Key Points
Question
What is the association between first flavored use of a given tobacco product and subsequent tobacco use, including progression of tobacco use, among US youth (aged 12-17 years), young adults (aged 18-24 years), and adults (aged ≥25 years)?
Findings
In this cohort study of 11 996 youth and 26 447 adults who participated in waves 1 and 2 of the Population Assessment of Tobacco and Health Study, most youth and young adult new tobacco users first tried a flavored product. First use of flavored tobacco products was positively associated with subsequent product use compared with first use of a nonflavored product.
Meaning
First use of flavored tobacco products may place youth and adults at risk of subsequent tobacco use.
This cohort study uses data from the Population Assessment of Tobacco and Health (PATH) Study to examine whether first use of flavored tobacco products is associated with subsequent tobacco use among US youth and adults.
Abstract
Importance
Flavors in tobacco products may appeal to young and inexperienced users.
Objective
To examine among youth (aged 12-17 years), young adults (aged 18-24 years), and adults (aged ≥25 years) the prevalence of first use of flavored tobacco products among new tobacco users and the association between first flavored use of a given tobacco product and tobacco use 1 year later, including progression of tobacco use.
Design, Setting, and Participants
This cohort study represents a longitudinal analysis of data from the Population Assessment of Tobacco and Health (PATH) Study, a nationally representative study with data collected in 2013 to 2014 (wave 1) and 2014 to 2015 (wave 2). Participants were noninstitutionalized individuals, including 11 996 youth and 26 447 adults, in selected households who participated in both waves of the PATH Study. Data analysis was conducted from July 2016 to June 2019.
Main Outcomes and Measures
Prevalence of tobacco product use at wave 2.
Results
The mean (SE) age of the participants was 14.5 (0.0) years for youth, 21.1 (0.0) years for young adults, and 50.3 (0.0) for adults. Most youth (71.9%; 95% CI, 69.7%-74.0%) and young adults (57.6%; 95% CI, 54.9%-60.3%) who were new users of tobacco products over the 10- to 13-month follow-up period used flavored products. First use of a menthol or mint or other flavored cigarette documented at wave 1 was positively associated with past 12-month and past 30-day cigarette use in all age groups at wave 2 compared with first use of a nonflavored cigarette (youth, flavored cigarette, past 12-month use adjusted prevalence ratio [aPR], 1.14 [95% CI, 1.05-1.25] and past 30-day use aPR, 1.15 [95% CI, 1.00-1.31]; youth, menthol or mint cigarette, past 12-month use aPR, 1.18 [95% CI, 1.08-1.29] and past 30-day use aPR, 1.19 [95% CI, 1.04-1.37]; young adult, flavored cigarette, past 12-month use aPR, 1.09 [95% CI, 1.04-1.15] and past 30-day use aPR, 1.13 [95% CI, 1.06-1.21]; young adult menthol or mint cigarette, past 12-month use aPR, 1.10 [95% CI, 1.05-1.16] and past 30-day use aPR, 1.15 [95% CI, 1.07-1.23]; adult flavored cigarette, past 12-month use aPR, 1.10 [95% CI, 1.05-1.15] and past 30-day use aPR, 1.09 [95% CI, 1.04-1.14]; adult menthol or mint cigarette, past 12-month use aPR, 1.13 [95% CI, 1.08-1.18] and past 30-day use aPR, 1.12 [95% CI, 1.07-1.17]). Among young adults, first use of flavored e-cigarettes (aPR, 2.05; 95% CI, 1.61-2.61), any cigars (aPR, 1.60; 95% CI, 1.26-2.02), cigarillos (aPR, 1.49; 95% CI, 1.08-2.05), filtered cigars (aPR, 3.69; 95% CI, 2.08-6.57), hookah (aPR, 1.91; 95% CI, 1.23-2.98), and any smokeless tobacco (aPR, 1.54; 95% CI, 1.08-2.20) was prospectively associated with current regular use of those products at wave 2 compared with first nonflavored use. Among adults aged 25 years and older, first use of flavored e-cigarettes (aPR, 1.60; 95% CI, 1.41-1.82), any cigars (aPR, 1.56; 95% CI, 1.29-1.87), cigarillos (aPR, 1.29; 95% CI, 1.01-1.64), filtered cigars (aPR, 1.79; 95% CI, 1.25-2.54), hookah (aPR, 5.66; 95% CI, 2.04-15.71), and any smokeless tobacco (aPR, 1.55; 95% CI, 1.32-1.82) was prospectively associated with current regular use of those products at wave 2 compared with first nonflavored use.
Conclusions and Relevance
In this longitudinal cohort study, flavors in tobacco products were associated with youth and young adult tobacco experimentation. First use of a flavored tobacco product may place youth, young adults, and adults at risk of subsequent tobacco use.
Introduction
Children prefer sweet flavors more than adults do,1 and tobacco industry documents2,3,4,5 confirm that flavors in tobacco products can increase their appeal to young and inexperienced tobacco users. Consistent with studies6,7,8 on menthol cigarettes and flavored cigars, data from the first wave of the Population Assessment of Tobacco and Health (PATH) Study9,10,11 revealed a strong inverse age gradient in the prevalence of flavored tobacco product use, with the highest use among youth aged 12 to 17 years, followed by young adults aged 18 to 24 years, and the lowest use among adults aged 25 years and older. These data9,10 also show a strong association between first use of a flavored tobacco product and current tobacco use among youth and adults.
Few longitudinal studies to date have examined the association between flavored tobacco product use and initiation or continuation of tobacco use, and these studies12,13,14 have largely been limited to menthol cigarettes. These studies highlight that menthol brand recognition is associated with smoking experimentation among youth,12 that adolescents who initiate smoking with menthol cigarettes are more likely to progress to established smoking by the end of 3 years than those who initiated with nonmenthol cigarettes,13 and that prior initiation with a menthol cigarette compared with a nonmenthol cigarette is associated with current cigarette smoking at follow-up among young adults.14 Five other cross-sectional studies10,15,16,17,18 support these findings.
The current study extends prior research by leveraging longitudinal data from waves 1 and 2 of the PATH Study to assess whether there is a prospective association between first flavored use of a given tobacco product and subsequent use of that specific product (eg, e-cigarettes). In addition, this study examines whether first use of a flavored tobacco product at wave 1 is associated with progression to greater frequency of tobacco use at wave 2. The primary aims of this study are to report the proportions of new tobacco users at wave 2 whose first use of a given tobacco product was flavored (ie, first flavored use) and to assess the association between first flavored use of a given tobacco product at wave 1 and subsequent tobacco use, including frequency of tobacco use, at wave 2 for youth (aged 12-17 years), young adults (aged 18-24 years), and adults (aged ≥25 years).
Methods
The National Institutes of Health, through the National Institute on Drug Abuse, is partnering with the US Food and Drug Administration’s Center for Tobacco Products to conduct the PATH Study under a contract with Westat. The PATH Study is an ongoing, nationally representative, longitudinal cohort study of adults and youth in the United States. The PATH Study uses audio computer-assisted self-interviews available in English and Spanish to collect self-reported information on tobacco-use patterns and associated health behaviors. Wave 1 data collection was conducted from September 12, 2013, to December 14, 2014; wave 2 data were collected from October 23, 2014, to October 30, 2015. The PATH Study recruitment used a stratified, address-based, area-probability sampling design at wave 1 that oversampled adult tobacco users, young adults (aged 18-24 years), and African American adults. An in-person screener was used at wave 1 to select youth and adults from households for participation.
The PATH study was conducted by Westat and approved by the Westat institutional review board. All participants aged 18 years and older provided written informed consent, with youth participants aged 12 to 17 years providing assent while their parent or legal guardian provided written informed consent. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.19
Population and replicate weights using the balanced repeated replication method with the Fay adjustment (ρ = 0.3) were created that adjusted for the complex study design characteristics (eg, oversampling at wave 1) and nonresponse at waves 1 and 2. Combined with the use of a probability sample, the weights allow analyses of the PATH Study data to compute estimates that are robust and representative of the noninstitutionalized, civilian US population aged 12 years and older. The longitudinal sampling weights provided for wave 2 are adjusted for wave 2 nonresponse to ensure that the wave 1 sample is representative of the population in the longitudinal estimates. Further details regarding the PATH Study design and methods have been published elsewhere.20 Details on survey interview procedures, questionnaires, sampling, weighting, and information on accessing the data are available online.21
At wave 1, the weighted response rate for the household screener was 54.0%. Among households that were screened, the overall weighted response rate at wave 1 was 74.0% for the adult interview and 78.4% for the youth interview. At wave 2, the overall weighted response rate was 83.2% for the adult interview and 87.3% for the youth interview.
At wave 1, interviews were completed with 32 320 adults (aged ≥18 years) and 13 651 youth (aged 12-17 years). At wave 2, interviews were completed with 28 362 adults and 12 172 youth. The differences in number of completed interviews between wave 1 and wave 2 reflect attrition due to nonresponse, mortality, and other factors. The sample at wave 2 also includes 1915 youth aged 17 years at wave 1 who responded to the youth questionnaire in wave 1 and then turned 18 and responded to the adult questionnaire in wave 2.21 Between waves 1 and 2, retention rates were 88.4% for continuing youth, 83.1% for continuing adults, and 85.7% for aged-up adults (18-year-olds at wave 2). Analyses in this study focus on respondents who provided data for both study waves (11 996 youth and 26 447 adults); unless otherwise stated, any references to age are based on age at wave 1.
Measures
Tobacco Product Use
Ever and current tobacco use was assessed at waves 1 and 2 among youth, young adults, and adults for cigarettes, e-cigarettes, traditional cigars, cigarillos, filtered cigars, hookah tobacco, pipe tobacco, smokeless tobacco (eg, moist snuff or chew), snus pouches, and dissolvable tobacco. Any cigar use was defined as using traditional cigars, cigarillos, or filtered cigars. Any smokeless tobacco use was defined as using smokeless tobacco or snus pouches. Youth, young adults, and adults who tried a tobacco product for the first time between waves 1 and 2 were defined as new users, with age at tobacco trial defined as their age at wave 1. Current use was defined in multiple ways as outlined in previous analyses22 and in the eTable in the Supplement. Participants missing data on moderate, frequent, or daily use of a product because of an instrument skip pattern were coded as not having the outcome and were included in the denominator.
Wave 1 First Flavored Tobacco Product Use
At wave 1, ever cigarette users were asked whether, when they first used a cigarette (youth) or when they first started smoking cigarettes (adults), it was “flavored to taste like menthol or mint.” Ever cigarette users who replied “no” to the menthol question were then asked whether their first cigarette was flavored to taste like “clove, spice, candy, fruit, chocolate, alcohol (such as wine or cognac), or other sweets.” Two comparisons were made for cigarettes: first use of any flavored cigarette (including menthol/mint, as indicated in the survey) vs first use of a nonflavored cigarette, and first use of a menthol or mint flavored cigarette vs first use of a nonflavored cigarette with individuals who reported other first flavored cigarette use excluded from the denominator. Ever users of other tobacco products were queried about whether, when they first used the product (youth) or when they first started using the product (adults), it was “flavored to taste like menthol, mint, clove, spice, candy, fruit, chocolate, alcohol (such as wine or cognac), or other sweets” (eTable in the Supplement).
Wave 1 Covariates
All covariates were assessed at wave 1 and were selected on the basis of previous work10 using this data set (eTable in the Supplement). Sociodemographic variables included self-reported age (adult only), sex, race/ethnicity, educational attainment, and annual household income (adults only). Past 30-day use of alcohol, marijuana, and other drugs (eg, painkillers, sedatives, tranquilizers, or stimulants) was assessed; never users were categorized as reporting no past 30-day use. Respondents also completed the Global Appraisal of Individual Needs–Short Screener,23 which measures severity of symptoms on 3 subscales (internalizing problems, externalizing problems, and substance use problems) in the past year (ie, 0-1 symptoms [no or low], 2-3 symptoms [moderate], and 4 or ≥4 symptoms [high], depending on the scale).
Statistical Analysis
Data analysis was conducted from July 2016 to June 2019. Analyses were conducted using svy procedures in Stata/SE statistical software version 14.2 (StataCorp) to account for the complex study design. Analysis of new users focused on the prevalence of using a flavored product at first tobacco use at wave 2 (Figure). For all other analyses, the main outcome was current product-specific use at wave 2 as defined in the eTable in the Supplement. The prevalence of each outcome was estimated for youth, young adults, and adults aged 25 years and older according to age at wave 1. Estimates with denominators less than 50 or relative SE greater than 30% were suppressed.24 Missing data on age, sex, race or Hispanic ethnicity, and adult education were imputed at wave 1 as described elsewhere.21 Participants missing any response to a composite variable (eg, any past 30-day tobacco use) were treated as missing; missing data were handled with listwise deletion. Multivariable models were built separately for youth, young adults, and adults aged 25 years or older; all models included sex, race/ethnicity, education, past 30-day alcohol, marijuana, or other drug use, and the 3 Global Appraisal of Individual Needs–Short Screener subscales as covariates. Adult models also included age and income. Modified Poisson regression models25 estimated the association between first flavored tobacco use among ever users at wave 1 and current tobacco use at wave 2, as well as moderate, frequent, and daily use at wave 2. For the 3 products for which there were sufficient sample sizes in each of the age groups (flavored cigarettes, menthol cigarettes, and flavored e-cigarettes), we conducted multivariable multinomial logistic regression models of increasing frequency of tobacco use compared with no use from the mutually exclusive categories of tobacco use frequency. For all analyses, α was set at P < .05 using 2-sided tests. Stata’s svy commands used a logit transformation to produce confidence intervals with limits between 0 and 1.26
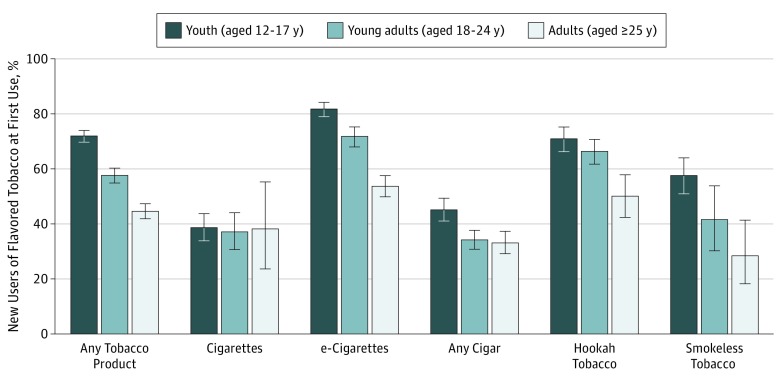
Figure. Weighted Proportions of New Tobacco Users at Wave 2 Who Reported Using a Flavored Product at First Use.
Percentages are weighted to represent the US population, and 95% CIs (whiskers) are estimated using the balanced repeated replication method. New use is ascribed to the participants’ age at wave 1. Respondents were categorized into age groups (youth aged 12-17 years, young adults aged 18-24 years, and adults aged ≥25 years) according to their ages at wave 1. New use of a tobacco product is defined as starting to use a product between waves 1 and 2. This can include never users at wave 1 who start tobacco use at wave 2 and ever users at wave 1 who report use of a new product or products at wave 2. Individuals who reported “don’t know” or refused to answer any part of the definition of ever use or first flavored use were excluded from the denominator. Unweighted numbers and unweighted percentages are presented for each age group: Among 11 996 youth, 2136 (17.8%) reported new use of a tobacco product, 9622 (80.2%) reported no new initiation, and 238 (2.0%) did not provide information on initiation between wave 1 and wave 2. Among 7325 young adults, 2058 (24.9%) reported new use of a tobacco product, 5232 (74.7%) reported no new initiation, and 35 (0.4%) did not provide information on initiation between wave 1 and wave 2. Among 19 116 adults aged 25 years and older, 2580 (8.1%) reported new use of a tobacco product, 16 407 (91.4%) reported no new initiation, and 129 (0.5%) did not provide information on initiation between wave 1 and wave 2. First flavored use is defined as reporting that the first product used was “flavored to taste like menthol, mint, clove, spice, candy, fruit, chocolate, alcohol (such as wine or cognac), or other sweets.” Individuals who did not report “yes,” “no,” or “I don’t know” or refused to answer whether their first product was flavored were excluded from the denominator. Flavored pipe tobacco and dissolvable tobacco use was not assessed among youth. Unweighted numbers and unweighted percentages are presented for each age group. For 2136 youth new tobacco users, 95 (4.5%) did not report whether they had used any flavored product between wave 1 and wave 2. For 2058 young adult new tobacco users, 58 (2.8%) did not report whether they had used any flavored product between wave 1 and wave 2. For 2580 adult (aged ≥25 years) new tobacco users, 58 (2.3%) did not report whether they had used any flavored product between wave 1 and wave 2. Any tobacco product included cigarettes, e-cigarettes, traditional cigars, cigarillos, filtered cigars, hookah, pipe (for adults only), smokeless tobacco, and snus or dissolvable tobacco (for adults only); any cigar use reflects use of a traditional cigar, cigarillo, or filtered cigar. Data are from the Population Assessment of Tobacco and Health (PATH) Study,9,10,11 waves 1 and 2.
Results
The mean (SE) age of the participants at wave 2 was 14.5 (0.0) years for youth, 21.1 (0.0) years for young adults, and 50.3 (0.0) for adults. For adults who completed interviews at both waves, the comparable weighted age distributions were 13.0% aged 18 to 24 years, 8.5% aged 25 to 29 years, 9.1% aged 30 to 34 years, 16.7% aged 35 to 44 years, 34.6% aged 45 to 64 years, and 18.0% aged 65 years and older at wave 1. Detailed characteristics of the adult sample are presented elsewhere.27
Prevalence of First Use Being a Flavored Tobacco Product, Among New Users at Wave 2
Between waves 1 and 2, 12.1% of youth (aged 12-17 years), 27.6% of young adults (aged 18-24 years), and 8.3% of adults (aged ≥25 years) became new users of a tobacco product (ie, were never users of a given product at wave 1; weighted percentages). Of these, first use of any product being flavored was inversely associated with age, with 71.9% (95% CI, 69.7%-74.0%) of youth, 57.6% (95% CI, 54.9%-60.3%) of young adults, and 44.6% (95% CI, 41.8%-47.4%) of adults aged 25 years and older whose first use of any tobacco product was flavored (Figure). First use of e-cigarettes and hookah being flavored was more prevalent among youth (e-cigarettes, 81.7% [95% CI, 78.9%-84.2%]; hookah, 70.9% [95% CI, 66.2%-75.2%]) and young adults (e-cigarettes, 71.7% [95% CI, 68.0%-75.2%]; hookah, 66.4% [95% CI, 61.8%-70.7%]) compared with adults aged 25 years and older (e-cigarettes, 53.7% [95% CI, 49.9%-57.5%]; hookah, 50.1% [95% CI, 42.3%-57.8%]); first use of any cigar and smokeless tobacco being flavored was more prevalent among youth (any cigar, 45.2% [95% CI, 41.1%-49.3%]; smokeless tobacco, 57.6% [95% CI, 50.9%-64.0%]) compared with adults aged 25 years and older (any cigar, 33.0% [95% CI, 29.1%-37.2%]; smokeless tobacco, 28.4% [95% CI, 18.2%-41.3%]).
Association Between First Use of a Given Tobacco Product Being Flavored at Wave 1 and Current Tobacco Use at Wave 2
Among ever tobacco users at wave 1, first use of a flavored cigarette was positively associated with past 12-month and past 30-day cigarette use, either flavored or unflavored, at wave 2 in all 3 age groups (Tables 1, 2, and 3) compared with first use of nonflavored cigarette, after adjusting for covariates (youth, past 12-month use adjusted prevalence ratio [aPR], 1.14 [95% CI, 1.05-1.25] and past 30-day use aPR, 1.15 [95% CI, 1.00-1.31]; young adult, past 12-month use aPR, 1.09 [95% CI, 1.04-1.15] and past 30-day use aPR, 1.13 [95% CI, 1.06-1.21]; adult, past 12-month use aPR, 1.10 [95% CI, 1.05-1.15] and past 30-day use aPR, 1.09 [95% CI, 1.04-1.14]). First use of a menthol or mint flavored cigarette among ever cigarette users at wave 1 was also positively associated with past 12-month and past 30-day cigarette use among youth (past 12-month use aPR, 1.18 [95% CI, 1.08-1.29] and past 30-day use aPR, 1.19 [95% CI, 1.04-1.37]), young adults (past 12-month use aPR, 1.10 [95% CI, 1.05-1.16] and past 30-day use aPR, 1.15 [95% CI, 1.07-1.23]), and adults aged 25 years and older (past 12-month use aPR, 1.13 [95% CI, 1.08-1.18] and past 30-day use aPR, 1.12 [95% CI, 1.07-1.17]) at wave 2 compared with first use of a nonflavored cigarette. First use of any flavored smokeless product was also prospectively associated with past 30-day any smokeless product use among youth aged 12 to 17 years (aPR, 1.76; 95% CI, 1.21-2.57) (Table 1). Low sample sizes limited estimation of the association between first use of a flavored tobacco product and moderate, frequent, daily, and current regular use of that product among youth (Table 1).
Table 1. Association Between First Tobacco Product Flavored Among Youth Ever Tobacco Users at Wave 1 and Product-Specific Tobacco Use at Wave 2 of the Population Assessment of Tobacco and Health Study.
| Ever Tobacco Use at Wave 1 | Past 12-mo Use at Wave 2a | Past 30-d Use at Wave 2b | ≥6 d Use at Wave 2c | ≥20 d Use at Wave 2c | Daily Product Use at Wave 2c | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No. (%) | aPR (95% CI)d | Participants, No. (%) | aPR (95% CI)d | Participants, No. (%) | aPR (95% CI)d | Participants, No. (%) | aPR (95% CI)d | Participants, No. (%) | aPR (95% CI)d | |
| Ever cigarette use | ||||||||||
| First product nonflavored | 453 (60) | 1 [Reference] | 293 (39) | 1 [Reference] | 178 (23.9) | 1 [Reference] | 130 (17.2) | 1 [Reference] | 99 (12.8) | 1 [Reference] |
| First product flavored | 531 (68.3) | 1.14 (1.05-1.25) | 342 (44.94) | 1.15 (1.00-1.31) | 193 (24.9) | 1.05 (0.85-1.29) | 142 (17.8) | 1.11 (0.85-1.47) | 107 (13.4) | 1.21 (0.92-1.59) |
| First product menthol or mint flavorede | 470 (69.9) | 1.18 (1.08-1.29) | 309 (46.8) | 1.19 (1.04-1.37) | 174 (25.9) | 1.09 (0.88-1.34) | 129 (18.6) | 1.17 (0.88-1.56) | 99 (14.2) | 1.30 (0.97-1.72) |
| Ever e-cigarette use | ||||||||||
| First product nonflavored | 122 (52.5) | 1 [Reference] | 58 (23.3) | 1 [Reference] | 22 (9) | 1 [Reference] | NAf | 1 [Reference] | NAf | 1 [Reference] |
| First product flavored | 569 (58.9) | 1.12 (0.96-1.29) | 266 (28.4) | 1.19 (0.91-1.55) | 105 (11.8) | 1.39 (0.82-2.36) | 55 (6.2) | 1.67 (0.77-3.63) | 42 (4.5) | 2.29 (0.81-6.50) |
| Ever any cigar use | ||||||||||
| First product nonflavored | 182 (64.9) | 1 [Reference] | 97 (36.3) | 1 [Reference] | NAf | 1 [Reference] | NAf | 1 [Reference] | NAf | NAf |
| First product flavored | 398 (69.8) | 1.08 (0.96-1.21) | 182 (33.7) | 0.94 (0.72-1.21) | 35 (6.5) | NAg | 18 (3.4) | NAg | NAf | NAf |
| Ever use of traditional cigar | ||||||||||
| First product nonflavored | 85 (65) | 1 [Reference] | 41 (31.3) | 1 [Reference] | NAf | NAf | NAf | NAf | NAf | NAf |
| First product flavored | 88 (69.4) | 1.16 (0.98-1.37) | 34 (27.6) | 0.92 (0.57-1.49) | NAf | NAf | NAf | NAf | NAf | NAf |
| Ever use of cigarillos | ||||||||||
| First product nonflavored | 160 (64.3) | 1 [Reference] | 83 (34.8) | 1 [Reference] | NAf | 1 [Reference] | NAf | NAf | NAf | NAf |
| First product flavored | 307 (63.4) | 1.00 (0.87-1.15) | 128 (27.7) | 0.81 (0.61-1.07) | 17 (3.7) | NAg | NAf | NAf | NAf | NAf |
| Ever use of filtered cigars | ||||||||||
| First product nonflavored | 51 (57.3) | 1 [Reference] | 13 (14.4) | 1 [Reference] | NAf | NAf | NAf | NAf | NAf | NAf |
| First product flavored | 118 (65) | 1.10 (0.84-1.44) | 29 (14.8) | 0.95 (0.39-2.34) | NAf | NAf | NAf | NAf | NAf | NAf |
| Ever use of pipe tobacco | ||||||||||
| First product nonflavored | 53 (35.9) | 1 [Reference] | 17 (11.9) | NAf | NAf | NAf | NAf | NAf | NAf | NAf |
| First product flavored | 21 (31.2) | 0.77 (0.41-1.46) | NAf | NAf | NAf | NAf | NAf | NAf | NAf | NAf |
| Ever use of hookah tobacco | ||||||||||
| First product nonflavored | 56 (56) | 1 [Reference] | 23 (24.8) | 1 [Reference] | NAf | 1 [Reference] | NAf | 1 [Reference] | NAf | NAf |
| First product flavored | 480 (64) | 1.09 (0.87-1.37) | 183 (24.5) | 0.91 (0.55-1.51) | 38 (4.8) | 0.62 (0.23-1.67) | 19 (2.5) | 0.62 (0.16-2.46) | NAf | NAf |
| Ever use of any smokeless tobaccoh | ||||||||||
| First product nonflavored | 51 (41.6) | 1 [Reference] | 24 (19.5) | 1 [Reference] | 13 (11) | 1 [Reference] | NAf | 1 [Reference] | NAf | 1 [Reference] |
| First product flavored | 216 (54.5) | 1.29 (0.99-1.68) | 132 (33.9) | 1.76 (1.21-2.57) | 77 (20.2) | 1.77 (0.92-3.40) | 65 (17.1) | 1.94 (0.92-4.12) | 49 (12.5) | NAg |
| Ever use of smokeless tobacco (excluding snus) | ||||||||||
| First product nonflavored | 65 (48.1) | 1 [Reference] | 34 (25.1) | 1 [Reference] | 23 (17.7) | 1 [Reference] | 21 (16.3) | 1 [Reference] | 18 (14) | 1 [Reference] |
| First product flavored | 169 (51.3) | 1.12 (0.88-1.41) | 106 (32.6) | 1.32 (0.98-1.79) | 63 (19.7) | 1.05 (0.67-1.65) | 54 (16.6) | 0.98 (0.61-1.59) | 40 (12.2) | NAg |
| Ever use of snus | ||||||||||
| First product nonflavored | NAf | 1 [Reference] | NAf | 1 [Reference] | NAf | NAf | NAf | NAf | NAf | NAf |
| First product flavored | 80 (50.1) | 1.37 (0.67-2.80) | 32 (19.7) | 1.22 (0.26-5.60) | NAf | NAf | NAf | NAf | NAf | NAf |
| Ever use of dissolvable tobacco | ||||||||||
| First product nonflavored | NAf | NAf | NAf | NAf | NAf | NAf | NAf | NAf | NAf | NAf |
| First product flavored | NAf | NAf | NAf | NAf | NAf | NAf | NAf | NAf | NAf | NAf |
Abbreviations: aPR, adjusted prevalence ratio; NA, not applicable.
Past 12-month use was defined as smoked or used product (even 1 or 2 times) in the past 12 months. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for past 12-month use were as follows: cigarettes, 0 (0.0%); e-cigarettes, 26 (2.1%); any cigar, 2 (0.2%); traditional cigars, 1 (0.4%); cigarillos, 2 (0.3%); filtered cigars, 1 (0.4%); pipe, 2 (0.9%); hookah, 1 (0.1%); any smokeless product, 6 (1.1%); smokeless product, 4 (0.8%); and snus, 2 (1.0%).
Past 30-day use was defined as smoked or used product (even 1 or 2 times) in the past 30 days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for past 30-day use were as follows: cigarettes, 3 (0.2%); e-cigarettes, 27 (2.2%); any cigar, 34 (3.9%); traditional cigars, 2 (0.8%); cigarillos, 31 (4.2%); filtered cigars, 1 (0.4%); pipe, 2 (0.9%); hookah, 4 (0.5%); any smokeless product, 8 (1.5%); smokeless product, 5 (1.0%); and snus, 1 (0.5%).
Moderate use was defined as having smoked or used the product on at least 6 of the past 30 days. Frequent product use was defined as having smoked or used the product on at least 20 of the past 30 days. Daily use among youth was defined as having smoked or used the product on 30 of the past 30 days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for moderate, frequent, and daily use were as follows: cigarettes moderate or frequent use, 25 (1.6%); cigarettes daily use, 12 (0.8%); e-cigarettes moderate or frequent use, 60 (4.9%); e-cigarettes daily use, 34 (2.8%); any cigar product moderate use, 54 (6.3%); any cigar product frequent use, 57 (6.7%); cigarillos moderate use, 29 (3.9%); hookah moderate or frequent use, 11 (1.3%); any smokeless tobacco product moderate use, 18 (3.4%); any smokeless tobacco product frequent use, 19 (3.5%); any smokeless tobacco product daily use, 10 (1.9%); smokeless tobacco moderate or frequent use, 11 (2.3%); and smokeless tobacco daily use, 7 (1.5%).
Multivariable modified Poisson regression models among youth were adjusted for sex, race/ethnicity, education, past 30-day alcohol, marijuana, or other drug use, and 3 Global Appraisal of Individual Needs–Short Screener subscales (internalizing problems, externalizing problems, and substance use problems). Individuals who reported “don’t know” or refused to answer any of these items were treated as missing. Among youth ever tobacco users, data were missing for sex (0 participants [0.0%]), race/ethnicity (0 participants [0.0%]), education (128 participants [5.2%]), past 30-day alcohol, marijuana, or other drug use (64 participants [2.6%]), internalizing problems subscale (52 participants [2.1%]), externalizing problems subscale (93 participants [3.8%]), and substance use problems subscale (91 participants [3.7%]). Respondents who reported never alcohol, marijuana, or other drug use were categorized as non–past 30-day users.
A separate comparison was made between wave 1 cigarette smokers who first smoked a menthol or mint flavored cigarette vs smokers who first smoked a nonflavored cigarette. Individuals who reported first other flavored cigarette use were excluded from the denominator.
The estimate was suppressed because it has low statistical precision. It is based on a sample size of less than 50, or the coefficient of variation of the estimate is larger than 30%.
There were insufficient observations to compute balanced repeated replication SEs.
Any smokeless tobacco use was defined as smokeless and/or snus use.
Table 2. Association Between First Tobacco Product Flavored Among Young Adult Ever Tobacco Users at Wave 1 and Product-Specific Tobacco Use at Wave 2 of the Population Assessment of Tobacco and Health Study.
| Ever Tobacco Use at Wave 1 | Past 12-mo Use at Wave 2a | Past 30-d Use at Wave 2b | ≥6 d Use at Wave 2c | ≥20 d Use at Wave 2c | Daily Use at Wave 2d | Current Regular Use at Wave 2d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | |
| Ever cigarette use | ||||||||||||
| First product nonflavored | 1577 (63.2) | 1 [Reference] | 1248 (48.7) | 1 [Reference] | 879 (33.7) | 1 [Reference] | 719 (26.8) | 1 [Reference] | 631 (22.9) | 1 [Reference] | 929 (35.2) | 1 [Reference] |
| First product flavored | 1772 (69.3) | 1.09 (1.04-1.15) | 1458 (55.7) | 1.13 (1.06-1.21) | 1060 (40.1) | 1.17 (1.07-1.29) | 884 (33.1) | 1.22 (1.10-1.36) | 773 (28.6) | 1.25 (1.11-1.41) | 1077 (40.8) | 1.17 (1.07-1.27) |
| First product menthol or mint flavoredf | 1635 (69.8) | 1.10 (1.05-1.16) | 1357 (56.5) | 1.15 (1.07-1.23) | 1005 (41.6) | 1.21 (1.10-1.32) | 841 (34.5) | 1.26 (1.13-1.41) | 742 (30) | 1.29 (1.15-1.46) | 1016 (41.7) | 1.18 (1.09-1.29) |
| Ever e-cigarette use | ||||||||||||
| First product nonflavored | 697 (61.3) | 1 [Reference] | 267 (24.1) | 1 [Reference] | 94 (8.6) | 1 [Reference] | 62 (5.9) | 1 [Reference] | 40 (3.7) | 1 [Reference] | 111 (9) | 1 [Reference] |
| First product flavored | 1324 (71.4) | 1.17 (1.10-1.25) | 571 (30.7) | 1.31 (1.10-1.55) | 238 (13.3) | 1.67 (1.27-2.20) | 163 (9.2) | 1.67 (1.21-2.31) | 141 (7.8) | 2.30 (1.61-3.28) | 330 (17.3) | 2.05 (1.61-2.61) |
| Ever any cigar use | ||||||||||||
| First product nonflavored | 703 (45) | 1 [Reference] | 445 (27.2) | 1 [Reference] | 82 (4.9) | 1 [Reference] | 46 (2.6) | 1 [Reference] | 33 (1.8) | 1 [Reference] | 128 (7.9) | 1 [Reference] |
| First product flavored | 1445 (52.8) | 1.19 (1.10-1.29) | 870 (30) | 1.13 (1.01-1.27) | 154 (5.4) | 1.17 (0.87-1.57) | 71 (2.3) | 0.84 (0.56-1.25) | 50 (1.5) | 0.88 (0.52-1.50) | 314 (11.4) | 1.60 (1.26-2.02) |
| Ever use of traditional cigars | ||||||||||||
| First product nonflavored | 440 (45.5) | 1 [Reference] | 195 (19.9) | 1 [Reference] | NAg | 1 [Reference] | NAg | NAg | NAg | NAg | 57 (5.9) | 1 [Reference] |
| First product flavored | 319 (48.8) | 1.15 (1.00-1.32) | 126 (18) | 0.96 (0.72-1.28) | 17 (2) | NAh | NAg | NAg | NAg | NAg | 56 (7.8) | 1.39 (0.92-2.10) |
| Ever use of cigarillos | ||||||||||||
| First product nonflavored | 553 (36.5) | 1 [Reference] | 354 (22.6) | 1 [Reference] | 54 (3.5) | 1 [Reference] | 25 (1.6) | 1 [Reference] | 18 (1.1) | 1 [Reference] | 87 (6.1) | 1 [Reference] |
| First product flavored | 1067 (43.3) | 1.22 (1.11-1.35) | 631 (24.2) | 1.13 (0.98-1.31) | 89 (3.4) | 1.02 (0.69-1.51) | 33 (1.1) | NAh | 24 (0.7) | NAh | 201 (7.9) | 1.49 (1.08-2.05) |
| Ever use of filtered cigars | ||||||||||||
| First product nonflavored | 146 (22.4) | 1 [Reference] | 70 (10.1) | 1 [Reference] | NAg | 1 [Reference] | NAg | NAg | NAg | NAg | 18 (2.3) | 1 [Reference] |
| First product flavored | 314 (32.1) | 1.50 (1.25-1.81) | 138 (12.8) | 1.32 (0.97-1.78) | 27 (2.5) | NAh | NAg | NAg | NAg | NAg | 68 (6.8) | 3.69 (2.08-6.57) |
| Ever use of pipe tobacco | ||||||||||||
| First product nonflavored | 168 (18.7) | 1 [Reference] | 72 (7.8) | 1 [Reference] | NAg | NAg | NAg | NAg | NAg | NAg | 32 (3.9) | 1 [Reference] |
| First product flavored | 72 (21.4) | 1.15 (0.86-1.55) | 34 (9.9) | 1.25 (0.76-2.06) | NAg | NAg | NAg | NAg | NAg | NAg | 25 (8.2) | 2.28 (1.23-4.24) |
| Ever use of hookah tobacco | ||||||||||||
| First product nonflavored | 186 (39.1) | 1 [Reference] | 89 (18.8) | 1 [Reference] | NAg | 1 [Reference] | NAg | 1 [Reference] | NAg | 1 [Reference] | 30 (6.4) | 1 [Reference] |
| First product flavored | 1868 (51.5) | 1.33 (1.14-1.54) | 783 (21.3) | 1.26 (0.99-1.61) | 116 (3) | 2.36 (1.00-5.54) | 43 (1) | NAh | 27 (0.6) | NAh | 451 (12.6) | 1.91 (1.23-2.98) |
| Ever use of any smokeless tobaccoi | ||||||||||||
| First product nonflavored | 122 (31.1) | 1 [Reference] | 91 (23.4) | 1 [Reference] | 49 (12.8) | 1 [Reference] | 40 (10.9) | 1 [Reference] | 37 (9.9) | 1 [Reference] | 62 (15.6) | 1 [Reference] |
| First product flavored | 509 (44.5) | 1.40 (1.16-1.69) | 360 (30.7) | 1.21 (0.96-1.54) | 220 (19.5) | NAh | 171 (15) | NAh | 144 (12.3) | NAh | 290 (24.7) | 1.54 (1.08-2.20) |
| Ever use of smokeless tobacco (excluding snus) | ||||||||||||
| First product nonflavored | 158 (37.3) | 1 [Reference] | 123 (29.2) | 1 [Reference] | 77 (18.8) | 1 [Reference] | 57 (14.1) | 1 [Reference] | 55 (12.9) | 1 [Reference] | 92 (21.9) | 1 [Reference] |
| First product flavored | 387 (47.2) | 1.29 (1.09-1.51) | 289 (34.2) | 1.18 (0.96-1.46) | 176 (21.2) | NAh | 144 (17.3) | NAh | 120 (14.4) | NAh | 231 (26.8) | 1.28 (0.99-1.66) |
| Ever use of snus | ||||||||||||
| First product nonflavored | 38 (18.4) | 1 [Reference] | 20 (9.1) | 1 [Reference] | NAg | 1 [Reference] | NAg | NAg | NAg | NAg | NAg | 1 [Reference] |
| First product flavored | 201 (28.2) | 1.61 (1.13-2.31) | 85 (11.3) | NAh | 23 (3.1) | NAh | NAg | NAg | NAg | NAg | 50 (7.1) | NAh |
| Ever use of dissolvable tobacco | ||||||||||||
| First product nonflavored | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg |
| First product flavored | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg |
Abbreviations: aPR, adjusted prevalence ratio; NA, not applicable.
Past 12-month use was defined as smoked or used product (even 1 or 2 times) in the past 12 months. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for past 12-month use are as follows: cigarettes, 1 (0.0%); e-cigarettes, 110 (3.5%); any cigar, 3 (0.1%); traditional cigars, 0 (0.0%); cigarillos, 1 (0.0%); filtered cigars, 2 (0.1%); pipe, 1 (0.1%); hookah, 2 (0.1%); any smokeless product, 4 (0.3%); smokeless product, 1 (0.1%); and snus, 2 (0.2%).
Past 30-day use was defined as smoked or used product (even 1 or 2 times) in the past 30 days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for past 30-day use were as follows: cigarettes, 0 (0.0%); e-cigarettes, 111 (3.6%); any cigar, 2 (0.1%); traditional cigars, 0 (0.0%); cigarillos, 4 (0.1%); filtered cigars, 1 (0.1%); pipe, 1 (0.1%); hookah, 2 (0.1%); any smokeless product, 3 (0.2%); smokeless product, 3 (0.2%); and snus, 1 (0.1%).
Moderate use was defined as having smoked or used the product on at least 6 of the past 30 days. Frequent product use defined as having smoked or used the product on at least 20 of the past 30 days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for moderate and frequent use were as follows: cigarettes moderate or frequent use, 81 (1.7%); e-cigarettes moderate or frequent use, 181 (5.8%); any cigar product moderate use, 305 (7.5%); any cigar product frequent use, 315 (7.7%); traditional cigars moderate use, 126 (7.9%); cigarillos moderate or frequent use, 152 (4.1%); filtered cigars moderate use, 60 (3.9%); hookah moderate or frequent use, 8 (0.2%); any smokeless tobacco product moderate use, 60 (4.0%); any smokeless tobacco product frequent use, 66 (4.4%); smokeless tobacco moderate or frequent use, 40 (3.3%); and snus moderate use, 28 (3.1%).
Daily use among adults was defined as now smokes or uses product every day. Current regular use was defined for cigarettes as having smoked at least 100 cigarettes in lifetime and now smokes every day or some days; for all other products, regular use was defined as having ever used a product “fairly regularly” and now uses it every day or some days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for daily and current regular use were as follows: cigarettes daily use, 30 (0.6%); cigarettes current established use, 3 (0.1%); e-cigarettes daily use, 71 (2.3%); e-cigarettes current established use, 10 (0.3%); any cigar product daily use, 116 (2.9%); any cigar product current established use, 247 (6.1%); traditional cigars current established use, 0 (0.0%); cigarillos daily use, 76, (2.1%); cigarillos current established use, 270 (7.4%); filtered cigars current established use, 1 (0.1%); pipe tobacco current established use, 0 (0.0%); hookah daily use, 5 (0.1%); hookah current established use, 0 (0.0%); any smokeless tobacco product daily use, 45 (3.0%); any smokeless tobacco product current established use, 6 (0.4%); smokeless tobacco daily use, 28 (2.3%); smokeless tobacco current established use, 0 (0.0%); and snus current established use, 0 (0.0%).
Multivariable modified Poisson regression models among young adults were adjusted for age, sex, race/ethnicity, education, income, past 30-day alcohol, marijuana, or other drug use, and 3 Global Appraisal of Individual Needs–Short Screener subscales (internalizing problems, externalizing problems, and substance use problems). Individuals who reported “don’t know” or refused to answer any of these items were treated as missing. Among young adults, data were missing for age (0 participants [0.0%]), sex (0 participants [0.0%]), race/ethnicity (0 participants [0.0%]), education (0 participants [0.0%]), income (589 participants [10.1%]), past 30-day alcohol, marijuana, or other drug use (54 participants [0.9%]), internalizing problems subscale (49 participants [0.8%]), externalizing problems subscale (75 participants [1.3%]), and substance use problems subscale (101 participants [1.7%]). Respondents who reported never alcohol, marijuana, or other drug use were categorized as non–past 30-day users.
A separate comparison was made between wave 1 cigarette smokers who first smoked a menthol or mint flavored cigarette vs smokers who first smoked a nonflavored cigarette. Individuals who reported first other flavored cigarette use were excluded from the denominator.
The estimate was suppressed because it has low statistical precision. It is based on a sample size of less than 50, or the coefficient of variation of the estimate is larger than 30%.
There was insufficient observations to compute balanced repeated replication SEs.
Any smokeless tobacco use was defined as smokeless and/or snus use.
Table 3. Association Between First Tobacco Product Flavored Among Adults Aged 25 Years and Older Ever Tobacco Users at Wave 1 and Product-Specific Tobacco Use at Wave 2 of the Population Assessment of Tobacco and Health Study.
| Ever Tobacco Use at Wave 1 | Past 12-mo Use at Wave 2a | Past 30-d Use at Wave 2b | ≥6 d Use at Wave 2c | ≥20 day Use at Wave 2c | Daily Use at Wave 2d | Current Regular Use at Wave 2d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No. (%) | aPR (95%CI)e | Participants, No. (%) | aPR (95%CI)e | Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | Participants, No. (%) | aPR (95% CI)e | |
| Ever cigarette use | ||||||||||||
| First product nonflavored | 5499 (31.5) | 1 [Reference] | 4946 (27.8) | 1 [Reference] | 4374 (24) | 1 [Reference] | 4028 (21.9) | 1 [Reference] | 3721 (20) | 1 [Reference] | 4430 (24.4) | 1 [Reference] |
| First product flavored | 3740 (41.9) | 1.10 (1.05-1.15) | 3371 (37.1) | 1.09 (1.04-1.14) | 2963 (31.9) | 1.08 (1.03-1.14) | 2688 (28.7) | 1.08 (1.02-1.14) | 2447 (26) | 1.09 (1.03-1.16) | 2986 (32.3) | 1.11 (1.06-1.17) |
| First product menthol or mint flavoredf | 3554 (42.9) | 1.13 (1.08-1.18) | 3218 (38.1) | 1.12 (1.07-1.17) | 2846 (32.9) | 1.12 (1.06-1.17) | 2590 (29.8) | 1.12 (1.06-1.18) | 2367 (27.1) | 1.13 (1.07-1.20) | 2867 (33.4) | 1.14 (1.09-1.20) |
| Ever e-cigarette use | ||||||||||||
| First product nonflavored | 1793 (57.7) | 1 [Reference] | 801 (25.1) | 1 [Reference] | 356 (10.8) | 1 [Reference] | 250 (7.6) | 1 [Reference] | 217 (6.3) | 1 [Reference] | 441 (13) | 1 [Reference] |
| First product flavored | 1818 (66.5) | 1.15 (1.10-1.20) | 838 (30.8) | 1.25 (1.13-1.38) | 440 (16.3) | 1.64 (1.42-1.89) | 338 (12.8) | 1.85 (1.54-2.22) | 299 (11) | 1.87 (1.55-2.26) | 580 (19.9) | 1.60 (1.41-1.82) |
| Ever any cigar use | ||||||||||||
| First product nonflavored | 1613 (20.1) | 1 [Reference] | 942 (11.1) | 1 [Reference] | 256 (2.8) | 1 [Reference] | 167 (1.8) | 1 [Reference] | 134 (1.4) | 1 [Reference] | 445 (4.9) | 1 [Reference] |
| First product flavored | 1636 (30.1) | 1.31 (1.22-1.41) | 1016 (18.1) | 1.34 (1.22-1.48) | 272 (4.7) | 1.36 (1.08-1.70) | 175 (3.1) | 1.44 (1.06-1.95) | 137 (2.3) | 1.49 (1.08-2.07) | 491 (8.4) | 1.56 (1.29-1.87) |
| Ever use of traditional cigars | ||||||||||||
| First product nonflavored | 1386 (19.6) | 1 [Reference] | 632 (8.7) | 1 [Reference] | 84 (1) | 1 [Reference] | 39 (0.5) | 1 [Reference] | 33 (0.4) | 1 [Reference] | 287 (3.7) | 1 [Reference] |
| First product flavored | 476 (23.2) | 1.09 (0.97-1.23) | 264 (12.8) | 1.36 (1.15-1.62) | 38 (1.6) | 1.15 (0.70-1.88) | 19 (0.8) | 1.37 (0.69-2.74) | 18 (0.7) | 1.71 (0.84-3.49) | 109 (4.7) | 1.44 (1.10-1.87) |
| Ever use of cigarillos | ||||||||||||
| First product nonflavored | 762 (12.6) | 1 [Reference] | 474 (7.8) | 1 [Reference] | 115 (1.9) | 1 [Reference] | 74 (1.2) | 1 [Reference] | 60 (1) | 1 [Reference] | 189 (3.1) | 1 [Reference] |
| First product flavored | 891 (20.6) | 1.37 (1.22-1.54) | 553 (12.4) | 1.23 (1.05-1.44) | 112 (2.5) | 1.02 (0.75-1.39) | 57 (1.3) | 0.88 (0.58-1.35) | 37 (0.8) | 0.65 (0.42-1.00) | 211 (4.6) | 1.29 (1.01-1.64) |
| Ever use of filtered cigars | ||||||||||||
| First product nonflavored | 362 (11.5) | 1 [Reference] | 202 (6.4) | 1 [Reference] | 95 (3) | 1 [Reference] | 80 (2.6) | 1 [Reference] | 70 (2.3) | 1 [Reference] | 118 (3.7) | 1 [Reference] |
| First product flavored | 373 (18.2) | 1.55 (1.31-1.83) | 203 (10) | 1.50 (1.16-1.95) | 87 (4.1) | 1.70 (1.16-2.50) | 65 (3.1) | 1.53 (0.98-2.38) | 58 (2.8) | 1.65 (1.03-2.63) | 131 (6.3) | 1.79 (1.25-2.54) |
| Ever use of pipe tobacco | ||||||||||||
| First product nonflavored | 240 (5.3) | 1 [Reference] | 136 (3.1) | 1 [Reference] | 43 (0.8) | 1 [Reference] | 34 (0.6) | 1 [Reference] | 30 (0.6) | 1 [Reference] | 92 (1.7) | 1 [Reference] |
| First product flavored | 146 (7.3) | 1.42 (1.07-1.87) | 72 (3.6) | 1.24 (0.86-1.78) | NAg | NAg | NAg | NAg | NAg | NAg | 47 (2.4) | 1.44 (0.92-2.25) |
| Ever use of hookah tobacco | ||||||||||||
| First product nonflavored | 127 (10.6) | 1 [Reference] | 57 (4.6) | 1 [Reference] | NAg | 1 [Reference] | NAg | NAg | NAg | NAg | NAg | 1 [Reference] |
| First product flavored | 738 (20.7) | 1.63 (1.25-2.13) | 264 (7.3) | 1.28 (0.88-1.87) | 34 (0.9) | NAh | NAg | NAg | NAg | NAg | 136 (3.7) | 5.66 (2.04-15.71) |
| Ever use of any smokeless tobaccoi | ||||||||||||
| First product nonflavored | 453 (15.9) | 1 [Reference] | 362 (12.9) | 1 [Reference] | 298 (10.4) | 1 [Reference] | 261 (9) | 1 [Reference] | 238 (8.2) | 1 [Reference] | 318 (11.2) | 1 [Reference] |
| First product flavored | 802 (26.1) | 1.50 (1.32-1.70) | 622 (20.4) | 1.49 (1.28-1.73) | 472 (15.3) | 1.43 (1.20-1.70) | 409 (13.3) | 1.44 (1.17-1.78) | 369 (11.8) | 1.44 (1.15-1.80) | 564 (18.1) | 1.55 (1.32-1.82) |
| Ever use of smokeless tobacco (excluding snus) | ||||||||||||
| First product nonflavored | 487 (16.5) | 1 [Reference] | 402 (13.7) | 1 [Reference] | 329 (11.2) | 1 [Reference] | 292 (9.8) | 1 [Reference] | 264 (8.8) | 1 [Reference] | 360 (12.2) | 1 [Reference] |
| First product flavored | 632 (24.8) | 1.38 (1.21-1.58) | 523 (20.6) | 1.43 (1.22-1.67) | 403 (15.5) | 1.37 (1.16-1.62) | 351 (13.5) | 1.36 (1.12-1.67) | 317 (12) | 1.38 (1.10-1.72) | 471 (18.1) | 1.43 (1.21-1.68) |
| Ever use of snus | ||||||||||||
| First product nonflavored | 53 (10.8) | 1 [Reference] | 25 (5.1) | 1 [Reference] | NAg | 1 [Reference] | NAg | 1 [Reference] | NAg | 1 [Reference] | NAg | 1 [Reference] |
| First product flavored | 209 (19.4) | 1.71 (1.20-2.44) | 102 (9.5) | 1.60 (0.95-2.71) | 47 (4.2) | 1.82 (0.81-4.09) | 34 (3.1) | 2.50 (0.80-7.76) | 31 (2.9) | 2.17 (0.71-6.59) | 75 (6.8) | 3.20 (1.65-6.20) |
| Ever use of dissolvable tobacco | ||||||||||||
| First product nonflavored | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg |
| First product flavored | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg | NAg |
Abbreviations: aPR, adjusted prevalence ratio; NA, not applicable.
Past 12-month use was defined as smoked or used product (even 1 or 2 times) in the past 12 months. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for past 12-month use were as follows: cigarettes, 4 (0.0%); e-cigarettes, 448 (7.2%); any cigar, 7 (0.1%); traditional cigars, 5 (0.1%); cigarillos, 3 (0.0%); filtered cigars, 3 (0.1%); pipe, 5 (0.1%); hookah, 1 (0.0%); any smokeless product, 4 (0.1%); smokeless product, 1 (0.0%); and snus, 1 (0.1%).
Past 30-day use was defined as smoked or used product (even 1 or 2 times) in the past 30 days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for past 30-day use were as follows: cigarettes, 5 (0.0%); e-cigarettes, 455 (7.3%); any cigar, 8 (0.1%); traditional cigars, 5 (0.1%); cigarillos, 9 (0.1%); filtered cigars, 4 (0.1%); pipe, 9 (0.2%); hookah, 1 (0.0%); any smokeless product, 4 (0.1%); smokeless product, 2 (0.0%); and snus, 2 (0.1%).
Moderate use was defined as having smoked or used the product on at least 6 of the past 30 days. Frequent product use was defined as having smoked or used the product on at least 20 of the past 30 days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for moderate and frequent use were as follows: cigarettes moderate or frequent use, 117 (0.7%); e-cigarettes moderate or frequent use, 326 (5.2%); any cigar product moderate use, 623 (6.2%); any cigar product frequent use, 638 (6.3%); traditional cigars moderate or frequent use, 388 (5.7%); cigarillos moderate or frequent use, 221 (2.9%); filtered cigars moderate or frequent use, 76 (1.9%); pipe tobacco moderate or frequent use, 84 (1.8%); hookah moderate use, 2 (0.0%); any smokeless product moderate use, 88 (2.0%); any smokeless product frequent use, 90 (2.0%); smokeless tobacco moderate or frequent use, 64 (1.6%); snus moderate or frequent use, 34 (2.4%).
Daily use among adults was defined as now smokes or uses product every day. Current regular use was defined for cigarettes as having smoked at least 100 cigarettes in lifetime and now smokes every day or some days; for all other products, current regular use was defined as having ever used product “fairly regularly” and now uses it every day or some days. Individuals who responded “don’t know” or refused to answer were excluded from the denominator. Unweighted numbers (percentages) of respondents excluded from the denominator for daily and current regular use were as follows: cigarettes daily use, 92 (0.6%); cigarettes current established use, 25 (0.2%); e-cigarettes daily use, 134 (2.2%); e-cigarettes current established use, 31 (0.5%); any cigar product daily use, 220 (2.2%); any cigar product current established use, 98 (1.0%); traditional cigars daily use, 112 (1.7%); traditional cigars current established use, 2 (0.0%); cigarillos daily use, 90 (1.2%); cigarillos current established use, 101 (1.3%); filtered cigars daily use, 29 (0.7%); filtered cigars current established use, 3 (0.1%); pipe tobacco daily use, 49 (1.0%); pipe tobacco current established use, 3 (0.1%); hookah current established use, 0 (0.0%); any smokeless product daily use, 56 (1.3%); any smokeless product current established use, 8 (0.2%); smokeless tobacco daily use, 38 (1.0%); smokeless tobacco current established use, 4 (0.1%); snus daily use, 20, (1.4%); and snus current established use, 1 (0.1%).
Multivariable modified Poisson regression models among adults were adjusted for age, sex, race/ethnicity, education, income, past 30-day alcohol, marijuana, or other drug use, and 3 Global Appraisal of Individual Needs–Short Screener subscales (internalizing problems, externalizing problems, and substance use problems). Individuals who reported “don’t know” or refused to answer any of these items were treated as missing. Among adults, data were missing for age (0 participants [0.0%]), sex (0 participants [0.0%]), race/ethnicity (0 participants [0.0%]), education (0 participants [0.0%]), income (1263 participants [7.6%]), past 30-day alcohol, marijuana, or other drug use (247 participants [1.5%]), internalizing problems subscale (199 participants [1.2%]), externalizing problems subscale (376 participants [2.3%]), and substance use problems subscale (427 participants [2.6%]). Respondents who reported never alcohol, marijuana, or other drug use were categorized as non–past 30-day users.
A separate comparison was made between wave 1 cigarette smokers who first smoked a menthol or mint flavored cigarette vs smokers who first smoked a nonflavored cigarette. Individuals who reported first other flavored cigarette use were excluded from the denominator.
The estimate was suppressed because it has low statistical precision. It is based on a sample size of less than 50, or the coefficient of variation of the estimate is larger than 30%.
There were insufficient observations to compute balanced repeated replication standard errors.
Any smokeless tobacco use was defined as smokeless and/or snus use.
Among young adults, first use of flavored e-cigarettes (aPR, 2.05; 95% CI, 1.61-2.61), any cigars (aPR, 1.60; 95% CI, 1.26-2.02), cigarillos (aPR, 1.49; 95% CI, 1.08-2.05), filtered cigars (aPR, 3.69; 95% CI, 2.08-6.57), hookah (aPR, 1.91; 95% CI, 1.23-2.98), and any smokeless tobacco (aPR, 1.54; 95% CI, 1.08-2.20) was prospectively associated with current regular use of those products at wave 2 compared with first nonflavored use (Table 2). Among adults aged 25 years and older, first use of flavored e-cigarettes (aPR, 1.60; 95% CI, 1.41-1.82), any cigars (aPR, 1.56; 95% CI, 1.29-1.87), cigarillos (aPR, 1.29; 95% CI, 1.01-1.64), filtered cigars (aPR, 1.79; 95% CI, 1.25-2.54), hookah (aPR, 5.66; 95% CI, 2.04-15.71), and any smokeless tobacco (aPR, 1.55; 95% CI, 1.32-1.82) was prospectively associated with current regular use of those products at wave 2 compared with first nonflavored use (Table 3). First use of any flavored cigar product was positively associated with each measure of subsequent use among adults aged 25 years and older (12-month use aPR, 1.31 [95% CI, 1.22-1.41]; 30-day use aPR, 1.34 [95% CI, 1.22-1.48]; ≥6-day use aPR, 1.36 [95% CI, 1.08-1.70]; ≥20-day use aPR, 1.44 [95% CI, 1.06-1.95]; daily use aPR, 1.49 [95% CI, 1.08-2.07]; current regular use aPR, 1.56 [95% CI, 1.29-1.87]) (Table 3) and with past 12-month (aPR, 1.19; 95% CI, 1.10-1.29), past 30-day (aPR, 1.13; 95% CI, 1.01-1.27), and current regular use (aPR, 1.60; 95% CI, 1.26-2.02) among young adults (Table 2).
Association Between First Use of a Given Tobacco Product Being Flavored at Wave 1 and Frequency of Current Tobacco Use at Wave 2
Among both youth and adults aged 25 years and older, first flavored or menthol cigarette use assessed at wave 1 was associated with a higher relative risk of cigarette use in the past 12 months (youth, flavored, relative risk ratio [RRR], 1.47 [95% CI, 1.09-1.98] and menthol, RRR, 1.60 [95% CI, 1.17-2.21]; adults, flavored, RRR, 1.34 [95% CI, 1.09-1.63] and menthol, RRR, 1.40 [95% CI, 1.14-1.73]), on 1 to 5 of the past 30 days (youth, flavored, RRR, 1.69 [95% CI, 1.20-2.40] and menthol, RRR, 1.93 [95% CI, 1.32-2.83]; adults, flavored RRR, 1.30 [95% CI, 1.07-1.58] and menthol, RRR, 1.36 [95% CI, 1.10-1.67]), and on all 30 of the past 30 days (youth, flavored, RRR, 1.61 [95% CI, 1.10-2.38] and menthol, RRR, 1.88 [95% CI, 1.25-2.82]; adults, flavored, RRR, 1.23 [95% CI, 1.11-1.35] and menthol, RRR, 1.32 [95% CI, 1.20-1.45]) compared with nonflavored cigarette use (Table 4). Among young adults, first flavored or menthol cigarette use was associated with use on all 30 days (RRR, 1.56 [95% CI, 1.27-1.93] for flavored and 1.66 [95% CI, 1.33-2.06] for menthol). Among young adults, first flavored e-cigarette use at wave 1 was associated with higher relative risk of e-cigarette use in the past 12 months (RRR, 1.52; 95% CI, 1.21-1.92), on 1 to 5 of the past 30 days (RRR, 1.61; 95% CI, 1.24-2.10), on 6 to 19 of the past 30 days (RRR, 2.35; 95% CI, 1.27-4.34), and on all 30 of the past 30 days (RRR, 3.24; 95% CI, 2.16-4.86) compared with nonflavored e-cigarette use. Among adults aged 25 years and older, first use of a flavored e-cigarette assessed at wave 1 was associated with greater frequency of e-cigarette use at wave 2 across all categories (past 12-month use, RRR, 1.38 [95% CI, 1.19-1.61]; 1-5 days in the past 30 days, RRR, 1.25 [95% CI, 1.02-1.53]; 6-19 days in the past 30 days, RRR, 1.44 [95% CI, 1.03-2.01]; 20-29 days in the past 30 days, RRR, 2.09 [95% CI, 1.09-4.00]; and all 30 of the past 30 days, RRR, 2.38 [95% CI, 1.90-3.00]).
Table 4. Multivariable Multinomial Logistic Regression Models of Frequency of Use at Wave 2 Among Ever Users of Specified Product at Wave 1 of the Population Assessment of Tobacco and Health Study, by Age Group.
| Age Group | Participants, No. | No Past 12-mo Use | RRR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Past 12-mo Use, No Past 30-d Use | 1-5 d in the Past 30 d | 6-19 d in the Past 30 d | 20-29 d in the Past 30 d | All 30 d in the Past 30 d | |||
| Youtha | |||||||
| First cigarette flavored | 1316 | 1 [Reference] | 1.47 (1.09-1.98) | 1.69 (1.20-2.40) | 1.22 (0.72-2.07) | 1.15 (0.61-2.18) | 1.61 (1.10-2.38) |
| First cigarette menthol or mint flavoredb | 1223 | 1 [Reference] | 1.60 (1.17-2.21) | 1.93 (1.32-2.83) | 1.33 (0.77-2.31) | 1.23 (0.65-2.32) | 1.88 (1.25-2.82) |
| First e-cigarette flavored | 1045 | 1 [Reference] | 1.26 (0.82-1.94) | 1.30 (0.78-2.16) | 1.40 (0.64-3.07) | 1.08 (0.21-5.71) | 2.85 (0.94-8.63) |
| Young adultsc | |||||||
| First cigarette flavored | 4109 | 1 [Reference] | 1.13 (0.90-1.41) | 1.24 (1.00-1.55) | 1.21 (0.93-1.57) | 1.26 (0.86-1.86) | 1.56 (1.27-1.93) |
| First cigarette menthol or mint flavoredb | 3925 | 1 [Reference] | 1.13 (0.89-1.44) | 1.21 (0.96-1.52) | 1.24 (0.95-1.63) | 1.30 (0.87-1.95) | 1.66 (1.33-2.06) |
| First e-cigarette flavored | 2622 | 1 [Reference] | 1.52 (1.21-1.92) | 1.61 (1.24-2.10) | 2.35 (1.27-4.34) | 0.81 (0.37-1.75) | 3.24 (2.16-4.86) |
| Adultsd | |||||||
| First cigarette flavored | 13 959 | 1 [Reference] | 1.34 (1.09-1.63) | 1.30 (1.07-1.58) | 1.22 (0.96-1.56) | 1.11 (0.86-1.43) | 1.23 (1.11-1.35) |
| First cigarette menthol or mint flavoredb | 13 594 | 1 [Reference] | 1.40 (1.14-1.73) | 1.36 (1.10-1.67) | 1.28 (1.00-1.63) | 1.15 (0.89-1.48) | 1.32 (1.20-1.45) |
| First e-cigarette flavored | 5188 | 1 [Reference] | 1.38 (1.19-1.61) | 1.25 (1.02-1.53) | 1.44 (1.03-2.01) | 2.09 (1.09-4.00) | 2.38 (1.90-3.00) |
Abbreviation: RRR, relative risk ratio.
Multivariable multinomial logistic regression models among youth were adjusted for sex, race/ethnicity, education, past 30-day alcohol, marijuana, or other drug use, and 3 Global Appraisal of Individual Needs–Short Screener (GAIN-SS) subscales (internalizing problems, externalizing problems, and substance use problems). Individuals who reported “don’t know” or refused to answer any of these items were treated as missing. Among youth ever tobacco users, data were missing for sex (0 participants [0.0%]), race/ethnicity (27 participants [1.5%]), education (114 participants [6.2%]), past 30-day alcohol, marijuana, or other drug use (47 participants [2.6%]), internalizing problems subscale (40 participants [2.2%]), externalizing problems subscale (67 participants [3.7%]), and substance use problems subscale (71 participants [3.9%]). Respondents who reported never alcohol, marijuana, or other drug use were categorized as non–past 30-day users.
A separate comparison was made between wave 1 cigarette smokers who first smoked a menthol or mint flavored cigarette vs smokers who first smoked a nonflavored cigarette. Individuals who reported first other flavored cigarette use were excluded from the denominator.
Multivariable multinomial logistic regression models among young adults were adjusted for age, sex, race/ethnicity, education, income, past 30-day alcohol, marijuana, or other drug use, and 3 GAIN-SS subscales (internalizing problems, externalizing problems, and substance use problems). Individuals who reported “don't know” or refused to answer any of these items were treated as missing. Among young adults, data were missing for age (0 participants [0.0%]), sex (0 participants [0.0%]), race/ethnicity (0 participants [0.0%]), education (0 participants [0.0%]), income (449 participants [9.5%]), past 30-day alcohol, marijuana, or other drug use (46 participants [1.0%]), internalizing problems subscale (42 participants [0.9%]), externalizing problems subscale (60 participants [1.3%]), and substance use problems subscale (83 participants [1.8%]). Respondents who reported never alcohol, marijuana, or other drug use were categorized as non–past 30-day users.
Multivariable multinomial logistic regression models among adults were adjusted for age, sex, race/ethnicity, education, income, past 30-day alcohol, marijuana, or other drug use, and 3 GAIN-SS subscales (internalizing problems, externalizing problems, and substance use problems). Individuals who reported “don’t know” or refused to answer any of these items were treated as missing. Among adults, data were missing for age (0 participants [0.0%]), sex (0 participants [0.0%]), race/ethnicity (0 participants [0.0%]), education (0 participants [0.0%]), income (1216 participants [7.6%]), past 30-day alcohol, marijuana, or other drug use (234 participants [1.5%]), internalizing problems subscale (185 participants [1.2%]), externalizing problems subscale (359 participants [2.3%]), and substance use problems subscale (411 participants [2.6%]). Respondents who reported never alcohol, marijuana, or other drug use were categorized as non–past 30-day users.
Discussion
The current study found that (1) youth and young adults who were new users of a tobacco product at wave 2 (over the 10- to 13-month follow-up period) were more likely to try flavored tobacco products than adults; (2) first use of a flavored cigarette documented at wave 1 was positively associated with past 12-month and past 30-day cigarette use among youth, young adults, and adults aged 25 years and older at wave 2; (3) first use of a menthol or mint flavored cigarette documented at wave 1 was positively associated with past 12-month and past 30-day cigarette use at wave 2 in all age groups; (4) first use of flavored e-cigarettes, cigars, hookah, and smokeless tobacco was associated with subsequent use of those products at wave 2 among young adults and adults aged 25 years and older; (5) first flavored use of a cigarette, e-cigarette, any cigar, cigarillo, filtered cigar, hookah, and any smokeless tobacco documented at wave 1 was associated with current regular use of those products among young adults and adults aged 25 years and older at wave 2 compared with first use of a nonflavored product; (6) first flavored or menthol cigarette use was associated with progression to daily cigarette use at wave 2 in all age groups; and (7) first flavored e-cigarette use was associated with progression of e-cigarette frequency among young adults and adults aged 25 years and older. The age gradient in first use of a flavored tobacco product and positive association with subsequent tobacco use are consistent with the findings from prior studies.9,10,12,13,14,15,16,17,18 These data support tobacco industry research2,3,4,5 on the role of flavors to promote uptake in nonusers. The observation of significant prospective associations between first use of a menthol or mint flavored cigarette at wave 1 and continued cigarette use at wave 2 across all age groups adds novel insight to previous longitudinal studies28 on the role of menthol in facilitating smoking initiation and progression and reducing cessation.
Limitations
This study has several limitations. First, tobacco product use and flavored tobacco use in the questionnaire are based on the respondent’s perception of and ability to recall whether past or current products were flavored. Second, analyses examined continued use or progression of use over the 10- to 13-month follow-up period; thus, the analyses excluded participants who were missing data at 1 of the waves. The extent of missing data and the small number of observations for specific products limited the detection of certain associations from wave 1 to wave 2; this was especially an issue for the youth findings. Third, progression of tobacco use is known to occur over several years among young people,29 and flavored use among adults was asked only of established tobacco users at wave 1; future studies that include experimental tobacco users and a longer follow-up period will inform estimates of the association of flavored tobacco with uptake and maintenance of tobacco use. Fourth, analyses were stratified by age, and, among adults, age was also included as a covariate; this does not fully account for potential cohort effects given differences in the availability of flavored tobacco products at the time of initiation, or the fact that estimates for the group of adults aged 25 years and older may be inflated because of greater smoking cessation among older adults.
Conclusions
The findings of this study suggest that flavors in most tobacco products are associated with youth and young adult tobacco experimentation; that first use of a menthol or mint flavored cigarette places youth, young adults, and adults aged 25 years and older at risk of subsequent cigarette smoking; and that first use of flavored e-cigarettes, cigars, hookah, and smokeless tobacco products can place young adults and adults at risk of regular tobacco use when examined prospectively. Additional longitudinal studies will allow for a better understanding of the role of flavors in tobacco use progression and trajectories over time.
eTable. Sociodemographic and Tobacco Use Constructs in the PATH Study
References
- 1.Hoffman AC, Salgado RV, Dresler C, Faller RW, Bartlett C. Flavour preferences in youth versus adults: a review. Tob Control. 2016;25(2)(suppl):-. doi: 10.1136/tobaccocontrol-2016-053192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostygina G, Glantz SA, Ling PM. Tobacco industry use of flavours to recruit new users of little cigars and cigarillos. Tob Control. 2016;25(1):66-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostygina G, Ling PM. Tobacco industry use of flavourings to promote smokeless tobacco products. Tob Control. 2016;25(2)(suppl):ii40-ii49. doi: 10.1136/tobaccocontrol-2016-053212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson SJ. Marketing of menthol cigarettes and consumer perceptions: a review of tobacco industry documents. Tob Control. 2011;20(2)(suppl):ii20-ii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donati J. Conference report #23: RJ Reynolds Tobacco. https://www.industrydocuments.ucsf.edu/docs/jfcb0102. Published June 5, 1974. Accessed September 21, 2019.
- 6.Giovino GA, Villanti AC, Mowery PD, et al. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control. 2015;24(1):28-37. doi: 10.1136/tobaccocontrol-2013-051159 [DOI] [PubMed] [Google Scholar]
- 7.Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004-2014. Tob Control. 2016;25(2)(suppl):ii14-ii20. doi: 10.1136/tobaccocontrol-2016-053329 [DOI] [PubMed] [Google Scholar]
- 8.Delnevo CD, Giovenco DP, Ambrose BK, Corey CG, Conway KP. Preference for flavoured cigar brands among youth, young adults and adults in the USA. Tob Control. 2015;24(4):389-394. doi: 10.1136/tobaccocontrol-2013-051408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrose BK, Day HR, Rostron B, et al. Flavored tobacco product use among US youth aged 12-17 years, 2013-2014. JAMA. 2015;314(17):1871-1873. doi: 10.1001/jama.2015.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villanti AC, Johnson AL, Ambrose BK, et al. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH Study (2013-2014). Am J Prev Med. 2017;53(2):139-151. doi: 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose SW, Johnson AL, Glasser AM, et al. Flavour types used by youth and adult tobacco users in wave 2 of the Population Assessment of Tobacco and Health (PATH) Study 2014-15 [published online September 21, 2019]. Tob Control. doi: 10.1136/tobaccocontrol-2018-054852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dauphinee AL, Doxey JR, Schleicher NC, Fortmann SP, Henriksen L. Racial differences in cigarette brand recognition and impact on youth smoking. BMC Public Health. 2013;13:170. doi: 10.1186/1471-2458-13-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nonnemaker J, Hersey J, Homsi G, Busey A, Allen J, Vallone D. Initiation with menthol cigarettes and youth smoking uptake. Addiction. 2013;108(1):171-178. doi: 10.1111/j.1360-0443.2012.04045.x [DOI] [PubMed] [Google Scholar]
- 14.Rath JM, Villanti AC, Williams VF, Richardson A, Pearson JL, Vallone DM. Patterns of longitudinal transitions in menthol use among US young adult smokers. Nicotine Tob Res. 2015;17(7):839-846. doi: 10.1093/ntr/ntu247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muilenburg JL, Legge JS Jr. African American adolescents and menthol cigarettes: smoking behavior among secondary school students. J Adolesc Health. 2008;43(6):570-575. doi: 10.1016/j.jadohealth.2008.08.017 [DOI] [PubMed] [Google Scholar]
- 16.Azagba S, Minaker LM, Sharaf MF, Hammond D, Manske S. Smoking intensity and intent to continue smoking among menthol and non-menthol adolescent smokers in Canada. Cancer Causes Control. 2014;25(9):1093-1099. doi: 10.1007/s10552-014-0410-6 [DOI] [PubMed] [Google Scholar]
- 17.Delnevo CD, Villanti AC, Wackowski OA, Gundersen DA, Giovenco DP. The influence of menthol, e-cigarettes and other tobacco products on young adults’ self-reported changes in past year smoking. Tob Control. 2016;25(5):571-574. doi: 10.1136/tobaccocontrol-2015-052325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DM, Bansal-Travers M, Huang J, Barker D, Hyland AJ, Chaloupka F. Association between use of flavoured tobacco products and quit behaviours: findings from a cross-sectional survey of US adult tobacco users. Tob Control. 2016;25(2)(suppl):ii73-ii80. doi: 10.1136/tobaccocontrol-2016-053313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017;26(4):371-378. doi: 10.1136/tobaccocontrol-2016-052934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Addiction & HIV Data Archive Program Population Assessment of Tobacco and Health (PATH) study series. https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/606. Published; 2018. Accessed April 12, 2018. doi: 10.3886/Series606. [DOI] [Google Scholar]
- 22.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342-353. doi: 10.1056/NEJMsa1607538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis ML, Chan YF, Funk RR. Development and validation of the GAIN Short Screener (GSS) for internalizing, externalizing and substance use disorders and crime/violence problems among adolescents and adults. Am J Addict. 2006;15(1)(suppl):80-91. doi: 10.1080/10550490601006055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein RJ, Proctor SE, Boudreault MA, Turczyn KM. Healthy People 2010 criteria for data suppression. Healthy People 2020 Stat Notes. 2002;24:1-12. [PubMed] [Google Scholar]
- 25.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 26.StataCorp Stata 14 Base Reference Manual. College Station, TX: Stata Press; 2015. [Google Scholar]
- 27.Kasza KA, Coleman B, Sharma E, et al. Correlates of transitions in tobacco product use by U.S. adult tobacco users between 2013-2014 and 2014-2015: findings from the PATH Study wave 1 and wave 2. Int J Environ Res Public Health. 2018;15(11):E2556. doi: 10.3390/ijerph15112556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villanti AC, Collins LK, Niaura RS, Gagosian SY, Abrams DB. Menthol cigarettes and the public health standard: a systematic review. BMC Public Health. 2017;17(1):983. doi: 10.1186/s12889-017-4987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Sociodemographic and Tobacco Use Constructs in the PATH Study