Abstract
This study reviews publications and the level of evidence for US Food and Drug Administration–approved and off-label indications for eculizumab.
Eculizumab is a monoclonal antibody against complement C5 that is manufactured by Alexion Pharmaceuticals. It first received US Food and Drug Administration (FDA) approval in 2007 for treatment of paroxysmal nocturnal hemoglobinuria, a rare disease with incidence of 1 to 10 per million and, when it debuted, was the most costly drug in the world, priced at $400 000 per year for indefinite use.1,2
Eculizumab has now been approved by the FDA for 3 indications: paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, and refractory generalized myasthenia gravis, each of which was granted an orphan designation by the agency given the rarity of the disease. However, according to 10-K filings to the Securities and Exchange Commission, worldwide net sales from eculizumab increased from $66 million in 2007 to more than $3.5 billion in 2018. Large and increasing revenue from eculizumab raises the question of off-label use for non–FDA-approved indications and the strength of the evidence supporting such use. We sought to review publications and level of evidence for FDA-approved (on-label) and off-label indications for eculizumab.
Methods
ClinicalTrials.gov was searched with keyword eculizumab, and ClinicalTrials.gov identifiers of all registered trials were used to search Google Scholar and PubMed to identify published clinical trials. To broaden the search to noninvestigational studies, such as observational studies or case series/case reports, PubMed was searched with the keyword eculizumab. All resulting articles published before February 2019 were reviewed. All publications that reported clinical efficacy of eculizumab based on patient data were included in the study. Data were extracted for number of patients in the study, type of study, date of study, and clinical indication for use of eculizumab. Studies were classified as investigational studies, observational studies, and case series/case reports. Investigational studies include confirmatory studies, defined as phase 3 trials, and exploratory studies, defined as phase 1 and phase 2 trials. This study was an investigation of published reports and public websites and therefore was not subject to institutional review board approval according to federal regulations. As such, patient consent was not obtained.
Results
Three hundred seventy-two publications were identified. The characteristics of the publications are summarized in the Table. These included 22 published clinical trials and 39 observational studies. There was a large number of case series and case reports (311 of 372 [84%]). Most clinical trials included fewer than 50 patients (17 of 22 [77%]).
Table. Characteristics of Publications.
| Characteristic | Investigational Studies (n = 22) | Observational Studies (n = 39) | Case Series/Case Reports (n = 311) | Total (n = 372) |
|---|---|---|---|---|
| No. of patients | ||||
| <5 | 1 | 0 | 294 | 295 |
| 5-49 | 16 | 26 | 17 | 59 |
| 50-499 | 5 | 12 | 0 | 17 |
| ≥500 | 0 | 1 | 0 | 1 |
| Type of study | ||||
| Phase 3 | 3 | NA | NA | NA |
| Phase 1/2 | 19 | NA | NA | NA |
| Year of study | ||||
| 2001-2005 | 1 | 0 | 1 | 2 |
| 2006-2010 | 2 | 0 | 13 | 15 |
| 2011-2015 | 9 | 13 | 142 | 164 |
| 2016-2019 | 10 | 26 | 155 | 191 |
Abbreviation: NA, not applicable.
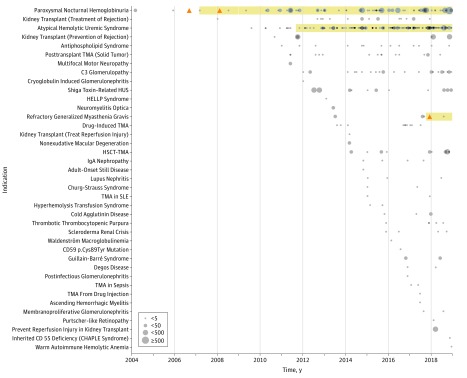
Publications reported on studies of eculizumab for 39 distinct indications (Figure), including 8 nonmalignant and 1 malignant hematologic disorders, 6 autoimmune disorders, 6 transplant-related indications, and 5 renal disorders.
Figure. The Landscape of Studies Supporting Eculizumab.
Studies describing clinical effects of eculizumab organized by clinical indication. Orange triangles represent confirmatory studies. Grey circles represent observational studies and other investigational studies that are not confirmatory. Grey circles vary in size based on number of patients in the study with 4 categories of less than 5, 5 to 49, 50 to 499, and 500 or more. For FDA-approved indications, the duration of FDA approval is highlighted in yellow.
CHAPLE indicates complement hyperactivation, angiopathic thrombosis, protein losing enteropathy; HELLP, hemolysis, elevated liver enzymes, low platelet count; HUS, hemolytic uremic syndrome; HSCT, hematopoietic stem cell transplantation; SLE, systemic lupus erythematosus; TMA, thrombotic microangiopathy.
Among FDA-approved indications, for only 2, paroxysmal nocturnal hemoglobinuria and myasthenia gravis, had confirmatory trials published. Twenty-seven of 39 indications (69%) did not have any randomized clinical trials reported with published literature and consist solely of observational studies, case series, and case reports.
Discussion
The clinical trial landscape of eculizumab shows a small number of confirmatory trials despite a large number of publications, mostly case series/case reports, describing use of eculizumab for non–FDA-approved indications. Prior research3,4 has shown how exploratory postapproval trials for unapproved indications may lead to widespread use of a drug without evidence of efficacy through confirmatory trials.
High and increasing net sales of eculizumab in the context of FDA approval for only 3 rare indications raises concern that off-label use may now represent a substantial proportion of revenue for eculizumab. However, patients and clinicians should be aware that the evidence supporting off-label use consists mostly of case series/case reports, along with small observational and interventional studies.
Our analysis is limited because we are unable to objectively assess the degree of off-label use of eculizumab. Previous studies have analyzed pharmaceutical benefits program data or hospitalization database to estimate off-label use of drugs.5,6 Future research may attempt to quantify what proportion of revenue from eculizumab is derived from on-label vs off-label use.
References
- 1.Gulbis B, Eleftheriou A, Angastiniotis M, et al. . Epidemiology of rare anaemias in Europe. Adv Exp Med Biol. 2010;686:375-396. doi: 10.1007/978-90-481-9485-8_22 [DOI] [PubMed] [Google Scholar]
- 2.Herper M. The world's most expensive drugs. https://www.forbes.com/2010/02/19/expensive-drugs-cost-business-healthcare-rare-diseases.html. Published 2010. Accessed June 25, 2019.
- 3.Federico CA, Wang T, Doussau A, Mogil JS, Fergusson D, Kimmelman J. Assessment of pregabalin postapproval trials and the suggestion of efficacy for new indications: a systematic review. JAMA Intern Med. 2019;179(1):90-97. doi: 10.1001/jamainternmed.2018.5705 [DOI] [PubMed] [Google Scholar]
- 4.Mattina J, Carlisle B, Hachem Y, Fergusson D, Kimmelman J. Inefficiencies and patient burdens in the development of the targeted cancer drug sorafenib: a systematic review. PLoS Biol. 2017;15(2):e2000487. doi: 10.1371/journal.pbio.2000487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kesselheim AS, Myers JA, Solomon DH, Winkelmayer WC, Levin R, Avorn J. The prevalence and cost of unapproved uses of top-selling orphan drugs. PLoS One. 2012;7(2):e31894. doi: 10.1371/journal.pone.0031894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castañeda-Sanabria J, Hajage D, Le Jouan M, Perozziello A, Tubach F. Off-label use of the expensive orphan drug eculizumab in France 2009-2013 and the impact of literature: focus on the transplantation field. Eur J Clin Pharmacol. 2016;72(6):737-746. doi: 10.1007/s00228-016-2027-z [DOI] [PubMed] [Google Scholar]