Abstract
This study characterizes the frequency and sex ratio of positive test results for myelin oligodendrocyte glycoprotein–IgG and aquaporin-4–IgG in adult and pediatric patient populations in a single center.
Myelin oligodendrocyte glycoprotein (MOG)–IgG is a biomarker associated with central nervous system–demyelinating disorders termed MOG-IgG–associated disorders. These disorders have overlapping clinical features, including optic neuritis and myelitis, with aquaporin-4 (AQP4)–IgG–positive neuromyelitis optica spectrum disorders. These disorders are hypothesized to be biologically distinct; AQP4-IgG–positive neuromyelitis optica spectrum disorders are autoimmune astrocytopathies, whereas MOG-IgG–associated disorders are postulated to be autoimmune oligodendrocytopathies.1 Despite their immunopathogenic differences, there are rare reports of patients with dual positivity of MOG-IgG and AQP4-IgG.2,3 We aimed to determine the frequency, sex ratio, and coexistence of glial antibodies (AQP4-IgG and MOG-IgG) in adults and children undergoing evaluation for suspected central nervous system–demyelinating diseases.
Methods
This is a retrospective cohort study approved by the Mayo Clinic institutional review board and ethics committee with a waiver of informed consent because of the restricted, deidentified nature of the data used. All patients who had serum samples tested for AQP4-IgG and MOG-IgG from October 2017 to May 2019 were evaluated using a clinically validated flow cytometric assay (BD FACSCanto II [BD Biosciences]).4 All samples were repeated to confirm positivity. We have used basic demographic data (age and sex) provided through the clinical testing request forms. We used χ2 tests to compare the proportions of female and male individuals within the antibody-positive groups. All tests were 2-sided, and P values less than .05 were considered significant. The statistical software R version 3.5.1 (R Foundation for Statistical Computing) was used.
Results
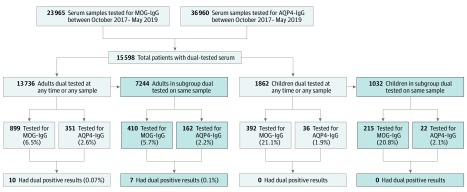
Over a 20-month period, 15 598 patients (1862 children [<18 years] and 13 736 adults) were tested for both AQP4-IgG and MOG-IgG (Figure 1). In 1291 patients (8.3%), MOG-IgG was detected; AQP4-IgG was detected in 387 patients (2.3%).
Figure 1. Flowchart of Cohort Selection and Identification of Glial Antibodies Within the Tested Population.
The frequency of detection of antibodies in 13 736 adults and 1862 children. AQP4-IgG indicates aquaporin-4 immunoglobulin G; MOG-IgG, myelin oligodendrocyte glycoprotein immunoglobulin G.
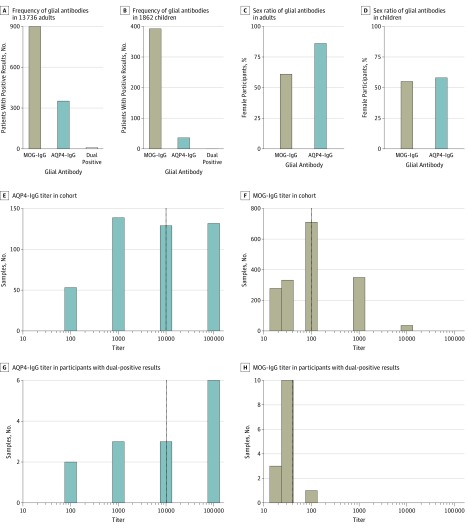
Of the adults, 899 (6.5%) were MOG-IgG positive, and 351 (2.6%) were AQP4-IgG positive (Figure 2A). Of the adults with MOG-IgG, 546 were female (60.7%); 303 of the adults with AQP4-IgG (86.3%) were female (P < .001) (Figure 2C). Of the children, 392 were MOG-IgG positive (21.1%), and 36 were AQP4-IgG positive (1.9%) (Figure 2B). Of all children with MOG-IgG, 214 were female (54.6%) vs 21 (58.3%) of all children with AQP4-IgG (P = .79) (Figure 2D).
Figure 2. Frequency of Myelin Oligodendrocyte Glycoprotein (MOG)–IgG and Aquaporin-4 (AQP4)–IgG Serum Titers on Flow Cytometry Assay.
A-D, sex ratio of glial antibodies in adults and children; E, AQP4-IgG–positive cohort; F, MOG-IgG–positive cohort; G, AQP4-IgG in individuals with dual positivity; and H, MOG-IgG in individuals with dual positivity.
In the subgroup of patients with serum testing completed on the same sample (n = 8276), similar proportions of antibody positivity were observed as in those with separate samples tested (Figure 1). The median serum titers for the cohort were 1:10 000 (range, 1:5 to 1:100 000) for AQP4-IgG and 1:100 (range, 1:20 to 1:100 000) for MOG-IgG (Figure 2E and F). The adult and children subgroups had the same median titers.
Of 15 598 patients, 10 patients (0.06%) had dual positivity. All 10 patients with dual positivity had high-titer AQP4-IgG (median, 1:10 000; range, 1:100 to 1:100 000) and low-titer MOG-IgG (median, 1:40; range, 1:20 to 1:100) (Figure 2G and H). All of the patients with dual positivity were adults (median age, 47 years [range, 30-61 years]), and 9 of the 10 patients were female (90%).
Conclusions
In this large cohort of patients undergoing evaluation for suspected central demyelinating diseases, MOG-IgG were detected almost 3 times as often as AQP4-IgG in adults; in children, MOG-IgG was detected more than 11 times as often as AQP4-IgG. In this study, MOG-IgG and AQP4-IgG rarely coexisted in a single patient (0.06%).
We have confirmed the previously described female predominance in adults who are positive for AQP4-IgG.5 Furthermore, we have demonstrated that there is a distinct difference in the female predominance in adults with AQP4-IgG compared with MOG-IgG. Together, these sex differences and the greater detection of MOG-IgG, especially in children, suggests that central demyelinating diseases associated with these biomarkers may have different drivers of autoimmunity.
The rare coexistence of these 2 antibody biomarkers also points to distinct immunopathogeneses of these diseases. It has been postulated that MOG-IgG is an epiphenomenon, occurring with exposure of antigen in the AQP4-IgG disease. However, the rarity of dual positivity is this large cohort is evidence against an epiphenomenon. All individuals with dual positivity had high titers of AQP4-IgG and low titers of MOG-IgG, suggesting the disease phenotype may be more compatible with AQP4-IgG–positive neuromyelitis optica spectrum disorders.
These findings suggest that MOG-IgG may occur more frequently than AQP4-IgG in patients tested for central demyelinating diseases, particularly in children. However, because the study population was derived from patients who had undergone a central demyelinating disease serological evaluation, these data should not be interpreted as representing seroprevalence in the general population. Further population-based studies are required to determine the prevalence and incidence of MOG-IgG–associated disorders. That MOG-IgG and AQP4-IgG rarely coexist highlights the immunopathogenic distinction of these biomarkers.
References
- 1.Pittock SJ, Lucchinetti CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann N Y Acad Sci. 2016;1366(1):20-39. doi: 10.1111/nyas.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyun J-W, Woodhall MR, Kim S-H, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88(10):811-817. doi: 10.1136/jnnp-2017-315998 [DOI] [PubMed] [Google Scholar]
- 3.Höftberger R, Sepulveda M, Armangue T, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler. 2015;21(7):866-874. doi: 10.1177/1352458514555785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters PJ, Komorowski L, Woodhall M, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology. 2019;92(11):e1250-e1255. doi: 10.1212/WNL.0000000000007096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quek AML, McKeon A, Lennon VA, et al. Effects of age and sex on aquaporin-4 autoimmunity. Arch Neurol. 2012;69(8):1039-1043. doi: 10.1001/archneurol.2012.249 [DOI] [PMC free article] [PubMed] [Google Scholar]