Abstract
Background:
The radiation emitted from electromagnetic fields (EMF) can cause biological effects on prokaryotic and eukaryotic cells, including non-thermal effects.
Objective:
The present study evaluated the non-thermal effects of wireless fidelity (Wi-Fi) operating at 2.4 GHz part of non-ionizing EMF on different pathogenic bacterial strains (Escherichia coli 0157H7, Staphylococcus aureus, and Staphylococcus epidermis). Antibiotic resistance, motility, metabolic activity and biofilm formation were examined.
Material and Methods:
In this case-control, a Wi-Fi router was used as a source of microwaves and also bacterial cells were exposed to Wi-Fi radiation continuously for 24 and 48 hours. The antibiotic susceptibility was carried out using a disc diffusion method on Müller Hinton agar plates. Motility of Escherichia coli 0157H7 was conducted on motility agar plates. Cell metabolic activity and biofilm formation were performed using 3-(4, 5-Dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay and crystal violet quantification, respectively.
Results:
The exposure to Wi-Fi radiation altered motility and antibiotic susceptibility of Escherichia coli 0157H7. However, there was no effect Wi-Fi radiation on antibiotic susceptibility of Staphylococcus aureus and Staphylococcus epidermis. On the other hand, the exposed cells, as compared to the unexposed control, showed an increased metabolic activity and biofilm formation ability in Escherichia coli 0157H7, Staphylococcus aureus and Staphylococcus epidermis.
Conclusion:
These results proposed that Wi-Fi exposure acted on bacteria in stressful manner by increasing antibiotic resistance and motility of Escherichia coli 0157H7, as well as enhancing biofilm formation by Escherichia coli 0157H7, Staphylococcus aureus and Staphylococcus epidermis. The findings may have implications for the management of serious diseases caused by these infectious bacteria.
Keywords: Non-thermal Effect , EMF , Wi-Fi , Disc Diffusion , Motility , MTT , Biofilms , Escherichia coli, Staphylococcus
Introduction
Over the past century, the natural environment has been altered by greater the use of telecommunication, such as global system for mobile communication (GSM) and wireless fidelity (Wi-Fi). Wi-Fi waves are a part of the non-ionizing radiation of the electromagnetic spectrum and they use radiofrequency typically operating at 2.4 GHz [1]. Non-ionizing electromagnetic radiation affects biological system through either thermal or non- thermal effects [1,2]. Many scientific studies have investigated biological effects of electromagnetic fields (EMF) [1-3]. Non-thermal EMF induced different responses on bacteria depending on frequency intensity, exposure time and organism model [4].
Several studies investigated the non-thermal effects of high frequency electromagnetic fields (GSM and Wi-Fi) on different strains of bacteria [5-11]. Exposure of Staphylococcus aureus, Staphylococcus epidermis, and Pseudomonas aeruginosa to GSM revealed no effects on their bacterial growth rate or antibiotic susceptibility [7]. However, others reported that exposure to Wi-Fi and GSM altered the inhibition zone diameters and growth rate for of Escherichia coli and Listeria monocytogenes [8]. In addition, Wi-Fi radiation significantly increased the sensitivity of klebsiella pneumoniae to different antibiotics followed by a decrease suggesting an adaptive response [9].
The aim of this study was to analyze the influences of electromagnetic fields (2.4 GHz) emitted by a WI-FI router on the antibiotic resistance, cell metabolic activity and ability to form biofilm by different pathogenic strains (Escherichia coli 0157H7, Staphylococcus aureus, and Staphylococcus epidermis).
Material and Methods
Microwave exposure system
The microwave (MW) source was generated continuously by 54 M wireless router Tp-Link extended range (TL-WR524G) corresponding to the appropriate frequency 2.4 GHz connected to an amplifier and monopole antenna, then mounted in an incubator which was kept at 37°C at 30 cm of distance from bacterial culture. The exposure protocol consisted of a phase of continuous exposure to the MW for 24 or 48 hours. For each experimental condition, a control was performed in Faraday bag at the same temperature to limit any exterior radiation.
Bacterial strains
The bacterial strains used in the current in vitro case-control study were Escherichia coli 0157H7, Staphylococcus aureus and Staphylococcus epidermis. They were provided by the Microbiology Laboratory, Faculty of Science, Beirut Arab University (BAU), Lebanon. The isolates were checked for their purity and identity. The growth of colorless transparent colonies on sorbitol MacConkey agar medium (Oxoid) confirmed the inability of E. coli O157H7 to ferment sorbitol [12]. API Staph was used to confirm the identification of S. aureus and S. epidermis.
Antimicrobial Susceptibility
Müller Hinton agar medium (Oxoid) was used for susceptibility testing based on the criteria of the National Committee for Clinical Laboratory Standards [13]. The isolated E. coli 0157H7 was tested for sensitivity to the antimicrobials (Oxoid), including Meropenem (MEM) 10 μg, Imipenem (IMI) 10 μg, Rifampicin (RD) 30 μg, Levofloxacin (Lev) 5 μg, Oflaxacin (OFX) 5 μg, and Chloramphenicol (C) 10 μg, Azithromycin. (AZM) 15 µg and Gentamicin (CN) 120 μg. S. aureus and S. epidermis strains were tested for sensitivity to antimicrobials, including Gentamicin (CN) 120 μg, Penicillin G (P) 10 μg, Oflaxacin (OFX) 5 μg, and Chloramphenicol (C) 10 μg. For both the control and exposed to 2.4 GHz, standard inocula of 0.5 McFarland was swabbed on Müller Hinton agar then incubated 24 hours at 37°C. The mean diameter of inhibition zones was recorded in mm.
Bacterial motility
Among the studied pathogenic strains, Escherichia coli 0157H7 was the only motile bacterium. Therefore, a single colony of E. coli 0157H7 was inoculated in 5 ml of Luria-Bertani (LB) broth (Sigma-Aldrich) and incubated at 37°C overnight with agitation at 200 rpm. Bacterial overnight culture (1μl) was injected into three spots on freshly prepared motility agar plates containing 0.5% agar (Motility Test Medium Himedia) [14]. The plates were incubated at 37°C for 24 and 48 hours under exposure of 2.4 GHz. The diameters of the bacterial growth rings were measured.
MTT and crystal violet assays
One colony forming unit (CFU) of each strain was transferred into 5 mL of Tryptic Soy Broth (TSB Oxoid) and incubated for 24 hours at 37 °C with shaking. The cultures were diluted to 1 /100 in TSB to obtain 0.1 optical density (OD), at 595 nm for all bacterial inocula. Two hundred microliters of each bacterial inocula were transferred into 96 well plates in duplicate (control and exposed to 2.4 GHz) and incubated for 24 hours at 37 °C; Biofilms were washed three times and subjected to 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay to evaluate the viability of the biofilms and crystal violet based on the quantification of biofilm biomass.
The MTT reduction assay, which determines the metabolic activities of bacteria, was done as previously described with minor modifications [15,16]. Upon washing step, 10 µl of 5 mg/ml MTT (Sigma-Aldrich) was added to 100 µl phosphate buffered saline (PBS) into each well, and the plates were incubated for 1 hour at 37 °C. A 100 µl of solubilizing isopropanol was added to each well, and the plates were vigorously shaken. The amount of MTT formazan formed was measured by the ELx800 Universal Microplate Reader at a wavelength of 570 nm. The pure TSB was used as blank. The results are shown as an index of cell metabolic activity and calculated by the formula: Index of cell metabolic activity = (OD sample − OD background)/ (OD control − OD background) [17].
The quantitative assessment of biofilm formation was determined as previously described with minor modification [18,19]. Biofilms were stained with 200 µL of 0.1% crystal violet. Excess stain was removed, and the wells were air-dried for 15 minutes and then the dye bounded to the adherent cells was solubilized with 200 µL of 33% acetic acid. The OD of each solubilized liquid was measured at wavelength of 595 nm. The pure TSB was used as blank. The results were shown as an index of biofilm formation and calculated by the same formula described above.
Statistical analysis
The data of this study are presented as means ± standard errors of the means calculated from three repetitions of each experiment. The statistical analysis was conducted using Graphpad Prism 8 by mean of paired t test. P values <0.05 was statistically significant.
Results
Effect of 2.4 GHz Wi-Fi exposure on antibiotic susceptibility
The susceptibility of Escherichia coli 0157H7, Staphylococcus aureus and Staphylococcus epidermis to various antibiotics was evaluated in the presence of Wi-Fi radiofrequency radiation. The data obtained for exposed and non-exposed (control) bacteria are represented in Tables 1 and 2.
Table 1.
Inhibition zone diameters (mm) for control and exposed Escherichia coli 0157H7 to 2.4 GHz Wi-Fi radiofrequency radiation.
| Antibiotic | Control | Wi-Fi Exposure | P-value |
|---|---|---|---|
| IPM 10 | 35.33±0.58 | 29.67±0.58 | 0.0002* |
| OFX 5 | 25.33±0.58 | 21.67±0.58 | 0.0014* |
| C 10 | 18.33±0.58 | 12.33±0.58 | 0.0002* |
| AZM 15 | 14.33±0.58 | 11.33±0.58 | 0.0031* |
| RD 30 | 16.67±0.58 | 14.67±0.58 | 0.0132* |
| MEM 10 | 29.33±0.58 | 23.67±0.58 | 0.0002* |
| LEV 5 | 31.67±0.58 | 28.33±0.58 | 0.0021* |
| CN120 | 30.33±0.58 | 28.33±0.58 | 0.0132* |
Each value is the mean ±SD of three repetitions.
statistically significant (p<0.05)
Table 2.
Inhibition zone diameters (mm) for control and exposed S. aureus and S. epidermis to 2.4 GHz Wi-Fi radiofrequency radiation.
| Antibiotic | S. aureus | S. epidermis | ||||
|---|---|---|---|---|---|---|
| Control | Wi-Fi Exposure | P-value | Control | Wi-Fi Exposure | P-value | |
| P 10 | 17.67±0.58 | 16±1 | 0.0667 | 13.67±0.58 | 14.33±0.58 | 0.2302 |
| OFX 5 | 29.67±0.58 | 28.67±0.58 | 0.1011 | 28.33±0.58 | 28.67±0.58 | 0.5185 |
| C 10 | 18.67±0.58 | 17.33±0.58 | 0.2302 | 15.33±0.58 | 15.67±0.58 | 0.5185 |
| CN120 | 22.67±0.58 | 22.33±0.58 | 0.5185 | 23.33±0.58 | 23.67±0.58 | 0.5185 |
The continuous exposure to Wi-Fi radiofrequency radiation decreased significantly the inhibition zone diameters of E. coli 0157H7 (p value<0.05) (Table 1). However, it revealed no significant changes in diameters of inhibition zones of S. aureus and S. epidermis (Table 2).
Effect of 2.4 GHz Wi-Fi exposure on motility of E. coli 0157H7
The effect of Wi-Fi radiofrequency radiation on the motility of E. coli 0157H7 was evaluated by measuring the diameter of bacterial growth rings on motility agar medium after 24 and 48 hours of Wi-Fi exposure. The data show significantly increased motility by 28 % and 29 % for 24 and 48 hours of 2.4 GHZ Wi-Fi exposure, respectively, as compared to unexposed E. coli 0157H7 (Figure 1).
Figure1.
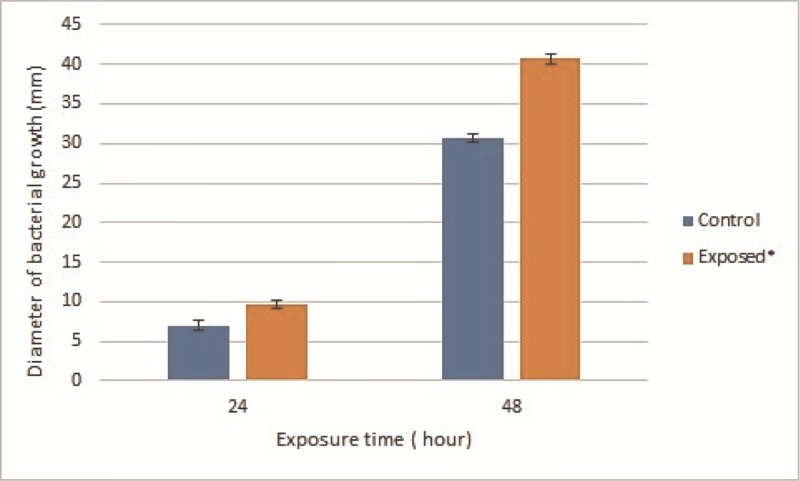
Motility of exposed E. coli 0157H7 to 2.4 GHz Wi-Fi radiofrequency radiation.
Each value is represented as mean ±SD (n=3). *Significance was considered at (p-value<0.05)
Effect of 2.4 GHz Wi-Fi exposure on cell metabolic activity and biofilm formation
In order to explore the effects of Wi-Fi radiofrequency on the bacterial metabolic activity and biofilm formation, the MTT assay and crystal violet quantification were performed. The MTT test is based on the reduction of the yellow MTT dye by dehydrogenase in living cells to purple MTT formazan, which can be solubilized and quantified by spectrophotometric measurements. The crystal violet assay is based on the values of optical density of the crystal violet stain bonded to biofilm. Data obtained from bacterial metabolic activity and biofilm formation index are summarized in Table 3. As consequence of 24 hours exposure to 2.4 GHz Wi-Fi radiofrequency radiation, results showed statistically significant 3 fold increase in cell metabolic activity and 1.9 fold raise in biofilm formation by exposed E. coli, S. aureus and S. epidermis as compared to controls (Table 3).
Table 3.
Effects of 2.4 GHz Wi-Fi radiofrequency radiation on metabolic activity and biofilm formation of E. coli, S. aureus, and S. epidermis.
| control | Exposed | P-value | ||
|---|---|---|---|---|
| E. coli 0157H7 | Cell metabolic activity | 1.018±0.023 | 2.432±0.102 | 0.002* |
| Biofilm formation | 1.009±0.080 | 1.413±0.203 | 0.042* | |
| Staphylococcus aureus | Cell metabolic activity | 1.026±0.081 | 3.323±0.280 | 0.004* |
| Biofilm formation | 1.053±0.068 | 1.918±0.223 | 0.022* | |
| Staphylococcus epidermis | Cell metabolic activity | 1.327±0.304 | 3.024±0.267 | 0.036* |
| Biofilm formation | 1.020±0.027 | 1.506±0.062 | 0.007* | |
Each value is the mean ±SD.
statistically significant (p<0.05)
Discussion
The results of this study showed that 2.4 GHz Wi-Fi radiofrequency radiation induced a rise in the antibiotic resistance of E. coli 0157H7 but revealed no significant changes in that of S. aureus and S. epidermis. This observation coincides with the previous reports shown that exposure to electromagnetic fields (EMF) makes some bacteria resistant to antibiotics [8] and the effects of EMF exposure depend on the morphology of exposed cells [20]. Moreover, continuous 24 hours exposure to mobile 1.8 GHz waves showed the presence of persisters Pseudomonas aeruginosa cells with enhanced antibiotic resistance [11]. In contrast, no significant effects of high frequency electromagnetic fields were revealed on antibiotic sensitivity of exposed Staphylococcus [7]. The different effect seen in the present study between E. coli 0157H7 and S. aureus & S. epidermis may be due to the difference in cell wall composition and how microwaves affect gram-positive and gram-negative bacteria. Several studies reported that radiation induced changes in membrane fatty acids and murein composition of bacteria as well as influenced antibiotic sensitivity [9,21]. Different antibiotics that act through various mechanisms were used in the current study, including inhibition of protein, DNA and cell wall synthesis. The radiation may alter the sensitivity of the efflux pumps or ion channels by permitting the entrance of the molecules through the bacterial cell wall [8,22].
In addition to antibiotic resistance, there was a significant increase in E. coli 0157H7 motility after 2.4 GHz Wi-Fi exposure, which may be a strategy of the pathogen’s survival in the face of radiation stress. These results are in agreement with prior studies that showed promoting E. coli 0157H7 motility under heat and acid stress [23,24]. Flagella are critical virulence factors, that permit bacterial motility and promote adherence to mucins, the major component of the mucus that lines the gastrointestinal tract [25].
The current study examined the effects of exposing E. coli 0157H7, S. aureus and S. epidermis to Wi-Fi radiofrequency radiation 2.4 GHz on their cell metabolic activity and ability to form biofilms where bacterial cells treated with Wi-Fi radiation continuously for 24 hours. Previous reports studied the effects of extremely low frequency EMF exposure and short time Wi-Fi radiofrequency exposure on biofilm formation [10,17]. Based on the current study, the exposure of bacteria to 2.4 GHz Wi-Fi radiofrequency radiation for 24 hours showed the statistically significant increase in the cell metabolic activity and ability to form biofilm of exposed E. coli, S. aureus and S. epidermis as compared to controls. Such finding agreed with previous observations for the influences of rotating magnetic field (RMF) on bacteria [17]. In contrast, short time exposure of Staphylococcus aureus to mobile phone electromagnetic waves did not affect their ability to form biofilm [10].
Conclusion
Based on our results, it can be concluded that Wi-Fi radiofrequency radiation affects bacterial strains in the stressful manner. Increasing antibiotic resistance, motility and biofilm formation which are pathogenic traits of bacteria is a worldwide threat to public health. There are some ambiguities that need further investigations regarding answering questions such as which cellular mechanism is responsible for this stress induced by EMF. Moreover, gene expression experiments are performed to clarify many uncertainties.
Acknowledgement
This work has been supported by grants from the Lebanese University.
Conflict of Interest:None
References
- 1.Ng K-H. Non-ionizing radiations–sources, biological effects, emissions and exposures. Proceedings of the international conference on non-ionizing radiation at UNITEN. 2003:1–16. [Google Scholar]
- 2.Belyaev I. Non-thermal biological effects of microwaves. Microwave Review. 2005;11:13–29. [Google Scholar]
- 3.Ishak N H, Ariffin R, Ali A, Sagiruddin M A, Tawi F M T. Biological effects of WiFi electromagnetic radiation. 25-27 Nov. 2011. Penang: IEEE International Conference on Control System, Computing and Engineering; 2011. [Google Scholar]
- 4.Salmen S H. Non-Thermal Biological Effects of Electromagnetic Field on Bacteria-A Review. Am J Res Commun. 2016;4:16–28. [Google Scholar]
- 5.Cranfield C, Wieser H G, Al Madan J, Dobson J. Preliminary evaluation of nanoscale biogenic magnetite-based ferromagnetic transduction mechanisms for mobile phone bioeffects. IEEE Trans Nanobioscience. 2003;2:40–3. doi: 10.1109/tnb.2003.810155. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Choi J, Gil H, Yang J, Lee E, Jeon Y, et al. Genotoxicity evaluation of electromagnetic fields generated by 835-MHz mobile phone frequency band. Eur J Cancer Prev. 2005;14:175–9. doi: 10.1097/00008469-200504000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Salmen S H, Alharbi S A, Faden A A, Wainwright M. Evaluation of effect of high frequency electromagnetic field on growth and antibiotic sensitivity of bacteria. Saudi J Biol Sci. 2018;25:105–10. doi: 10.1016/j.sjbs.2017.07.006. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taheri M, Mortazavi S M, Moradi M, Mansouri S, Hatam G R, Nouri F. Evaluation of the Effect of Radiofrequency Radiation Emitted From Wi-Fi Router and Mobile Phone Simulator on the Antibacterial Susceptibility of Pathogenic Bacteria Listeria monocytogenes and Escherichia coli. Dose Response. 2017;15:1559325816688527. doi: 10.1177/1559325816688527. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taheri M, Mortazavi S, Moradi M, Mansouri S, Nouri F, Mortazavi S, et al. Klebsiella pneumonia, a microorganism that approves the non-linear responses to antibiotics and window theory after exposure to Wi-Fi 2.4 GHz electromagnetic radiofrequency radiation. J Biomed Phys Eng. 2015;5:115. [PMC free article] [PubMed] [Google Scholar]
- 10.Mohd-Zain Z, Mohd-Ismail M, Buniyamin N. Effects of mobile phone generated high frequency electromagnetic field on the viability and biofilm formation of Staphylococcus aureus. World Acad Sci Eng Technol. 2012;70:221–4. [Google Scholar]
- 11.Nakouti I, Hobbs G, Teethaisong Y, Phipps D. A demonstration of athermal effects of continuous microwave irradiation on the growth and antibiotic sensitivity of Pseudomonas aeruginosa PAO1. Biotechnol Prog. 2017;33:37–44. doi: 10.1002/btpr.2392.. [DOI] [PubMed] [Google Scholar]
- 12.March S B, Ratnam S. Sorbitol-MacConkey medium for detection of Escherichia coli 0157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1986;23:869–72. doi: 10.1128/jcm.23.5.869-872.1986. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cockerill F R, Wikler M A, Alder J, Dudley M, Eliopoulos G, Ferraro M, et al. Performance standards for antimicrobial susceptibility testing: twenty-second informational supplement. Clinical and Laboratory Standards Institute. 2012;32:M100–S22. [Google Scholar]
- 14.Elmer W, Stephen D, William M, Paul C, Washington C. Color atlas and textbook of diagnostic microbiology. Philadelphia: Lippincott; 1992. [Google Scholar]
- 15.Wang H, Cheng H, Wang F, Wei D, Wang X. An improved 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J Microbiol Methods. 2010;82:330–3. doi: 10.1016/j.mimet.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Saising J, Dube L, Ziebandt A K, Voravuthikunchai S P, Nega M, Gotz F. Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2012;56:5804–10. doi: 10.1128/AAC.01296-12. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fijalkowski K, Nawrotek P, Struk M, Kordas M, Rakoczy R. The effects of rotating magnetic field on growth rate, cell metabolic activity and biofilm formation by Staphylococcus aureus and Escherichia coli. Journal of Magnetics. 2013;18:289–96. doi: 10.4283/jmag.2013.18.3.289. [DOI] [Google Scholar]
- 18.Peeters E, Nelis H J, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72:157–65. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Kwasny S M, Opperman T J. Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr Protoc Pharmacol. 2010;50:13A.8.1–23. doi: 10.1002/0471141755.ph13a08s50. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strašák L, Vetterl V, Fojt L. Effects of 50 Hz magnetic fields on the viability of different bacterial strains. Electromagn Biol Med. 2005;24:293–300. doi: 10.1080/15368370500379715. [DOI] [Google Scholar]
- 21.Ayari S, Dussault D, Millette M, Hamdi M, Lacroix M. Changes in membrane fatty acids and murein composition of Bacillus cereus and Salmonella Typhi induced by gamma irradiation treatment. Int J Food Microbiol. 2009;135:1–6. doi: 10.1016/j.ijfoodmicro.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Segatore B, Setacci D, Bennato F, Cardigno R, Amicosante G, Iorio R. Evaluations of the Effects of Extremely Low-Frequency Electromagnetic Fields on Growth and Antibiotic Susceptibility of Escherichia coli and Pseudomonas aeruginosa. Int J Microbiol. 2012;2012:587293. doi: 10.1155/2012/587293. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung H, Bang W, Drake M. Stress response of Escherichia coli. Compr Rev Food Sci Food Saf. 2006;5:52–64. [Google Scholar]
- 24.House B, Kus J V, Prayitno N, Mair R, Que L, Chingcuanco F, et al. Acid-stress-induced changes in enterohaemorrhagic Escherichia coli 0157: H7 virulence. Microbiology. 2009;155:2907–18. doi: 10.1099/mic.0.025171-0. [DOI] [PubMed] [Google Scholar]
- 25.Erdem AL, Avelino F, Xicohtencatl-Cortes J, Giron J A. Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J Bacteriol. 2007;189:7426–35. doi: 10.1128/JB.00464-07. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


