Abstract
The introduction of CD38-targeting monoclonal antibodies (CD38 MoABs), daratumumab and isatuximab, has significantly impacted the management of patients with multiple myeloma (MM). Outcomes of patients with MM refractory to CD38 MoABs have not been described. We analyzed outcomes of 275 MM patients at 14 academic centers with disease refractory to CD38 MoABs. Median interval between MM diagnosis and refractoriness to CD38 MoAB (T0) was 50.1 months. The median overall survival (OS) from T0 for the entire cohort was 8.6 [95% C.I. 7.5–9.9] months, ranging from 11.2 months for patients not simultaneously refractory to an immunomodulatory (IMiD) agent and a proteasome inhibitor (PI) to 5.6 months for “penta-refractory” patients (refractory to CD38 MoAB, 2 PIs and 2 IMiDs). At least one subsequent treatment regimen was employed after T0 in 249 (90%) patients. Overall response rate to first regimen after T0 was 31% with median progression-free survival (PFS) and OS of 3.4 and 9.3 months, respectively. PFS was best achieved with combinations of carfilzomib and alkylator (median 5.7 months), and daratumumab and IMiD (median 4.5 months). Patients with MM refractory to CD38 MoAB have poor prognosis and this study provides benchmark for new therapies to be tested in this population.
Introduction
Proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs) have significantly improved survival in patients with multiple myeloma (MM) 1, 2. However, MM eventually becomes refractory to these two classes of drugs. In the rapidly evolving treatment landscape, with several modern classes of compounds and combinations approved in the past 5 years 3, 4, double refractoriness to PIs and IMiDs still portends poor outcomes with a median overall survival (OS) of about 13 months based on a recent multicenter analysis 5.
Daratumumab and isatuximab are CD38-targeting monoclonal antibodies (CD38 MoABs) with remarkable activity in relapsed and/or refractory MM (RRMM) 6. Isatuximab has demonstrated single agent activity 7 as well as high response rates when combined with IMiDs or PIs 8–10. Similarly, daratumumab has demonstrated activity as a single agent 11 and in combination with IMiDs 12, 13 and PIs 14. When combined with lenalidomide and dexamethasone or with bortezomib and dexamethasone, daratumumab produces objective responses in over 80% of MM patients in early relapse and reduces the risk of progression or death by over 60% in such patients 13, 14. Daratumumab is commercially available having received FDA approvals as monotherapy (4th line; 2015) as well as in combination with lenalidomide (2nd line; 2016), bortezomib (2nd line; 2016) and pomalidomide (3rd line; 2017) for RRMM; recently, it also received approval in combination with bortezomib and melphalan in transplant-ineligible patients (1st line; 2018).
Acknowledging CD38 MoABs as a new class of agents in MM with a profound impact on the disease course, we hypothesized that patients with MM refractory to CD38 MoABs would have limited effective treatment options available and represented a new subset of patients with an unmet need for treatment. We therefore conducted a multicenter, retrospective study to investigate the natural history and outcomes of patients with MM refractory to CD38 MoABs (Monoclonal Antibodies in Multiple Myeloma: Outcomes after Therapy Failure, the MAMMOTH study).
Methods
Patient population
We identified patients at 14 academic institutions in the US with diagnosis of active MM and refractory to daratumumab or isatuximab, administered alone or in combination (henceforth referred to as the index regimen). Such an index regimen could have been administered as part of a clinical trial or routine clinical practice in the management of relapse or refractory MM (i.e. not first regimen employed for treatment of MM). Eligibility for the study required patients with MM be treated for at least 4 weeks with a CD38 MoAB-containing index regimen and with evidence of progressive disease (PD), as defined by the International Myeloma Working Group (IMWG) Response Criteria 15, 16, having progressed while on therapy or within 60 days after last dose of the index regimen. The time point when patients met the above criteria of progression was referred to as time zero (T0). Since the study focused on patients refractory to CD38 MoAB, those with an ongoing response to a CD38 MoAB-containing regimen and those who discontinued such therapy due to reasons other than PD were excluded. Data were collected retrospectively, and included patient-(age, sex, race/ethnicity, renal function) and disease characteristics [staging, cytogenetic abnormalities present at most recent assessment, level of lactic dehydrogenase (LDH)], all therapies administered before and after T0 (agents, best response, duration of response) and survival status. High-risk cytogenetics were defined as presence of t(4;14), t(14;16), or del 17p. This research received approval from the Institutional Review Boards from the coordinating institution (University of Alabama at Birmingham) and subsequently from all participating institutions. The research was performed in compliance with the terms from the declaration of Helsinki and was waived from the obligation to obtain written informed consent.
Data collection and statistical analysis
Study data were collected between January 2017 and June 2018 and managed using REDCap electronic data capture tools hosted at Vanderbilt University Medical Center 17. All data underwent peer-based quality check for completeness and internal consistencies. For cases with incomplete information or internal inconsistencies, queries were generated, and the cases were not included in the final dataset until all queries were satisfactorily resolved.
For analysis, patients were classified into three groups based on refractoriness to the anti-MM agents: “Penta-refractory” (refractory to 1 CD38 MoAB + 2 PIs + 2 IMiDs), “triple-refractory and quad-refractory” (refractory to 1 CD38 MoAB + 1 PI + 1 or 2 IMiDs, or 1 CD38 MoAB + 1 or 2 PIs + 1 IMiD), and “not triple-refractory” (refractory to 1 CD38 MoAB, and not both of a PI and an IMiD). Responses were evaluated using the IMWG criteria. Overall response rate (ORR) was calculated by combining rates of partial response (PR) and ≥ very good partial response (VGPR).
We compared categorical variables between two or more groups utilizing chi-square test and continuous variables utilizing the Kruskal-Wallis non-parametric test. We described OS using the method of Kaplan and Meier for the entire population and for subgroups of patients. OS time was measured from T0 until death or last follow up. For the subset of patients who underwent further therapy after T0, we also analyzed response to subsequent therapy and progression-free survival (PFS), here defined as the time between the onset of the next line of therapy and disease progression or death. Comparisons of OS and PFS between groups were performed using log-rank test.
We subsequently explored patient-, disease-and treatment features influencing OS by performing Cox proportional hazards model multivariable analysis. Variables considered in this analysis were age, sex, cytogenetic abnormalities, refractoriness subset (as defined above), specific CD38 MoAB in the index regimen (isatuximab vs. daratumumab), renal function, and LDH at onset of index regimen. Variables were chosen using a stepwise forward selection process with a probability of entry of 0.05 and a probability of removal of 0.10. Formal testing revealed that predictors included in Cox proportional hazards models satisfied the assumption of proportionality. For the subset of patients who underwent additional therapy after T0, we performed binary logistic multivariate regression to study factors influencing the likelihood of objective response and Cox proportional hazards model multivariable analysis to explore factors affecting PFS. In addition to the variables cited above, we also evaluated the impact of different classes or agents and combinations utilized in the first regimen post-T0. A two-sided p-value ≤ 0.05 was considered statistically significant. All analyses were conducted using IBM SPSS (v22.0).
Results
Two hundred and seventy-five patients were included in this study; median age at T0 was 65 years (range 27–90) and 55% patients were male. Median interval from diagnosis of MM to T0 was 50.1 months (range 2.5–230.1). Patients received a median of 4 lines of therapy (range 1–16) prior to the index regimen; 72% underwent prior autologous hematopoietic cell transplantation (AHCT). None of the patients received CD38 MoAB as their first-line of therapy. Daratumumab-refractory patients constituted the majority (93%) of this cohort. Most of the patients were refractory to lenalidomide (77%), pomalidomide (65%), and bortezomib (68%). Seventy patients (25%) were “penta-refractory” and 148 (54%) were “triple-refractory and quad-refractory” (Table 1).
Table 1-.
Characteristics of Patients
| All | Non-triple refractory |
Triple and quad refractory |
Penta refractory | |
|---|---|---|---|---|
| N=275 | N=57 | N=148 | N=70 | |
| Age (years)* | 65 (27–90) | 63 (24–74) | 60 (23–85) | 58.5 (35–76) |
| Male Gender | 152 (55.3%) | 28 (49.1%) | 85 (57.4%) | 39 (55.7%) |
| Race/ethnicity | ||||
| White | 202 (73.5%) | 39 (68.4%) | 116 (78.4%) | 47 (67.1%) |
| Black | 45 (16.4%) | 13 (22.8%) | 19 (12.8%) | 13 (18.6%) |
| Hispanic | 6 (2.2%) | 3 (5.3%) | 1 (0.7%) | 2 (2.9%) |
| Other | 22 (8.0%) | 2 (3.5%) | 12 (8.1%) | 8 (11.4%) |
| Immunoglobulin subtype | ||||
| IgG | 143 (52.0%) | 34 (59.7%) | 73 (49.3%) | 36 (51.5%) |
| IgA | 56 (20.4%) | 8 (14.1%) | 33 (22.3%) | 15 (21.4%) |
| Light chain | 67 (24.4%) | 13 (22.8%) | 38 (25.7%) | 16 (22.9%) |
| Other | 9 (3.3%) | 2 (3.5%) | 4 (2.7%) | 3 (4.3%) |
| ISS at diagnosis | ||||
| 1 | 69 (25.1%) | 12 (21.1%) | 40 (27.0%) | 17 (24.3%) |
| 2 | 84 (30.5%) | 20 (35.1%) | 40 (27.0%) | 24 (34.3%) |
| 3 | 80 (29.1%) | 20 (35.1%) | 47 (31.8%) | 13 (18.6%) |
| N.A. | 42 (15.3%) | 5 (8.8%) | 21 (14.2%) | 16 (22.9%) |
| Cytogenetic risk on most recent assessment | ||||
| Standard | 175 (63.6%) | 39 (68.4%) | 96 (64.9%) | 40 (57.1%) |
| High [t(4;14), t(14;16) or del17p] | 80 (29.1%) | 13 (22.8%) | 42 (28.4%) | 25 (35.7%) |
| N.A. | 20 (7.3%) | 5 (8.8%) | 10 (6.8%) | 5 (7.1%) |
| LDH at diagnosis | ||||
| > ULN | 40 (14.5%) | 8 (14.0%) | 25 (16.9%) | 7 (10.0%) |
| <= ULN | 121 (44.0%) | 24 (42.1%) | 66 (44.6%) | 31 (44.3%) |
| N.A. | 114 (41.5%) | 25 (43.9%) | 57 (38.5%) | 32 (45.7%) |
| Creatinine at diagnosis | ||||
| <= 2 mg/dL | 227 (82.5%) | 48 (84.2%) | 119 (80.4%) | 60 (85.7%) |
| > 2 mg/dL | 48 (17.5%) | 9 (15.8%) | 29 (19.6%) | 10 (14.3%) |
| LDH at start of index regimen | ||||
| > ULN | 54 (19.6%) | 11 (19.3%) | 27 (18.2%) | 16 (22.9%) |
| <= ULN | 103 (37.5%) | 26 (45.6%) | 50 (33.8%) | 27 (38.6%) |
| N.A. | 118 (42.9%) | 20 (35.1%) | 71 (48.0%) | 27 (38.6%) |
| Creatinine at start of index regimen | ||||
| <= 2 mg/dL | 250 (90.9%) | 50 (87.7%) | 135 (91.2%) | 65 (92.9%) |
| > 2 mg/dL | 22 (8.0%) | 6 (10.5%) | 11 (7.4%) | 5 (7.1%) |
| Number of lines of therapy prior to index regimen* | 4 (1–16) | 3 (1–6) | 4 (1–11) | 5 (2–16) |
| Prior autologous HCT | 198 (72.0%) | 47 (82.5%) | 104 (70.3%) | 47 (67.1%) |
| CD38 MoAB in index regimen | ||||
| Daratumumab | 256 (93.1%) | 50 (87.7%) | 138 (93.2%) | 68 (97.1%) |
| Isatuximab | 19 (6.9%) | 7 (12.3%) | 10 (6.8%) | 2 (2.9%) |
| Bortezomib exposed | 271 (98.6%) | 56 (98.2%) | 146 (98.6%) | 69 (98.6%) |
| Bortezomib refractory | 188 (68.4%) | 13 (22.8%) | 107 (72.3%) | 68 (97.1%) |
| Ixazomib exposed | 38 (13.9%) | 2 (3.6%) | 24 (16.2%) | 12 (17.1%) |
| Ixazomib refractory | 34 (12.4%) | 1 (1.8%) | 23 (15.5%) | 10 (14.3%) |
| Carfilzomib exposed | 178 (64.8%) | 25 (43.8%) | 85 (57.4%) | 68 (97.1%) |
| Carfilzomib refractory | 130 (47.3%) | 6 (10.5%) | 57 (38.5%) | 67 (95.7%) |
| Thalidomide exposed | 55 (20.0%) | 6 (10.6%) | 26 (17.6%) | 23 (32.9%) |
| Thalidomide refractory | 23 (8.4%) | 3 (5.3%) | 6 (4.1%) | 14 (20.0%) |
| Lenalidomide exposed | 270 (98.2%) | 54 (94.7%) | 146 (98.6%) | 70 (100%) |
| Lenalidomide refractory | 211 (76.7%) | 25 (43.9%) | 117 (79.1%) | 69 (98.6%) |
| Pomalidomide exposed | 189 (68.7%) | 29 (50.9%) | 91 (61.5%) | 69 (98.6%) |
| Pomalidomide refractory | 179 (65.1%) | 23 (40.4%) | 87 (58.8%) | 69 (98.6%) |
| CD38 MoAb monotherapy as index regimen | 120 (43.6%) | 31 (54.4%) | 67 (45.3%) | 22 (31.4%) |
| Penta-exposed (1 CD38 MoAB, 2 IMiD, 2 PI ) | 157 (57.1%) | 17 (29.8%) | 70 (47.3%) | 70 (100%) |
| Time from diagnosis to T0 (years)* | 4.5 (0.4–19.4) | 3.8 (1.0–12.7) | 4.4 (0.4–19.4) | 5.7 (0.6–14.4) |
| Duration of index regimen (months)* | 3.1 (0.5–27.2) | 3.6 (0.7–27.4) | 3.1 (0.5–18.4) | 2.9 (0.5–12.6) |
| Best response on index regimen | ||||
| PD | 77 (28.0%) | 12 (21.1%) | 38 (25.7%) | 27 (38.6%) |
| SD | 115 (41.8%) | 26 (45.6%) | 64 (43.2%) | 25 (35.7%) |
| PR | 63 (22.9%) | 16 (28.1%) | 33 (22.3%) | 14 (20.0%) |
| VGPR | 17 (6.2%) | 1 (1.8%) | 12 (8.1%) | 4 (5.7%) |
| CR/sCR | 1 (0.4%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) |
| N.A | 2 (0.7%) | 1 (1.8%) | 1 (0.7%) | 0 (0.0%) |
Median (range); N.A.= not available
Natural history of CD38 MoAb RRMM
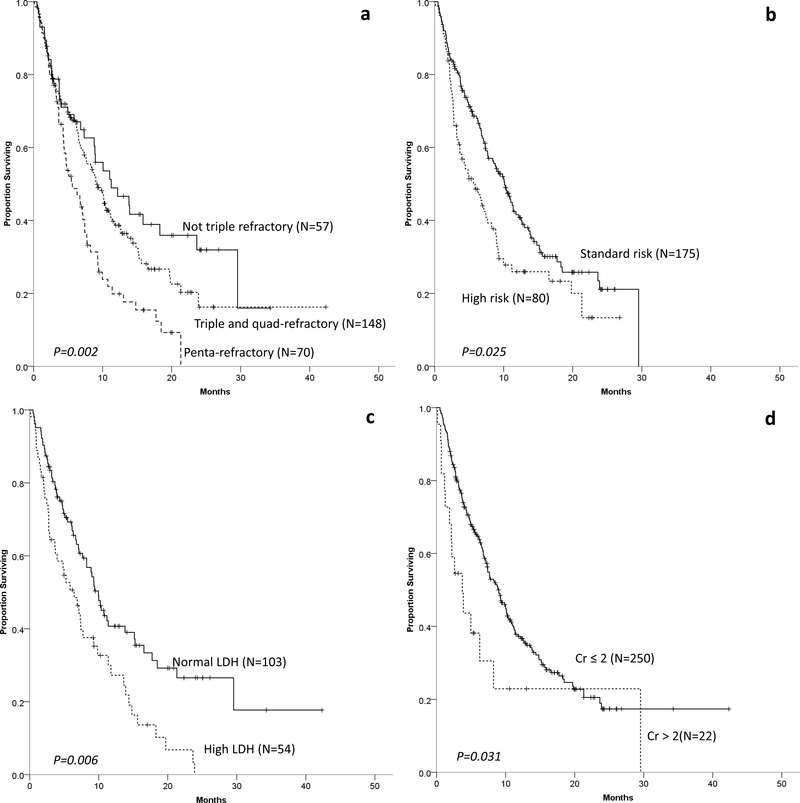
Median follow-up (from T0) of survivors was 10.6 months (range 1.9–42.3 months). The median OS (mOS) from T0 for the entire cohort was 8.6 months (95% C.I. 7.2–9.9). OS according to refractoriness group is shown in Figure 1a. mOS was 11.2 months (95% C.I. 5.4–17.1) for “not triple-refractory” group, 9.2 months (95% C.I. 7.1–11.2) for “triple-and quad-refractory” and 5.6 months (95% C.I. 3.5–7.8) for “penta-refractory” groups.
Figure 1–
OS of MM patients refractory to CD38 MoAB according to refractoriness to PIs and IMiDs (panel a), cytogenetic risk (panel b), LDH (panel c) and renal function (panel d) at time of initiation of index regimen.
OS was also affected by cytogenetic risk (median, 10.1 months for standard-risk vs. 5.6 months for high-risk, Figure 1b); LDH (median, 10.0 months for normal vs. 6.4 months for elevated LDH, Figure 1c) and renal function (median, 9.0 months for creatinine ≤ 2 mg/dL vs. 3.7 months for creatinine > 2 mg/dl, Figure 1d) recorded at the start of index regimen.
On multivariable analysis, disease refractoriness group, cytogenetic risk, LDH and renal function were associated with OS (Table 2). Age, sex, and CD38 MoAB in the index regimen (daratumumab vs. isatuximab) were not found to be predictive of OS.
Table 2-.
Univariate and multivariable analysis for factors affecting OS from T0
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% C.I.) | P | HR (95% C.I.) | P | |
| Refractoriness group | 0.002 | 0.001 | ||
| Not triple-refractory | 1.00 (Reference ) | 1.00 (Reference ) | ||
| Triple or quad-refractory | 1.31 (0.88–1.95) | 0.19 | 1.30 (0.86–1.95) | 0.21 |
| Penta-refractory | 2.10 (1.35–3.27) | 0.001 | 2.25 (1.44–3.53) | <0.001 |
| Cytogenetic risk on most recent assessment | 0.027 | 0.04 | ||
| Standard | 1.00 (Reference ) | 1.00 (Reference ) | ||
| High [t(4;14), t(14;16) or del17p] | 1.55 (1.13–2.14) | 0.007 | 1.51 (1.09–2.08) | 0.013 |
| N.A. | 1.24 (0.73–2.11) | 0.43 | 1.32 (0.78–2.27) | 0.30 |
| Creatinine at start of index regimen | 0.036 | 0.002 | ||
| ≤ 2 mg/dL | 1.00 (Reference ) | 1.00 (Reference ) | ||
| > 2 mg/dL | 1.98 (1.18–3.33) | 0.01 | 2.42 (1.42–4.11) | 0.001 |
| N.A. | 1.00 (0.25–4.04) | 0.99 | 1.15 (0.28–4.73) | 0.84 |
| LDH at start of index regimen | 0.007 | 0.001 | ||
| Normal | 1.00 (Reference ) | 1.00 (Reference ) | ||
| Increased | 1.86 (1.26–2.75) | 0.002 | 2.01 (1.36–2.97) | <0.001 |
| N.A. | 1.22 (0.87–1.72) | 0.25 | 1.30 (0.92–1.85) | 0.16 |
Outcomes of subsequent therapy
Most patients (249, 90%) received at least one line of therapy after T0, (median 2, range 1–10) and their clinical characteristics were similar to the larger group (Supplemental Table 1). Among patients who received ≥ 1 subsequent treatment, the median PFS (mPFS) and mOS from T0 were 3.4 months (95% C.I. 2.8–4.0) and 9.3 months (95% C.I. 8.1–10.6) respectively; the mOS for the 26 patients who received no further treatment was only 1.3 (95% C.I. 0.6–1.9) months (i.e. 39 days).
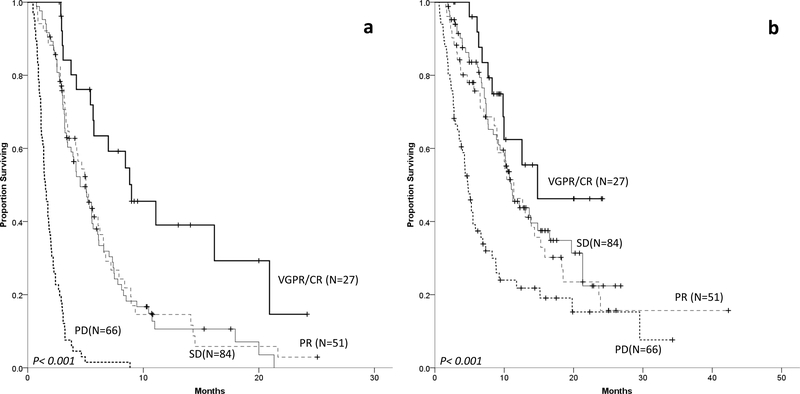
Among the 249 patients treated beyond T0, 47% had an objective response to at least one of the subsequent therapies. The ORR was 31% to the first regimen after T0, including an ORR of 38% for patients “not triple-refractory”, 29% for “triple-or quad-refractory” and 30% for “penta-refractory”. ORR declined with each subsequent regimen, reaching 18% with the 5th subsequent line (Supplemental Table 2). Depth of response to the line of therapy after T0 was predictive of response duration and survival. The mPFS and mOS for patients achieving ≥VGPR were 9 and 14.8 months, 5.1 and 11.4 months for patients achieving PR, 4.5 and 11.9 months for patients with stable disease (SD), and 1.5 and 4.8 months for patients with PD, respectively (Figure 2).
Figure 2–
OS (panel a) and PFS (panel b) of MM patients refractory to CD38 MoAB therapy according to depth of response to next line of therapy.
Outcomes according to treatment choice
We intended to gain insight into the efficacy of specific MM agents and their combinations after failure of CD38 MoAB as well as the merit of re-treatment with CD38 MoAB in combination with other classes of drugs. Therefore, we grouped the cohort of 249 patients who had received at least one line of therapy post-T0 into categories according to the agent or combination of agents used in the first subsequent line of therapy to report key characteristics and therapeutic outcomes (Table 3).
Table 3-.
Patient Characteristics and outcomes of specific regimens* at next line after T0
| All regimens | Any Daratumumab | Daratumumab + IMiD | Daratumumab + PI | Elotuzumab + IMiD | Any Carfilzomib | Carfilzomib + IMiD | Carfilzomib + Alkylator | Any Alkylator | “PACE”-like | Bendamustine | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N=249 | N=57 | N=41 | N=13 | N=19 | N=68 | N=34 | N=19 | N=90 | N=24 | N=15 | |
| Characteristics | |||||||||||
| High risk FISH | 71 (28.5%) | 16 (28.1%) | 11 (26.8%) | 5 (38.5%) | 3 (15.8%) | 16 (23.2%) | 7 (20.0%) | 6 (31.6%) | 34 (37.8%) | 8 (33.3%) | 6 (40.0%) |
| Prior lines# | 5 (2–17) | 5 (2–17) | 5 (2–9) | 5 (2–17) | 4 (2–10) | 4 (2–9) | 4 (2–7) | 4 (2–9) | 5 (2–12) | 4.5 (3–9) | 6 (4–12) |
| ≥Triple-refractory | 197 (79.1%) | 49 (86.0%) | 33 (80.5%) | 12 (92.3%) | 18 (94.7%) | 43 (62.3%) | 19 (54.3%) | 14 (73.7%) | 77 (85.6%) | 21 (87.5%) | 15 (100%) |
| Penta-refractory | 63 (25.6%) | 9 (15.8%) | 3 (7.3%) | 5 (38.5%) | 6 (31.6%) | 8 (11.6%) | 2 (5.9%) | 2 (10.5%) | 29 (32.2%) | 5 (20.8%) | 6 (40.0%) |
| Carfilzomib-refractory | 116 (46.6%) | 25 (43.9%) | 17 (41.5%) | 4 (30.8%) | 13 (68.4%) | 10 (14.7%) | 2 (5.9%) | 2 (10.5%) | 49 (54.4%) | 14 (58.3%) | 9 (60.0%) |
| Pomalidomide-refractory | 162 (65.1%) | 33 (57.9%) | 23 (56.1%) | 9 (69.2%) | 13 (68.4%) | 38 (55.9%) | 18 (52.9%) | 12 (63.2%) | 69 (76.7%) | 18 (75.0%) | 13 (86.7%) |
| Carfilzomib and Pomalidomide-refractory | 89 (35.7%) | 17 (29.8%) | 10 (24.4%) | 4 (30.8%) | 10 (52.6%) | 8 (11.8%) | 1 (2.9%) | 2 (10.5%) | 43 (47.8%) | 11 (45.8%) | 8 (53.3%) |
| Response to next line of therapy after T0 | |||||||||||
| PD | 66 (26.5%) | 19 (33.3%) | 10 (24.4%) | 7 (53.8%) | 5 (26.3%) | 16 (23.5%) | 6 (17.6%) | 5 (26.3%) | 18 (20.0%) | 5 (20.8%) | 6 (40.0%) |
| SD | 84 (33.7%) | 22 (38.6%) | 17 (41.5%) | 4 (30.8%) | 6 (31.6%) | 23 (33.8%) | 13 (38.2%) | 4 (21.1%) | 26 (28.9%) | 6 (25.0%) | 4 (26.7%) |
| PR | 51 (20.5%) | 9 (15.8%) | 8 (19.5%) | 0 (0.0%) | 4 (21.0%) | 11 (16.2%) | 5 (14.7%) | 4 (21.1%) | 25 (27.8%) | 8 (33.3%) | 3 (20%) |
| VGPR | 22 (8.8%) | 5 (8.8%) | 5 (12.2%) | 0 (0.0%) | 0 (0.0%) | 10 (14.7%) | 5 (14.7%) | 4 (21.1%) | 11 (12.2%) | 2 (8.3%) | 1 (6.7%) |
| CR/sCR | 5 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.5%) | 1 (2.9%) | 1 (5.3%) | 4 (4.4%) | 1 (4.2%) | 1 (6.7%) |
| ORR | 78 (31.3%) | 14 (24.6%) | 15 (36.6%) | 0 (0.0%) | 4 (21.1%) | 22 (32.3%) | 11 (32.4%) | 9 (47.4%) | 40 (44.4%) | 11 (45.8%) | 5 (33.3%) |
| N.A | 15 (6.0%) | 2 (3.5%) | 1 (2.4%) | 2 (15.4%) | 4 (21.0%) | 7 (10.3%) | 4 (11.8%) | 1 (5.9%) | 6 (6.7%) | 1 (5.6%) | 0 (0.0%) |
| PFS and OS [in months (95% C.I.)] on next line after T0 | |||||||||||
| PFS | 3.4 (2.8–4.0) | 3.9 (2.7–5.1) | 4.5 (2.8–6.3) | 1.8 (1.2–2.5) | 2.6 (1.1–4.1) | 4.2 (2.3–6.2) | 4.1 (3.1–5.1) | 5.7 (1.6–9.7) | 3.2 (2.9–3.5) | 3.0 (2.5–3.4) | 3.2 (2.3–4.1) |
| OS | 9.31 (8.1–10.6) | 11.4 (8.8–14.0) | 12.6 (8.5–16.6) | 7.7 (0.0–19.6) | 8.3 (1.9–14.6) | 10.9 (9.5–12.4) | 11.2 (8.9–13.6) | 12.7 (5.9–19.5) | 7.7 (4.9–10.5) | 5.9 (2.6–9.1) | 9.3 (5.0–13.6) |
Regimen categories are not mutually exclusive, # median (range); N.A. = not available; PACE = cisplatin, adriamycin, cyclophosphamide and etoposide.
Alkylator (excluding high dose melphalan conditioning)-(n=90), carfilzomib-(n=68), and daratumumab-based (n=57) regimens were most commonly used as first subsequent lines of therapies post-T0: these groups were not mutually exclusive. Carfilzomib-based regimens (N=68) resulted in ORR of 32% with mPFS of 4.2 months and mOS of 10.9 months; carfilzomib + alkylator combination (n=19) yielded an ORR of 47%, with mPFS 5.7 months and mOS 12.7 months. Multivariable analysis showed that the PFS of carfilzomib-based regimens was significantly better than non-carfilzomib-based regimens (HR 0.60, p=0.004; Supplementary Figure 1). Alkylator-based regimens resulted in ORR of 44%, while the mPFS and mOS were 3.2 months and 7.7 months, respectively.
The combination of daratumumab + IMiD (n=41) yielded ORR of 37% with mPFS of 4.5 months and mOS of 12.6 months despite these patients being refractory to a prior CD38 MoAB-based regimen. However, there were no responses seen among those who received daratumumab + PI as the subsequent post-T0 regimen. We found that patients progressing on an index regimen of single-agent CD38 MoAB had mPFS of 5.1 months when subsequently treated with a daratumumab combination, compared with a mPFS of 1.9 months for patients who received CD38 MoAB in a combination therapy as the index regimen (Supplementary Figure 2). Use of elotuzumab-based treatment (n=19) resulted in a mPFS 2.6 months and mOS 8.3 months.
In order to adjust for intrinsic differences between the groups in terms of risk factors and prior therapeutic exposures, we performed multivariable analysis for attainment of endpoints of objective response and PFS on first therapy after T0. Interestingly, high cytogenetic risk (OR = 0.14, 95% C.I. 0.03–0.65) and alkylator-based regimen (OR=3.14, 95% C.I. 1.75–5.66) were the only factors predictive of objective response. With regards to PFS, after adjustment for LDH level at start of index regimen and refractoriness group, the use of carfilzomib-based therapy (HR 0.60, 95% C.I. 0.42–0.85) and daratumumab + IMiD combination regimens (HR 0.64, 95% C.I. 0.43–0.94) were associated with reduction in risk of progression or death.
Discussion
To our knowledge, this is the first study to investigate the natural history and outcomes in a cohort of MM patients with refractoriness to a CD38 MoAB-based line of treatment. Given the multicenter design, this study captures a cohort representative of the currently evolving state of RRMM, with patients who have not only progressed on multiple lines of treatments including PIs, IMiDs, or high-dose alkylating chemotherapy/AHCT, but also became refractory to a novel class of highly active agents, the CD38 MoABs. Based on our analysis, the mOS of patients refractory to CD38 MoAB was 8.6 months, while the mOS of our cohort of “penta-refractory” patients was a dismal 5.6 months. The mPFS of those who receive at least one subsequent line of therapy was 3.4 months, with objective responses seen in less than half of those patients. Interestingly, outcomes of subsequent therapy point towards a potential benefit with certain agents and combinations.
In a recent multicenter, retrospective study, Kumar et al reported outcomes on patients with MM diagnosed since 2006, who had received at least three prior lines of therapy, were refractory to both IMiD and a PI, and had been exposed to an alkylating agent 5. More than half of their patients were from Europe (318/543), and only a small proportion had received monoclonal antibody therapy. In that cohort, the median time from diagnosis to T0 was 37.2 months and the mOS of their entire cohort was 13 months. In our study, the median time from diagnosis to T0 was longer at 50.1 months, and the mOS of the entire cohort was 8.6 months. To some extent, this might reflect the improvement in outcomes conferred by the availability of CD38 MoABs as a treatment option in past few years, but invariably exposes the limited subsequent treatment options and worse outcomes after progression on CD38 MoAB. It is interesting to note that while the “penta-refractory” patients have extremely poor outcomes with mOS of <6 months, the group of patients who are “not triple-refractory” also tend to fare poorly with mOS not reaching even one year. This implies that once patients progress on a CD38 MoAB-based regimen, their prognosis is unfavorable even if they might still be responsive to a PI or IMiD. The MAMMOTH study results, thus, establish a contemporary benchmark for comparison of the outcomes of forthcoming clinical trials of newer agents and combinations.
The analysis of outcomes from treatments subsequent to progression on CD38 MoABs (post-T0) yielded intriguing findings. Alkylator-based regimens, the most-widely employed first subsequent treatment-, had high overall response rates, but these responses were not durable. On the other hand, carfilzomib-based regimens resulted in superior PFS and OS outcomes, especially when combined with an alkylator or an IMiD. Such relatively favorable outcomes with carfilzomib-based treatment may partially be explained by the fact that only 14.7% of those patients were carfilzomib refractory as compared to 46.6% in the entire population (Table 3). Nevertheless, these findings warrant further investigation of carfilzomib-based combination regimens in CD38 MoAB-refractory population, particularly if no prior carfilzomib refractoriness.
The combination of daratumumab-IMiD might be particularly relevant for those who progress on single-agent CD38 MoAB. In fact, we observed that median PFS of patients of patients treated with daratumumab combination after progressing on single-agent CD38 MoAb was 5.1 months (driven mostly by results of combination of daratumumab and IMiD), compared with 1.9 months for those who had received a CD38 MoAb-combination as their index regimen. These findings corroborate well with the results from a single-center, retrospective study that reported response rate of 35% with daratumumab-pomalidomide-dexamethasone in patients refractory to single-agent daratumumab 18. These results further justify the need for investigating the CD38 MoAB-IMiD combination in CD38 MoAB-refractory (different or same CD38 MoAB; IMiD dictated by prior patient experience) patients. Of note, the MMY1001 study that led to the FDA approval of daratumumab-pomalidomide-dexamethasone combination included only daratumumab-naïve patients 19.
Due to the retrospective and observational design, the patient population is quite heterogeneous. While a cox-regression analysis was used to adjust for this, it is possible the patient heterogeneity had some uncompensated impact on the analysis. The relatively small number of patients in most of the post-T0 treatment subsets also forms a major limitation of the study. Similarly, isatuximab-refractory patients represent less than 10% of our study population. Also, the results of the study may not be generalizable as restricting the study population to the patients from major academic centers in the US can potentially reflect a referral bias and as a result, the real-world outcomes may be different than found in the study. Finally, the survival patterns observed in this study are likely to evolve as these agents are used earlier in the disease course including as part of upfront therapy20–22.
In summary, the present study highlights a new and important challenge in MM therapy for finding strategies that improve the survival of CD38 MoAB-refractory patients. There is enthusiasm that novel immunotherapeutic approaches such as CAR-T cells 23, bispecific antibodies (BiTEs)24 and antibody-drug conjugates25 will provide clinical benefit in this challenging population and while that remains to be demonstrated in clinical trials, the MAMMOTH study population may serve as a contemporary control for the comparison of outcomes.
Supplementary Material
Acknowledgement
We are thankful to the Institutional Review Boards and hospital staff at each participating academic center, REDCap IT support at Vanderbilt University Medical Center, and all the patients and their families. This study was not funded commercially.
EhM: Consultancy and Speakers Bureau (Takeda, Celgene, Amgen, Janssen); BP: Stock and pension plan (Bristol Myer Squibb); AN: Consultancy (Celgene, Amgen); ML: Consultancy (Amgen/Onyx), Honoraria (Amgen/Onyx, Pfizer, Prothena, Takeda), Research Funding (Amgen/Onyx, BlueBirdBio, Celgene, Genentech/Roche, Gilead, Pfizer, Prothena, Takeda), Membership on an entity’s Board of Directors or advisory committees (Caelum, Pfizer, Prothena, Takeda); PH: Consultancy and Research Funding (Amgen, Celgene), Honoraria (Celgene); RV: Honoraria (Celgene, Bristol Myers Squibb, Takeda, Amgen, Janssen, Karyopharma, Jazz), Research Funding (Celgene, Bristol Myers Squibb, Takeda); SU: Consultancy (Abbvie, Amgen, Celgene, Genmab, Merck, MundiPharma, Seattle Genetics), Research Funding (Amgen, BMS, Celgene, Janssen, Merck, Pharmacyclics, Takeda), Membership on an entity’s Board of Directors or advisory committees (Sanofi); ShK: Consultancy (AbbVie, Celgene, Janssen, Kite Pharma, Merck, Takeda); LC: Honoraria (Amgen, Celgene, AbbVie), Research Funding (Amgen, Celgene, Janssen).
The work presented in this manuscript did not receive any financial or material support.
Footnotes
Conflicts of Interest
The remaining authors have no conflict of interest to disclose.
References
- 1.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012; 120: 947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014. May; 28(5): 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau P. How I treat myeloma with new agents. Blood 2017. September 28; 130(13): 1507–1513. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV, Kumar S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clinic proceedings 2016. January; 91(1): 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar SK, Dimopoulos MA, Kastritis E, Terpos E, Nahi H, Goldschmidt H, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia 2017. November; 31(11): 2443–2448. [DOI] [PubMed] [Google Scholar]
- 6.van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood 2018; 131: 13–29. [DOI] [PubMed] [Google Scholar]
- 7.Richter JR, Martin TG, Vij R, Cole C, Atanackovic D, Zonder JA, et al. Updated data from a phase II dose finding trial of single agent isatuximab (SAR650984, anti-CD38 mAb) in relapsed/refractory multiple myeloma (RRMM). Journal of Clinical Oncology 2016; 34(15_suppl): 8005–8005. [Google Scholar]
- 8.Martin T, Baz R, Benson DM, Lendvai N, Wolf J, Munster P, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood 2017; 129: 3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin TG, Mannis GN, Chari A, Munster P, Campana F, Hui A-M, et al. Phase Ib Study of Isatuximab and Carfilzomib in Relapse and Refractory Multiple Myeloma. Blood 2016; 128: 2111–2111.27789435 [Google Scholar]
- 10.Mikhael J, Richardson PG, Usmani SZ, Raje NS, Bensinger W, Dubin F, et al. Final results of a phase Ib study of isatuximab (ISA) plus pomalidomide (Pom) and dexamethasone (dex) in relapsed/refractory multiple myeloma (RRMM). Journal of Clinical Oncology 2018; 36(15_suppl): 8038–8038. [Google Scholar]
- 11.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet (London, England) 2016. April 9; 387(10027): 1551–1560. [DOI] [PubMed] [Google Scholar]
- 12.Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017. August 24; 130(8): 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. New England Journal of Medicine 2016; 375(14): 1319–1331. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. New England Journal of Medicine 2016; 375(8): 754–766. [DOI] [PubMed] [Google Scholar]
- 15.Durie BGM, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia 2006. 07/20/online; 20: 1467. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Harousseau J-L, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011; 117: 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009. April; 42(2): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nooka AK, Joseph N, Boise LH, Gleason C, Kaufman JL, Lonial S. Clinical Efficacy of Daratumumab, Pomalidomide and Dexamethasone in Relapsed, Refractory Myeloma Patients: Utility of Retreatment with Daratumumab Among Refractory Patients. Blood 2016; 128: 492–492. [DOI] [PubMed] [Google Scholar]
- 19.Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017; 130: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar SK, Kapoor P, Laplant B, Muchtar E, Buadi FK, Gonsalves WI, et al. Phase 2 Trial of Ixazomib, Lenalidomide, Dexamethasone and Daratumumab in Patients with Newly Diagnosed Multiple Myeloma. Blood 2018; 132(Suppl 1): 304–304. [Google Scholar]
- 21.Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Phase 3 Randomized Study of Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Patients with Newly Diagnosed Multiple Myeloma (NDMM) Ineligible for Transplant (MAIA). Blood 2018; 132(Suppl 1): LBA-2-LBA-2. [Google Scholar]
- 22.Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med 2018. February 8; 378(6): 518–528. [DOI] [PubMed] [Google Scholar]
- 23.Raje NS, Berdeja J, Lin Y, Munshi NC, Spigel D, Liedtke M, et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: Updated results from a multicenter phase I study. J Clin Oncol 36, 2018. (suppl; abstr 8007) 2018. [Google Scholar]
- 24.Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Treatment with AMG 420, an Anti-B-Cell Maturation Antigen (BCMA) Bispecific T-Cell Engager (BiTE®) Antibody Construct, Induces Minimal Residual Disease (MRD) Negative Complete Responses in Relapsed and/or Refractory (R/R) Multiple Myeloma (MM) Patients: Results of a First-in-Human (FIH) Phase I Dose Escalation Study. Blood 2018; 132(Suppl 1): 1010–1010. [Google Scholar]
- 25.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol 2018. December; 19(12): 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.