T cells are targeted very early in the blood and gut. In humans, a high proportion of initial HIV-target cells are likely α4β7+, and the rapid gut depletion of CD4+ T cells may be driven by the preferential depletion of cells expressing α4β7 (even earlier than CCR5). Therefore, in individuals with higher frequency of α4β7+ cells one may expect increased viral replication and associated destruction of many CD4 compartments including the GI mucosa, the latter of which has a major effect on disrupting immune homeostasis. While we were not able to link rapid gut depletion with pre-HIV α4β7, due to lack of pre-HIV samples in RV254 and lack of gut sampling in CAPRISA004, our finding of raised LBP levels at all stages of untreated HIV infection in individuals with higher α4β7 expression supports this concept, linking α4β7 expression with subsequent microbial translocation and gut damage.
Initiation of antiretroviral therapy (ART), particularly at early stages of HIV infection(35), restores some immune cell populations, but rarely to their pre-infection frequency and/or function(36). The majority of CD4 depletion occurs in the GALT during primary infection(3, 37) and ART administration at first detection of VL failed to prevent depletion or facilitate reconstitution of gut α4β7+ CD4+ T cells in RV254. The fact that ART alone does not lead to immune-restoration, but ART in combination with anti- α4β7 did so in NHPs(19), suggests that interventions in addition to ART may be needed to achieve functional CD4+ T cell restoration.
One of the limitations of our study is the lack of paired cervical and blood sampling, which is not available in CAPRISA 004. Nevertheless, data from two independent East African cohorts demonstrate that the frequency of α4β7+ cells correlated strongly between blood and cervical CD4+ T cells, suggesting that the correlation between increased frequencies of blood α4β7+ CD4+ T cells and HIV acquisition may be explained, at least in part, by a higher concentration of target cells at the site of HIV exposure(38). While the results presented here are specific to heterosexual transmission of HIV, previous NHP studies support a similar role for α4β7 during other modes of exposure.
This study defines the importance of α4β7 in HIV acquisition and disease progression in a prospective natural history study of high-risk women. One model to explain these data is that preferential infection of α4β7+ CD4+ T cells at the time of HIV exposure leads to more pronounced local infection of these cells, which then migrate rapidly to the gut mucosal and lymphoid tissue, where rapid viral replication contributes to establishment of the latent HIV reservoir. Recent work by our group has suggested that the administration of a primatized analogue of the anti-α4β7 mAb in SIV-infected macaques receiving early ART led to sustained spontaneous control of SIV and repopulation of GI tract with CD4+ T cells in the absence of further treatment(19). Combined with the findings of the current study, these data suggest that α4β7 integrin might be a useful target for HIV prevention and/or treatment in humans. Further evaluation of this concept is clinically feasible, given that a humanized version of the Act1 monoclonal antibody clone (called Vedolizumab) has been proven safe and effective and is FDA-approved for the treatment of adults with moderate to severe ulcerative colitis and Crohn’s disease(39, 40).
Materials and Methods
Study design
We carried out a nested retrospective case-control analysis to correlate the expression of intergrin β7Hi on CD4+ T cells to rates of HIV acquisition. Cases were then followed prospectively to compare disease progression outcomes stratified by β7Hi CD4+ T cells at pre-HIV infection time points. The main outcomes for disease progression were set point and peak HIV viral loads measured post-infection prior to the initiation of ART. Survival analyses were used to compare the time to CD4 decline below 500 cells/μl. In RV254 study different acute HIV stages were compared cross-sectionally at diagnosis, and followed longitudinally following early ART initiation. All experiments were performed in a blinded fashion. The detailed study cohorts, sample collection and processing information is provided in Supplementary Material and Methods. The sample size for each experiment is included in the figures and/or figure legends.
Flow cytometry analysis
PBMC were thawed, washed to remove the cryopreserving fluid and then rested for 3 hours (RPMI 1640 supplemented with 10% fetal bovine serum) at 37°C, 5% CO2 , and stained with a panel of antibodies designed to profile β7 expression on different CD4+ T cell subsets in terms of their memory, activation, and target cell properties. Detailed methods can be found in Supplementary Material and Methods.
Soluble biomarker analysis
Plasma LBP levels were measured using Human LBP DuoSet ELISA, DY870-05 (R&D Systems Inc., Minneapolis, USA). All assays were performed following manufacturer’s instructions. Samples with values below the lower detection limit were assigned the value half the lower limit of quantification, LLOQ/2.
Viral sequencing
The sequence of transmitted/founder envelopes were inferred as the consensus of acute/early sequences obtained from a median of 5 weeks post-HIV infection (IQR: 3 – 7.25, range: 2 – 13 weeks). Viral envelope sequences were generated by Sanger sequencing of amplicons generated by single genome amplication of viral RNA, performed as previously described(45, 46). Additional methods can be found in Supplementary Material and Methods.
Statistical approaches
To compare HIV acquisition risk, we carried out conditional logistic regression with strata defined by matching criteria that was used to select cases and controls. The main explanatory variables in all models included 3 integrin β7-defined subsets, each modeled separately in bivariate and multivariable models adjusting for a number of potential confounding variables. Disease progression rates were evaluated using several readouts, including correlation analyses (Spearman rank correlation) with HIV VL and CD4:CD8 ratio, measured both as peak (highest value in the first 180 days of infection) and set point (average value in measurements made after 180 days infection until ART initiation). Rates of CD4 decline were compared in bivariate and multivariable Cox regression models with the endpoint defined as any two CD4 counts below 500/μl prior to ART initiation. Survival analyses excluded CD4 counts measured during the first 180 days of follow up; these were censored to exclude transient CD4 drops during acute HIV infection, as previously described(48). D’Agostino and Pearson omnibus normality test was utilized for Gaussian distribution of the data. For data that did not follow normal distribution, non-parametric tests including Kruskal-Wallis test and Spearman correlation were performed. All statistics are two-tailed. Linear mixed models were used to compare LBP levels prospectively, with β7Hi above and below the median as the primary predictor, and adjustments made for progression variables including VL and CD4:CD8 ratio.
Supplementary Material
Acknowledgments:
We thank all the study participants and the clinic and laboratory staff that participated in the CAPRISA 004 and 002 studies in Durban South Africa, RV254 study in Thailand and Nairobi and Uganda studies. Special thank you to Lynn Morris and Shelly Krebs for critical review of the manuscript.
Funding: The CAPRISA 004 part of this project was funded by NIH R21 AI115978-01. The original CAPRISA 004 Tenofovir gel trial was funded principally by the United States Agency for International Development (USAID) through FHI360 and CONRAD, with additional support provided by the South African Department of Science and Technology (DST). RV254 is supported by cooperative agreements (W81XWH-07-2-0067, W81XWH-11-2-0174) between The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of the Army and by an intramural grant from the Thai Red Cross AIDS Research Center. The ART in RV254 was supported by The Government Pharmaceutical Organization (GPO), Thailand, Gilead, Merck and ViiV Healthcare. The Majengo cohort in Nairobi has been funded by Gates Grand Challenges and US PEPFAR.
Footnotes
Competing interests: The authors do not declare any conflicts of interest.
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense or other institutions listed.
References:
- 1.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA, Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection, Science 280, 427–431 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Schneider T, Jahn HU, Schmidt W, Riecken EO, Zeitz M, Ullrich R, Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group, Gut 37, 524–529 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M, Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract, J. Exp. Med 200, 761–770 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt PW, Sinclair E, Rodríguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM, Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection, Journal of Infectious Diseases 210, 1228–1238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1, Cell 74, 185–195 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, Mckinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. The integrin forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptable to infection by HIV-1. PNAS 105, 49, 20877–20882 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peachman KK, Karasavvas N, Chenine A-L, McLinden R, Rerks-Ngarm S, Jaranit K, Nitayaphan S, Pitisuttithum P, Tovanabutra S, Zolla-Pazner S, Michael NL, Kim JH, Alving CR, Rao M, Ansari AA, Ed. Identification of New Regions in HIV-1 gp120 Variable 2 and 3 Loops that Bind to α4β7 Integrin Receptor, PLoS ONE 10, e0143895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Jin W, Du T, Wu B, Liu Y, Shattock RJ, Hu Q, Binding of HIV-1 virions to α4β7 expressing cells and impact of antagonizing α4β7 on HIV-1 infection of primary CD4+ T cells, Virol. Sin 29, 381–392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS, HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells, Nat. Immunol 9, 301–309 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Perez LG, Chen H, Liao H-X, Montefiori DC, Envelope glycoprotein binding to the integrin α4β7 is not a general property of most HIV-1 strains, J Virol 88, 10767–10777 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Tasker C, Lespinasse P, Dai J, Fitzgerald-Bocarsly P, Lu W, Heller D, Chang T, D-105 Integrin a4b7 expression increases HIV susceptibility in activated cervical CD4+ T cells via an HIV attachment- independent mechanism, JAIDS Journal of Acquired Immune Deficiency Syndromes 71, 54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joag VR, McKinnon LR, Liu J, Kidane ST, Yudin MH, Nyanga B, Kimwaki S, Besel KE, Obila JO, Huibner S, Oyugi JO, Arthos J, Anzala O, Kimani J, Ostrowski MA, Kaul R, Identification of preferential CD4+ T-cell targets for HIV infection in the cervix, Mucosal Immunology 1,1–12 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, Mattapallil JJ, |[alpha]|4|[plus]||[beta]|7hiCD4|[plus]| memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection, Mucosal Immunol 2, 439–449 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelli E, Veglia F, Goode D, Guerra-Pérez N, Aravantinou M, Arthos J, Piatak M, Lifson JD, Blanchard J, Gettie A, Robbiani M, The frequency of α₄β₇(high) memory CD4⁺ T cells correlates with susceptibility to rectal simian immunodeficiency virus infection, J. Acquir. Immune Defic. Syndr 64, 325–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson SI, Gray ES, Mkhize NN, Sheward DJ, Lambson BE, Wibmer CK, Masson L, Werner L, Garrett N, Passmore J-AS, Karim QA, Karim SSA, Williamson C, Moore PL, Morris L, South African HIV-1 subtype C transmitted variants with a specific V2 motif show higher dependence on α4β7 for replication, Retrovirology 12, 1965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, Arthos J, Kimani J, Kaul R, Characterization of a Human Cervical CD4+ T Cell Subset Coexpressing Multiple Markers of HIV Susceptibility, J Immunol. 187, 6032–42 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, Wang X, Li J, Price AA, Little DM, Zaidi M, Lyles R, Villinger F, Blocking of α4β7 Gut-Homing Integrin during Acute Infection Leads to Decreased Plasma and Gastrointestinal Tissue Viral Loads in Simian Immunodeficiency Virus-Infected Rhesus Macaques, The Journal of Immunology 186, 1044–1059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Targeting [alpha]4[beta]7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection, Nature medicine 12, 1397–400 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, Sidell N, Kane MA, Yu J, Jones JW, Santangelo PJ, Zurla C, McKinnon LR, Arnold KB, Woody CE, Walter L, Roos C, Noll A, Van Ryk D, Jelicic K, Cimbro R, Gumber S, Reid MD, Adsay V, Amancha PK, Mayne AE, Parslow TG, Fauci AS, Ansari AA, Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy, Science 354, 197–202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Xu H, Gill AF, Pahar B, Kempf D, Rasmussen T, Lackner AA, Veazey RS, Monitoring alpha4beta7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal of CD4+ T-cell loss in SIV infection, Mucosal Immunol 6, 518–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masson L, Passmore J-AS, Liebenberg LJ, Werner L, Baxter C, Arnold KB, Williamson C, Little F, Mansoor LE, Naranbhai V, Lauffenburger DA, Ronacher K, Walzl G, Garrett NJ, Williams BL, Couto-Rodriguez M, Hornig M, Lipkin WI, Grobler A, Karim QA, Karim SSA, Genital Inflammation and the Risk of HIV Acquisition in Women, Clin. Infect. Dis 2, 260–9(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NPM, Cameron M, Keele BF, Shen X, Tomaras GD, Billings E, Rao M, Chung AW, Dowell KG, Bailey-Kellogg C, Brown EP, Ackerman ME, Vargas-Inchaustegui DA, Whitney S, Doster MN, Binello N, Pegu P, Montefiori DC, Foulds K, Quinn DS, Donaldson M, Liang F, Loré K, Roederer M, Koup RA, McDermott A, Ma Z-M, Miller CJ, Phan TB, Forthal DN, Blackburn M, Caccuri F, Bissa M, Ferrari G, Kalyanaraman V, Ferrari MG, Thompson D, Robert-Guroff M, Ratto-Kim S, Kim JH, Michael NL, Phogat S, Barnett SW, Tartaglia J, Venzon D, Stablein DM, Alter G, Sekaly R-P, Franchini G, Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition, Nature medicine 22, 762–770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peachman K, Alving C, Rao M, P-D8 HIV-1 Envelope Variable Loop V2 and V3 Peptides Inhibit a4b7 Integrin Receptor Binding to MAdCAM-1, JAIDS Journal of Acquired Immune Deficiency Syndromes 67, 90 (2014). [Google Scholar]
- 24.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei D, Fauci AS, Arthos J, The Genotype of Early-Transmitting HIV gp120s Promotes α 4 β 7 –Reactivity, Revealing α 4 β 7 + /CD4 + T cells As Key Targets in Mucosal Transmission, PLoS Pathog. 7, e1001301 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy J-P, CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients, Journal of the International AIDS Society 18, 20052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adriaanse MPM, Mubarak A, Riedl RG, Ten Kate FJW, Damoiseaux JGMC, Buurman WA, Houwen RHJ, Vreugdenhil ACE, Celiac Disease Study Group, Progress towards non-invasive diagnosis and follow-up of celiac disease in children; a prospective multicentre study to the usefulness of plasma I-FABP, Sci Rep 7, 8671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K, Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans, Gastroenterology 110, 339–343 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Thuijls G, Derikx JPM, van Wijck K, Zimmermann LJI, Degraeuwe PL, Mulder TL, Van der Zee DC, Brouwers HAA, Verhoeven BH, van Heurn LWE, Kramer BW, Buurman WA, Heineman E, Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis, Ann. Surg 251, 1174–1180 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Shive CL, Jiang W, Anthony DD, Lederman MM, Soluble CD14 is a nonspecific marker of monocyte activation, AIDS 29, 1263–1265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ananworanich J, Fletcher JL, Pinyakorn S, van Griensven F, Vandergeeten C, Schuetz A, Pankam T, Trichavaroj R, Akapirat S, Chomchey N, Phanuphak P, Chomont N, Michael NL, Kim JH, de Souza M, A novel acute HIV infection staging system based on 4 th generation immunoassay, Retrovirology 10, 1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicala C, Arthos J, Fauci AS, HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV, J Transl Med 9, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julien J-P, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, Ramos A, Diwanji DC, Pejchal R, Cupo A, Katpally U, Depetris RS, Stanfield RL, McBride R, Marozsan AJ, Paulson JC, Sanders RW, Moore JP, Burton DR, Poignard P, Ward AB, Wilson IA, Broadly Neutralizing Antibody PGT121 Allosterically Modulates CD4 Binding via Recognition of the HIV-1 gp120 V3 Base and Multiple Surrounding Glycans, PLoS Pathog. 9, e1003342 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhiman JN, Anthony C, Doria-Rose NA, Karimanzira O, Schramm CA, Khoza T, Kitchin D, Botha G, Gorman J, Garrett NJ, Karim SSA, Shapiro L, Williamson C, Kwong PD, Mascola JR, Morris L, Moore PL, Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies, Nature medicine 21, 1332–1336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H-X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH, Immune-correlates analysis of an HIV-1 vaccine efficacy trial, N Engl J Med 366, 1275–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, Estes JD, Sandler NG, Sukhumvittaya S, Marovich M, Jongrakthaitae S, Akapirat S, Fletscher JLK, Kroon E, Dewar R, Trichavaroj R, Chomchey N, Douek DC, O Connell RJ, Ngauy V, Robb ML, Phanuphak P, Michael NL, Excler J-L, Kim JH, de Souza MS, Ananworanich J, RV254/SEARCH 010 and RV304/SEARCH 013 Study Groups, Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation, PLoS Pathog. 10, e1004543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan ES, Micci L, Fromentin R, Paganini S, McGary CS, Easley K, Chomont N, Paiardini M, Loss of Function of Intestinal IL-17 and IL-22 Producing Cells Contributes to Inflammation and Viral Persistence in SIV-Infected Rhesus Macaques, PLoS Pathog. 12, e1005412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S, Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue during Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration following Highly Active Antiretroviral Therapy, J Virol 77, 11708–11717 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinnon LR, Kaul R, Quality and quantity: mucosal CD4+ T cells and HIV susceptibility, Current Opinion in HIV and AIDS 7, 195–202 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel J-F, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A, GEMINI 2 Study Group, Vedolizumab as induction and maintenance therapy for Crohn's disease, N Engl J Med 369, 711–721 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel J-F, Sandborn WJ, Van Assche G, Axler J, Kim H-J, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A, GEMINI 1 Study Group, Vedolizumab as induction and maintenance therapy for ulcerative colitis, N Engl J Med 369, 699–710 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Karim QA, Kharsany AB, Frohlich JA, Baxter C, Yende N, Mansoor LE, Mlisana KP, Maarschalk S, Arulappan N, Grobler A, Sibeko S, Omar Z, Gengiah TN, Mlotshwa M, Samsunder N, Karim SSA, Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial, Trials 12, 67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, Abdool Karim Q, Grobler A, Barnabas N, Iriogbe I, Abdool Karim SS, CAPRISA 002 Acute Infection Study Team, Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study, PLoS ONE 3, e1954 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Souza MS, Phanuphak N, Pinyakorn S, Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection, AIDS 7, 793–800 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection, AIDS 13, 1871–9 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, Athreya GS, Treurnicht FK, Keele BF, Wood N, Salazar-Gonzalez JF, Bhattacharya T, Chu H, Hoffman I, Galvin S, Mapanje C, Kazembe P, Thebus R, Fiscus S, Hide W, Cohen MS, Karim SA, Haynes BF, Shaw GM, Hahn BH, Korber BT, Swanstrom R, Williamson C, for the CAPRISA Acute Infection Study Team and the Center for HIV-AIDS Vaccine Immunology Consortium, Quantitating the Multiplicity of Infection with Human Immunodeficiency Virus Type 1 Subtype C Reveals a Non-Poisson Distribution of Transmitted Variants, J Virol 83, 3556–3567 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salazar-Gonzalez JF, Bailes E, Pham KT, Salazar MG, Guffey MB, Keele BF, Derdeyn CA, Farmer P, Hunter E, Allen S, Manigart O, Mulenga J, Anderson JA, Swanstrom R, Haynes BF, Athreya GS, Korber BTM, Sharp PM, Shaw GM, Hahn BH, Deciphering Human Immunodeficiency Virus Type 1 Transmission and Early Envelope Diversification by Single-Genome Amplification and Sequencing, J Virol 82, 3952–3970 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villinger F, Bucur S, Chikkala NF, Brar SS, Bostik P, Mayne AE, Adams J, Lee ME, Novem FJ, Gately MK, Ansari AA, Hillyer CD, In Vitro and in Vivo Responses to Interleukin 12 Are Maintained until the Late SIV Infection Stage but Lost during AIDS, http://www.liebertpub.com.uml.idm.oclc.org/aid 16, 751–763 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Mlisana K, Werner L, Garrett NJ, McKinnon LR, van Loggerenberg F, Passmore JAS, Gray CM, Morris L, Williamson C, Abdool Karim SS, for the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 002 Study Team, Rapid Disease Progression in HIV-1 Subtype C-Infected South African Women, Clin. Infect. Dis 59, 1322–1331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.