Abstract
Long non-coding RNAs (lncRs), by virtue of their versatility and multilevel gene regulation, have emerged as attractive pharmacological targets for treating heterogenous and complex malignancies like triple-negative breast cancer (TNBC). Despite multiple studies on lncRNA functions in tumor pathology, systemic targeting of these “undruggable” macromolecules with conventional approaches remains a challenge. Here, we demonstrate effective TNBC therapy by nanoparticle-mediated RNAi of the oncogenic lncRNA DANCR, which is significantly overexpressed in TNBC. Tumor-targeting RGD-PEG-ECO/siDANCR nanoparticles were formulated via self-assembly of multifunctional amino lipid ECO, cyclic RGD peptide-PEG, and siDANCR for systemic delivery. MDA-MB-231 and BT549 cells treated with the therapeutic RGD-PEG-ECO/siDANCR nanoparticles exhibited 80–90% knockdown in the expression of DANCR for up to 7 days, indicating efficient intracellular siRNA delivery and sustained target silencing. The RGD-PEG-ECO/siDANCR nanoparticles mediated excellent in vitro therapeutic efficacy, reflected by the significant reduction in the invasion, migration, survival, tumor spheroid formation, and proliferation of the TNBC cell lines. At the molecular level, functional ablation of DANCR dynamically impacted the oncogenic nexus by downregulating PRC2-mediated H3K27-trimethylation and Wnt/EMT signaling, and altering the phosphorylation profiles of several kinases in the TNBC cells. Furthermore, systemic administration of the RGD-PEG-ECO/siDANCR nanoparticles at a dose of 1 mg/kg siRNA in nude mice bearing TNBC xenografts resulted in robust suppression of TNBC progression with no overt toxic side-effects, underscoring the efficacy and safety of the nanoparticle therapy. These results demonstrate that nanoparticle-mediated modulation of onco-lncRNAs and their molecular targets is a promising approach for developing curative therapies for TNBC and other cancers.
Keywords: Targeted ECO/siRNA nanoparticles, systemic therapy, lncRNA, DANCR, TNBC
Graphical Abstract
Introduction
Triple-negative breast cancer (TNBC) is highly heterogenous with distinct molecular sub-types and refractory to endocrine and other targeted therapies.1 Although chemotherapy forms the mainstay of clinical intervention for TNBC, it is known to further exacerbate tumor heterogeneity, resulting in disease relapse, metastases, and drug resistance in most patients.2 In addition, conventional targeted therapies, including antibodies or small molecule inhibitors of oncoproteins, have met with limited success in achieving curative therapy for TNBC, due to the emergence of compensatory mitogenic pathways.3–4 The emerging consensus on the dynamic and evolving nature of triple-negative breast neoplasms has shifted the treatment paradigm to the development of new therapies against novel molecular targets that are associated with the complex biological characteristics of the disease.2, 5
Long non-coding RNAs (lncRNAs) have recently emerged as critical players in the development of multiple oncogenic processes, including EMT, inflammation, cancer stemness, metastasis, and drug-resistance.6–8 Aberrant overexpression of oncogenic lncRNAs (onco-lncRNAs) is disease- and tissue-specific, and can influence global gene signatures and clinical outcomes by regulating multi-level gene expression.9–10 The onco-lncRNA DANCR (Differentiation Antagonizing Non-Coding RNA)11 was recently implicated in the progression of multiple cancers, including gliomas,12–13 prostate,14 colorectal,15–16 gastric,17 liver,15, 18–19, breast,20 and ovarian21 cancers. With a broad spectrum of molecular targets and well-defined temporal and spatial expression,22 DANCR has the potential to overcome the limitations of single-target therapies and is a promising candidate for therapeutic targeting of multiple oncogenic TNBC networks with high efficacy and reduced risk of toxic side-effects in healthy tissues. Although DANCR was shown to be involved in TNBC,20 its potential as a therapeutic target for systemic treatment and its dynamic functions in TNBC biology need to be explored.
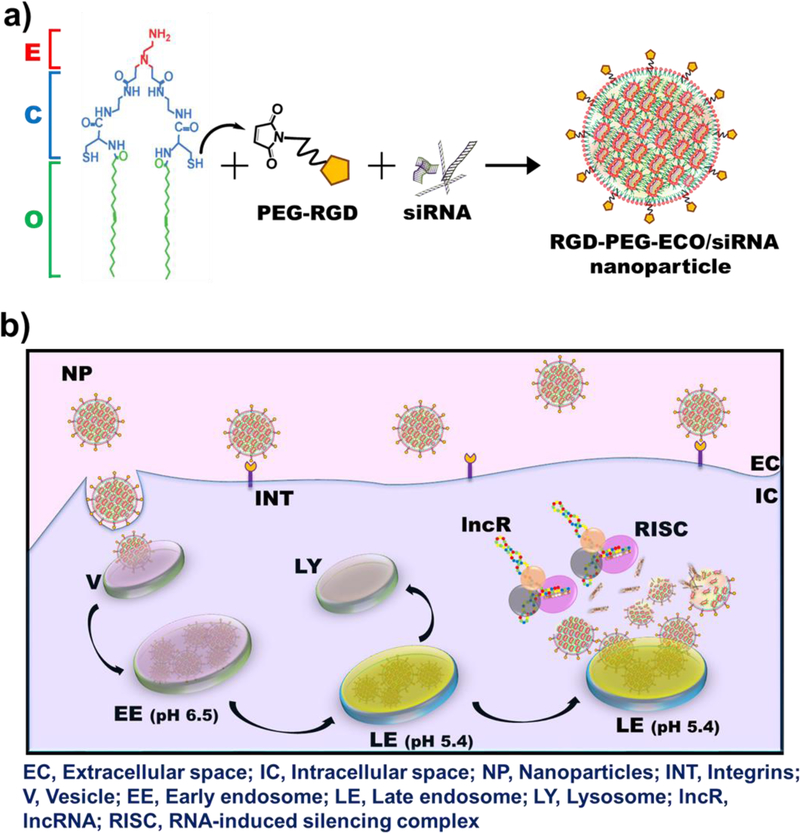
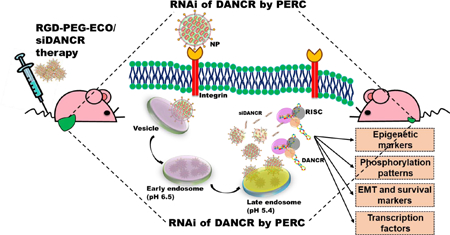
In addition, DANCR is an “undruggable” macromolecule that cannot be targeted with conventional pharmacological approaches, necessitating the use of RNA interference (RNAi). However, the primary challenge for RNAi therapy is specific delivery of therapeutic siRNA into the cytosol of target cells via systemic administration. The clinical translation of most non-viral siRNA delivery systems is impeded, particularly by their limited cellular uptake, low transfection efficiency, toxicity, and transient gene expression,23 underscoring the urgent need for a safe and efficient gene delivery platform for cancer therapy. To address these concerns, we implemented the multifunctional amino lipid carrier ECO, (1-aminoethyl)iminobis[N-oleicylcysteinyl-1-aminoethyl)propionamide], which can form stable self-assembly nanoparticles with therapeutic nucleic acids, including siRNA, DNA, and CRISPR/Cas.24–27 Fig. 1 illustrates the schematic for the formation and working of the nanoparticle system. ECO undergoes electrostatic complexation with siRNA to form ECO/siRNA nanoparticles that are further stabilized by the hydrophobic condensation of the oleic acid tails and auto-oxidation of cysteine residues to form disulfide bonds. The ECO/siRNA nanoparticles can also be functionalized by conjugation with polyethylene glycol (PEG) for improved biocompatibility and with cyclic RGD peptide for tumor targeting for in vivo gene delivery (Fig. 1a).25–26 The nanoparticles are known to mediate efficient cytosolic siRNA delivery and robust knockdown of target mRNAs through the PERC mechanism: pH-sensitive amphiphilic endosomal escape, followed by reductive dissociation and cytosolic release of the siRNA cargo, to enable effective RNAi (Fig. 1b).23, 28
Figure 1.
Schematic representation of formation and working of RGD-PEG-ECO/siRNA nanoparticles. (a) Amino lipid carrier ECO (E) is mixed with the targeting moiety RGD-PEG-Mal followed by electrostatic condensation with siRNA molecules. Oxidation of cysteine residues (C) to form disulfide bonds and hydrophobic condensation of the oleic acid tails (O) further stabilize the formation of RGD-PEG-ECO/siRNA nanoparticles. (b) RGD-PEG-ECO/siRNA nanoparticles mediate efficient siRNA delivery by the PERC mechanism. Nanoparticles are internalized into cells by receptor-mediated endocytosis and travel through the intracellular trafficking pathway. As the pH in the endosomes decreases, the pH-sensitive amphiphilicity of the nanoparticles enables them to induce endosomal membrane destabilization and endosomal escape into the cytosol. The nanoparticles then undergo reductive dissociation, releasing the siRNA cargo into the cytoplasm where it encounters the RNAi machinery to silence the target lncRNA.
Here, we demonstrate efficient and effective nanoparticle-mediated targeting of the onco-lncRNA DANCR for TNBC therapy. We established the significant overexpression of DANCR in human breast cancer (BCa) cells, tumors, and The Cancer Genome Atlas (TCGA) database. We then investigated DANCR as a therapeutic target for effective TNBC therapy in two cell lines and mouse models by silencing its expression using tumor-targeting RGD-PEG-ECO/siDANCR nanoparticles to facilitate efficient cytosolic delivery of siDANCR. Transfections of RGD-PEG-ECO/siDANCR nanoparticles showed significant and prolonged DANCR silencing and inhibited invasion and proliferation of TNBC cells. Systemic injections of the RGD-PEG-ECO/siDANCR nanoparticles led to suppression of TNBC proliferation in mice with high efficacy and no overt side-effects. We also investigated the mechanism of action of DANCR and observed its pleiotropic roles in regulating multiple cancer-associated signaling pathways in TNBC.
Results
DANCR is overexpressed in triple-negative breast cancer (TNBC)
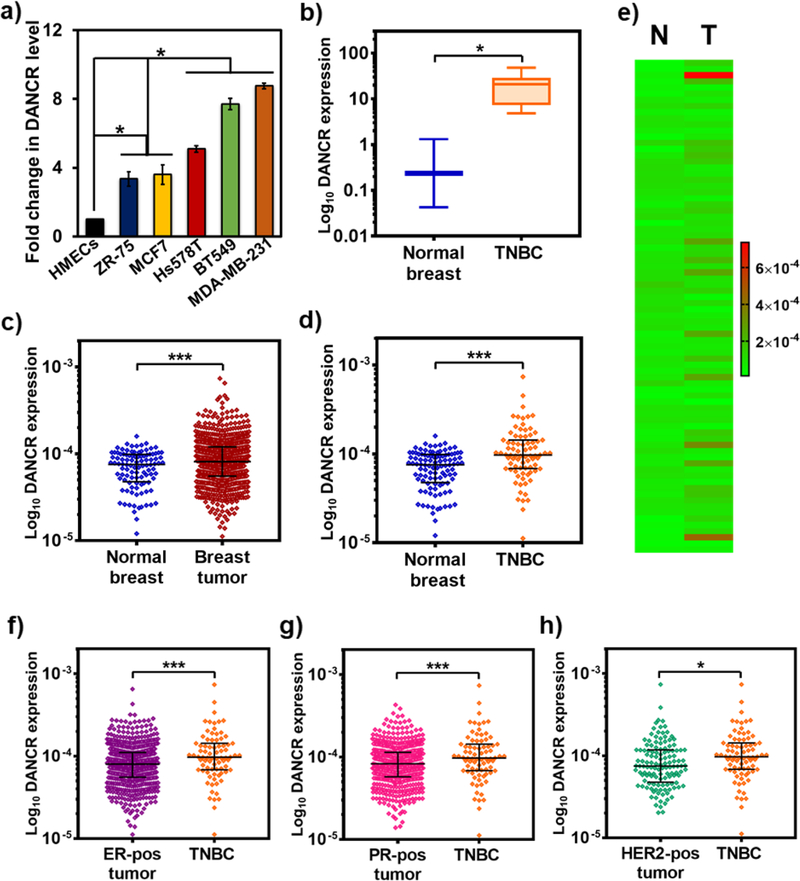
We examined the endogenous levels of DANCR in a wide range of human BCa cells, tumors, and healthy tissues. Using qRT-PCR, we compared the expression of DANCR in BCa cell lines with distinct molecular profiles: hormone receptor (HR)-positive MCF7 and ZR-75, and TNBC Hs578T, BT549, and MDA-MB-231 cells.29 Compared to normal human mammary epithelial cells (HMECs), the BCa cells showed significantly elevated (4–9 fold) levels of DANCR (Fig. 2a). Among the cancer lines, the TNBC cells (particularly, BT549 and MDA-MB-231) overexpressed DANCR more (2-fold) than the HR-positive cells. Next, we quantified DANCR levels using a cDNA array and found that DANCR is significantly upregulated (40-fold) in TNBC tumors than in normal breast tissues (Fig. 2b). Analysis of the RNA-Seq data from TCGA database for DANCR expression in 104 normal and 790 BCa samples showed consistent results, where DANCR was significantly overexpressed in BCa patients (Fig. 2c), and particularly in TNBC patients (Fig. 2d), compared to healthy controls. The heat map in Fig. 2e shows a diverse range of DANCR expression in the individual normal (N) and TNBC (T) patients, indicating the heterogeneity of the lncRNA. An additional comparison revealed higher DANCR expression in TNBC samples than in the ER+, PR+, and Her2+ tumors (1.34-fold, 1.33-fold, and 1.27-fold, respectively) (Fig. 2f–h). These results demonstrate that DANCR is substantially upregulated in BCa, and especially more so in TNBC.
Figure 2.
DANCR is significantly overexpressed in TNBC. (a) Endogenous expression of DANCR is significantly elevated in all breast cancer cell lines: MCF7 (luminal A; ER+, PR+/−, Her2−), ZR-75 (luminal B, ER+, PR+/−, Her2+), and Hs578T, BT549, and MDA-MB-231 (claudin-low ER−, PR−, Her2−), compared to HMECs, and even more so in the TNBC cell lines. (b) Quantification of DANCR expression from a breast cancer array containing cDNA from normal subjects (n=4) and breast cancer patients shows that DANCR is highly upregulated in tumor samples from TNBC patients (n=12), in comparison with breast tissues from normal subjects. (c) Differential gene expression analysis for DANCR performed for 104 normal and 790 breast cancer samples from TCGA database shows a significant upregulation of DANCR in breast cancer samples, particularly in 80 TNBC samples (d), compared to normal levels. (e) Heat map comparing and demonstrating the heterogeneity of DANCR expression in the individual normal (N) and TNBC (T) samples from TCGA database. DANCR expression is significantly higher in TNBC samples than breast cancer samples expressing (f) ER (n=584), (g) PR (n=508), and (h) HER2 (n=128) receptors (error bars denote s.e.m., *p<0.05, ***p<0.0005).
Targeted RGD-PEG-ECO/siDANCR nanoparticles facilitate robust and prolonged DANCR silencing in TNBC cells
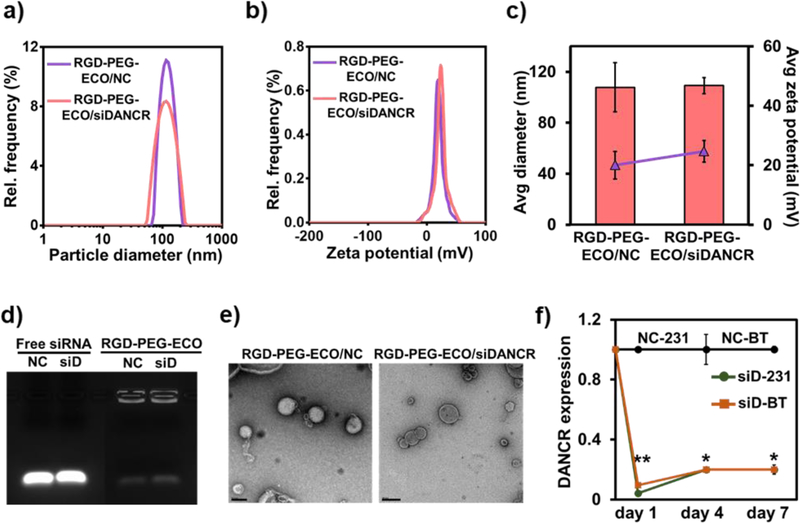
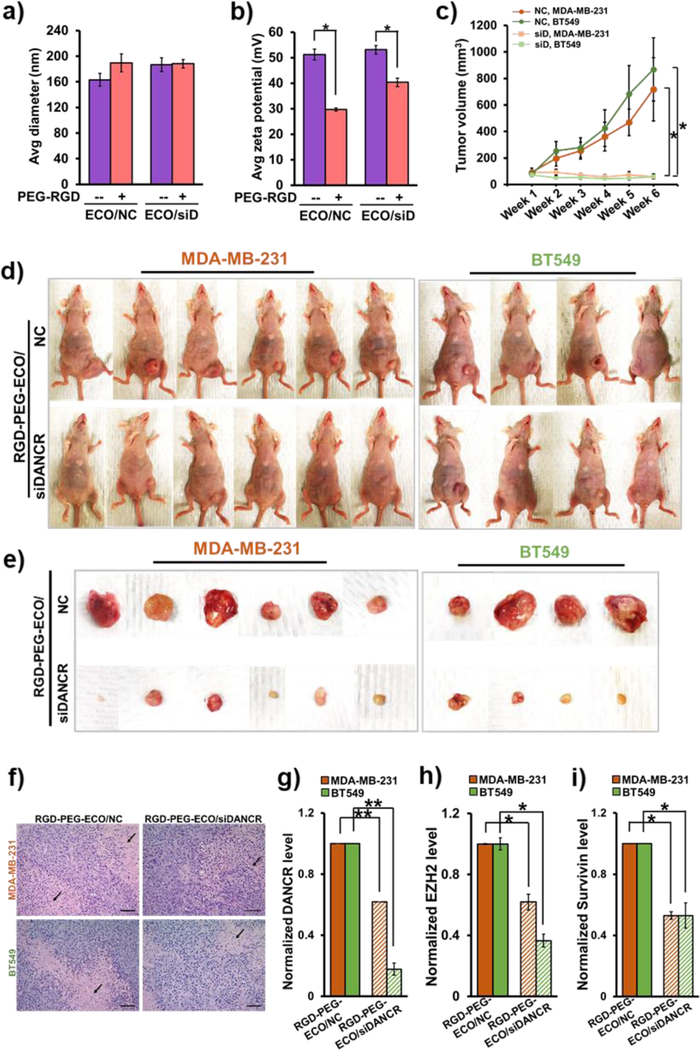
To effectively downregulate DANCR expression in TNBC, we formulated stable targeted nanoparticles using the lipid ECO and unmodified siDANCR via self-assembly. The nanoparticles were functionalized with cyclic RGD peptide via a PEG spacer (3,400 Da) for tumor targeting and improved biocompatibility during systemic delivery. These RGD-PEG-ECO/siDANCR nanoparticles mediate efficient cytosolic siRNA delivery and robust knockdown of target mRNAs through the PERC mechanism (Fig. 1).23, 28 RGD-PEG-ECO/siDANCR nanoparticles were formulated ([siRNA]=100 nM, N/P=10, RGD-PEG/ECO=2.5 mol%,) as previously described.25 Control RGD-PEG-ECO/NC (NC) nanoparticles were similarly prepared using siLuciferase as the non-specific siRNA (NC). The targeted nanoparticles possessed a narrow size and charge distribution, as characterized by dynamic light scattering (Fig. 3a,b). The RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles had a zeta potential of 20 ± 4.6 mV and 24.67 ± 3.6 mV and hydrodynamic diameter of 107.83 ± 19.28 nm and 109.12 ± 6.34 nm, respectively, Fig. 3c. The siRNA loading efficiency and encapsulation in the nanoparticles were evaluated by gel retardation assay, Fig. 3d. Both the nanoparticles demonstrated efficient siRNA encapsulation, with negligible free siRNA bands. The structure and morphology of the nanoparticles were visualized by transmission electron microscopy (Fig. 3e) and consistent sizes (ca. 100 nm) were observed.
Figure 3.
RGD-PEG-ECO/siDANCR nanoparticles mediate robust and sustained silencing of DANCR expression in MDA-MB-231 and BT549 cells. RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles were formulated at N/P = 10, RGD-PEG/ECO = 2.5 mol%, and [siRNA] = 100 nM and characterized on LiteSizer. The particles show (a) and (b) single intensity peaks indicating uniform distribution, with (c) predicted size of about 108 nm and zeta potential of 22 mV. (d) Gel retardation assay of RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles shows high encapsulation efficiency, with free siRNA as control. (e) Morphology of RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles is visualized by transmission electron microscopy. (f) MDA-MB-231 and BT549 cells transfected with RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles were harvested on days 1, 4, and 7, for measurement of DANCR knockdown by qRT-PCR. DANCR expression by siDANCR treatment is normalized to NC treatment for both the cell lines (scale bar = 200 nm, error bars denote s.e.m., **p<0.00005, *p<0.005).
The efficiency and kinetics of DANCR silencing by RGD-PEG-ECO/siDANCR nanoparticles were tested in MDA-MB-231 and BT549 cells with high DANCR expression on days 1, 4, and 7 post-treatment. Compared to the control, RGD-PEG-ECO/siDANCR nanoparticles induced 80–90% silencing of DANCR in both the cell lines for at least 7 days after a single transfection (Fig. 3f). These results demonstrate that RGD-PEG-ECO/siDANCR nanoparticles can facilitate efficient, robust, and prolonged silencing of DANCR in TNBC cells.
RGD-PEG-ECO/siDANCR nanoparticles suppress the invasion and proliferation of TNBC cells
To determine if DANCR depletion plays a role in TNBC suppression, we performed loss-of-function studies in MDA-MB-231 and BT549 cells treated with RGD-PEG-ECO/siDANCR and RGD-PEG-ECO/NC to evaluate functional changes. After confirming ~80% knockdown of DANCR expression after 24 h of siDANCR-nanoparticle treatment (Fig. 4a), the transfected cells were tested for their migratory and invasive capacity. The RGD-PEG-ECO/siDANCR-treated cells showed a significant reduction in their ability to invade Matrigel™-coated membranes in standard transwell assays compared to the control-treated cells, evidenced by the reduced number of migrated cells stained using crystal violet (Fig. 4b) and counted using ImageJ (Fig. 4c). The siDANCR-treated cells also showed a significant decrease in their ability to close the wounds scratched into a confluent monolayer, as compared to those treated with the control (Fig. 4d). These results demonstrate that DANCR downregulation inhibits the invasion and migration of TNBC cells.
Figure 4.
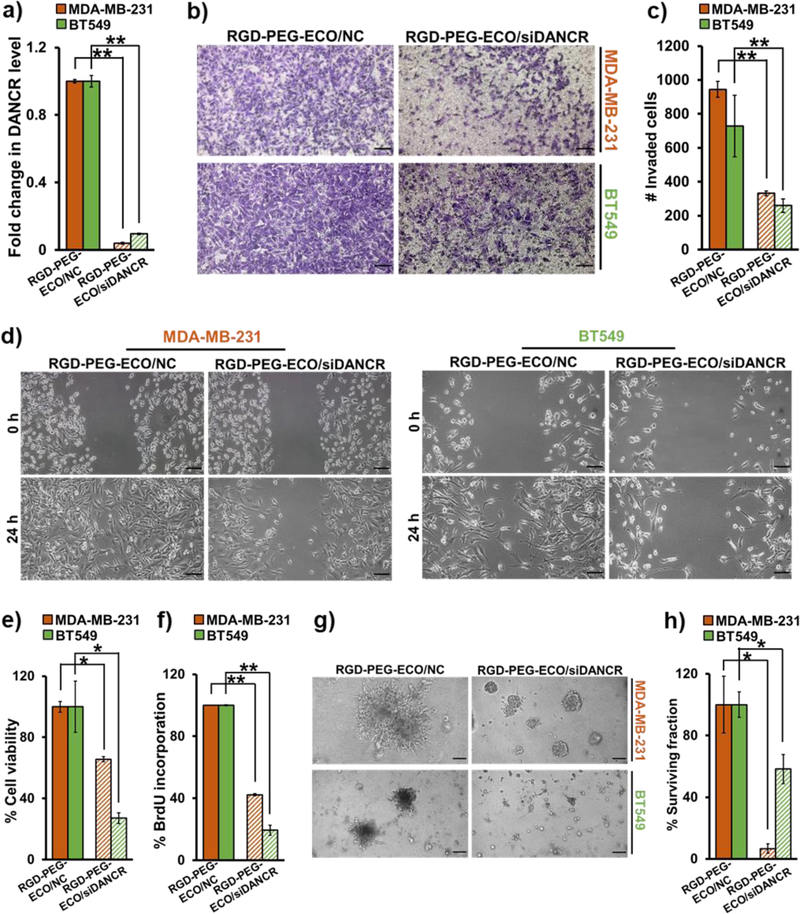
RGD-PEG-ECO/siDANCR nanoparticles inhibit TNBC invasion, migration, and proliferation. (a) siDANCR-treated MDA-MB-231 and BT549 cells show 80–90% knockdown in DANCR expression after 24 h, compared to NC-treated cells (**p<0.0005). (b) DANCR silencing leads to substantial reduction in the invasive potential of MDA-MB-231 and BT549 cells plated in Matrigel-coated Transwell inserts in serum-free media for 24 h. The migrated cells were fixed with 10% formalin, stained with 0.05% crystal violet, and counted using ImageJ software (**p<0.0005). (c & d) Standard scratch-wound assays demonstrate that siDANCR-treated MDA-MB-231 and BT549 cells have reduced ability to close the scratched wounds in 24 h, while the NC-treated cells readily migrate and close the wounds. (e) MTT assay demonstrates significant reduction in cell viability of MDA-MB-231 and BT549 cells treated with siDANCR nanoparticles, compared to NC (*p<0.01). (f) DANCR silencing in MDA-MB-231 and BT549 cells results in significant decrease in cell proliferation, as indicated by the decreased BrdU incorporation, in comparison to NC (**p<0.0001). (g) MDA-MB-231 and BT549 cells treated with RGD-PEG-ECO/siDANCR nanoparticles show considerable reduction in their ability to form 3D tumor spheroids, compared to NC-treated cells. (h) Clonogenic assay shows significant suppression of proliferative potential of MDA-MB-231 and BT549 cells treated with RGD-PEG-ECO/siDANCR nanoparticles, compared to NC nanoparticles (*p<0.01). Colonies formed after plating 2000 siDANCR- and NC-treated cells were stained with 0.01% crystal violet after 10 days, counted, and represented as % of survived colonies. Error bars denote s.e.m., scale bar = 100 μm.
Next, we determined the effect of RGD-PEG-ECO/siDANCR-mediated DANCR silencing on the survival, growth, and proliferative abilities of MDA-MB-231 and BT549 cells. The cells were treated with the nanoparticles for 48 h, and their viability was measured using the MTT assay. Compared to control, DANCR knockdown caused a significant decrease (30–70%) in the viability of both the cell lines (Fig. 4e), which was further validated by BrdU incorporation assay. RGD-PEG-ECO/siDANCR-treated cells showed significant reduction in BrdU incorporation (Fig. 4f), indicating that DANCR knockdown suppresses the proliferation of TNBC cells.
The effect of RGD-PEG-ECO/siDANCR treatment in MDA-MB-231 and BT549 cells was also assessed in 3D Matrigel culture. Silencing of DANCR significantly reduced the ability of the cells to form 3D tumor spheroids, as compared to control-treated cells (Fig. 4g), at 5 days post-treatment. Finally, the ability of single TNBC cells to grow and proliferate into colonies following DANCR silencing was evaluated by standard clonogenic assay. Control and siDANCR-treated cells were serially-diluted and allowed to grow for 10–15 days. Individual colonies formed were stained with 0.01% crystal violet and counted. As shown in Fig. 4h and Fig. S1, RGD-PEG-ECO/siDANCR-treated cells showed significant reduction of colonies than the NC-treated cells, indicating that loss of DANCR can inhibit the ability of TNBC cells to establish new colonies.
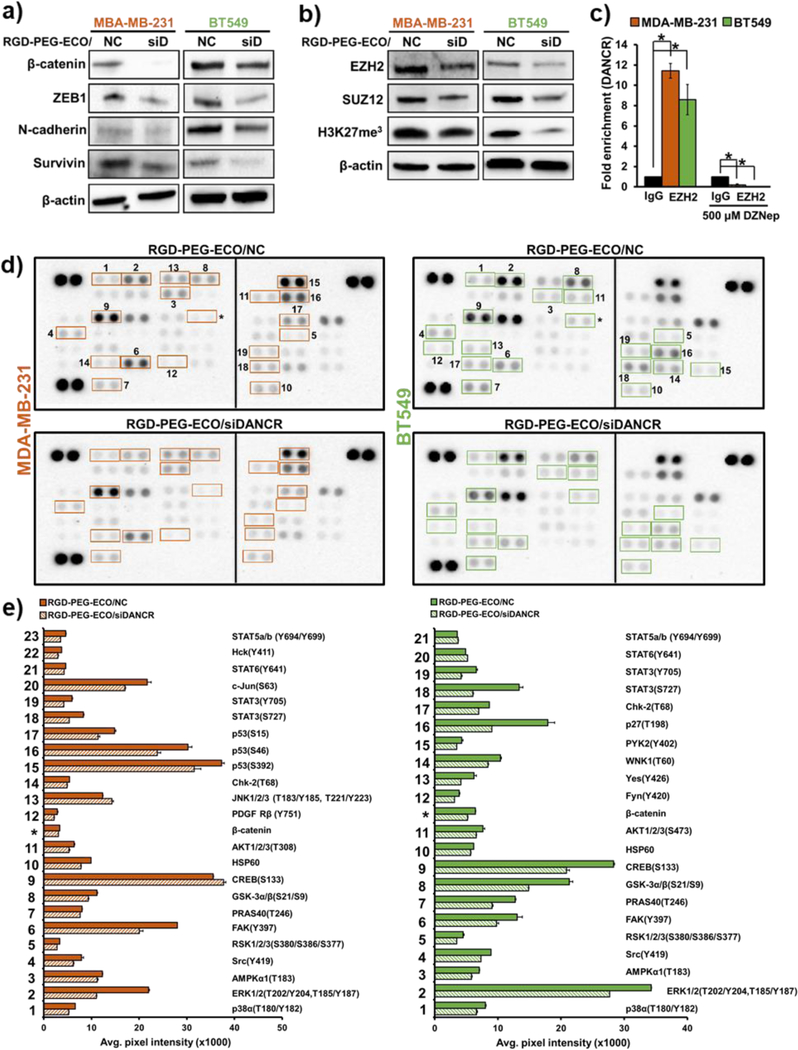
RGD-PEG-ECO/siDANCR nanoparticles suppress TNBC invasion and proliferation by influencing multiple oncogenic targets
The mechanism of biological functions of DANCR was investigated by evaluating the expression of multiple oncoproteins in MDA-MB-231 and BT549 cells treated with RGD-PEG-ECO/siDANCR or ECO-PEG-RGD/NC. These cell lines represent the claudin-low mesenchymal TNBC subtype, which is particularly aggressive, and is characterized by highly active EMT and Wnt signaling.3, 29 As shown in Fig. 5a, DANCR silencing downregulated the expression of the Wnt signaling protein β-catenin, the EMT markers ZEB1 and N-cadherin, as well as the anti-apoptotic marker survivin. DANCR is known to mediate its effects through EZH2-mediated epigenetic regulation.14, 20, 30 Since EZH2 is a part of Polycomb repressive complex 2 (PRC2) that facilitates target gene silencing through trimethylation of H3K27 residues,31 we tested the expression of EZH2 and SUZ12 in siDANCR-treated cells and found a significant decrease in the expression of the two proteins. This was also accompanied by a reduction in the total levels of H3K27-trimethylation (Fig. 5b), suggesting that DANCR may play a role in PRC2-mediated trimethylation and repression of target genes.
Figure 5.
RGD-PEG-ECO/siDANCR nanoparticles suppress the expression of proteins involved in multiple oncogenic pathways. (a) DANCR silencing in MDA-MB-231 and BT549 cells induces significant decrease in the expression of β-catenin (Wnt signaling); ZEB1 and N-cadherin (EMT markers); and the anti-apoptotic protein, survivin. β-Actin is used as the loading control. (b) RGD-PEG-ECO/siDANCR nanoparticle-mediated silencing of DANCR in MDA-MB-231 and BT549 cells downregulates the expression of the PRC2 complex proteins EZH2 and SUZ12, along with decreased H3K27 tri-methylation marks. (c) DANCR regulates PRC2 complex by directly binding to EZH2 in MDA-MD-231 and BT549 cells (*p<0.005). (d) Phospho-kinase arrays demonstrate the differential phosphorylation patterns of 43 kinases with treatment of RGD-PEG-ECO/siDANCR nanoparticles in MDA-MB-231 and BT549 cells. Proteins highlighted in the boxes show significant differences in phosphorylation status between NC and DANCR knockdown. (e) The bar graphs represent the corresponding kinases/proteins that show significant changes (p<0.05) in expression between control and DANCR silencing, calculated by comparing the mean pixel intensities of the duplicate spots.
Since siDANCR strongly inhibits PRC2 expression, we performed RNA immunoprecipitation (RIP) for EZH2. As shown in Fig. 5c, DANCR directly binds to EZH2 in both MDA-MB-231 and BT549 cells, showing a 12-fold and 8-fold enrichment with anti-EZH2 antibody pull-down, respectively. This enrichment was lost when the cells were treated an EZH2-inhibitor, DZNep. This direct binding coupled with the methylation changes suggests that DANCR influences the expression of its target genes by epigenetic control.
Because lncRNAs are known to play dynamic roles in multiple oncogenic signaling pathways,32 we evaluated the effect of DANCR silencing on molecular alterations in the TNBC phosphorylation network using a Phospho-kinase array. The functional status of most oncogenes and tumor suppressors is governed by post-translational modifications like phosphorylation, which are widely dysregulated in TNBC.33–34 Among these, the phosphorylation status of over 40 kinases expressed in MDA-MB-231 and BT549 cells were analyzed after treatment with RGD-PEG-ECO/siDANCR and RGD-PEG-ECO/NC, Fig. 5d and Fig. S2. The kinases that showed a significant change in phosphorylation with siDANCR treatment are highlighted by numbered boxes and depicted in Fig. 5e. A common subset of tumor-promoting kinases, including p38α, ERK1/2, AMPKα1, Src, RSK, FAK, and PRAS40 (#1–7), was significantly less phosphorylated with DANCR silencing in both cell lines.35–36
Interestingly, DANCR knockdown showed a differential phosphorylation pattern between the 2 TNBC cell lines, with reduced phosphorylation of 2 distinct residues of AKT1/2/3 (T308 in MDA-MB-231 vs S473 in BT549 cells, #11), contrasting S133 phosphorylation levels of CREB (#9), phosphorylation of JNK1/2/3 and PDGF-Rβ in MDA-MB-231 (#12,13) only, and phosphorylation of Fyn, Yes, WNK1, and PYK in BT549 (# 12–15) only. DANCR silencing reduced the total level of β-catenin (*) in both cell lines, independently validating the results in Fig. 5a, and also altered the phosphorylation patterns of tumor suppressors like p53, p27 and transcription factors (TFs) like Stat proteins. The endogenous kinase profiles and the siDANCR-mediated changes in these profiles are distinct between the two cell lines (Fig. S2), highlighting the heterogeneity of the disease and broad functions of the lncRNA. Our results underscore the dynamic and complex role of DANCR in impacting multiple oncogenic and tumor suppressor proteins in different TNBC models.
Systemic administration of RGD-PEG-ECO/siDANCR nanoparticles mediates effective therapy of TNBC in vivo
The efficacy of RGD-PEG-ECO/siDANCR nanoparticles in treating TNBC was investigated in mice bearing MDA-MB-231 and BT549 xenografts. For the in vivo experiments, RGD-PEG-ECO/siDANCR and RGD-PEG-ECO/NC nanoparticles were formulated at N/P=8, RGD-PEG/ECO = 2.5 mol%, and siRNA dose=1.0 mg/kg. Given the higher siRNA concentration, the nanoparticles were larger than those formed for in vitro transfections (Fig. 3), (average diameter 189.46 ± 14.3 nm and 188.11 ± 6.82 nm for RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles, respectively) (Fig. 6a). Compared to unmodified ECO/NC and ECO/siDANCR nanoparticles (51.15 ± 2.15 mV and 53.1 ± 1.7 mV), the RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles showed a reduction in the zeta potential (29.7 ± 0.6 mV and 40.35 ± 1.65 mV, respectively), indicating proper RGD-PEG conjugation (Fig. 6b). Mice were intravenously injected with RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles weekly for 6 weeks. The treatment with RGD-PEG-ECO/siDANCR resulted in a complete suppression of tumor proliferation and reduced tumor volumes (90.3 ± 18.77 to 59.79 ± 18.73 mm3 for MDA-MB-231 and 72.9 ± 14.75 to 58.79 ± 18 mm3 for BT549), while the RGD-PEG-ECO/NC-treated mice showed a rapid tumor growth (91.5 ± 32 to 718 ± 237 mm3 for MDA-MB-231 and 77.77 ± 17.4 to 868.1 ± 238.3 mm3 for BT549), Fig. 6c–e and Fig. S3.
Figure 6.
Systemic therapy of RGD-PEG-ECO/siDANCR nanoparticles significantly reduces primary tumor burden in TNBC xenografts. RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles were formulated at N/P = 8, PEG-RGD/ECO = 2.5 mol% and [siRNA] = 0.3 mg/mL, and characterized for (a) particle size and (b) zeta potential (*p<0.05). (c) Weekly intravenous injections of RGD-PEG-ECO/siDANCR nanoparticles at a siRNA dose of 1.0 mg/kg for 6 weeks result in significant reduction in tumor volumes (*p<0.05). MDA-MB-231 and BT549 xenografts (2 & 4 × 106 cells, respectively) were implanted into the mammary fat pads of athymic mice and treatment was started at average tumor volume of 70–90 mm3. (d) Compared to RGD-PEG-ECO/NC, RGD-PEG-ECO/siDANCR nanoparticles mediate significant inhibition of tumor progression. (e) Tumor status at the end of 6 weeks. (f) H&E staining of paraffin-fixed tumor samples from mice treated with RGD-PEG-ECO/siDANCR and NC (scale bar = 100 μm) shows large zonal necrosis (arrows) in NC but only focal necrosis with siDANCR treatment. Compared to RGD-PEG-ECO/NC-treated tumors, RGD-PEG-ECO/siDANCR-treated tumors demonstrate (g) significantly reduced DANCR levels (**p<0.005), and significantly lower mRNA expression of (h) EZH2 and (i) survivin, indicating that the targeted RGD-PEG-ECO-siDANCR therapy likely causes epigenetic alterations and apoptosis in the TNBC tumors (*p<0.05).
H&E staining of the tumors showed poorly differentiated high-grade malignancy (Fig. 6f). While the NC-treated tumors showed large areas of zonal necrosis, the siDANCR-treated tumor sections showed only focal areas of necrosis. The efficacy of RGD-PEG-ECO/siDANCR was also validated by measuring DANCR expression in total RNA extracted from the primary tumors. Significant downregulation of DANCR was observed in the siDANCR-treated tumors, compared to the NC-treated tumors (Fig. 6g), confirming that RGD-PEG-ECO/siDANCR effectively delivered siDANCR into the cancer cells to mediate efficient DANCR silencing and anti-tumor activity.
As quantified by qRT-PCR, significant reduction of EZH2 (Fig. 6h) and survivin (Fig. 6i) was observed in the siDANCR-treated tumors, suggesting that the tumor suppression and regression are likely due to epigenetic changes and apoptotic/cytostatic effects of systemic siDANCR therapy.
No changes in the body weight were observed in the siDANCR- and NC-treated mice during the entire treatment period (Fig. 7a). The vital organs of the mice were assessed by H&E-staining. As shown in Fig. 7b–d, the liver, kidneys, and lungs showed normal hepatocyte, renal, and alveolar histology, with no evidence of inflammation and fibrosis/interstitial disease, respectively. No significant difference in morphology was observed between the siDANCR- and NC-treated mice, indicating that repeated injections of the nanoparticles did not adversely affect the vital organs and health of the mice.
Figure 7.
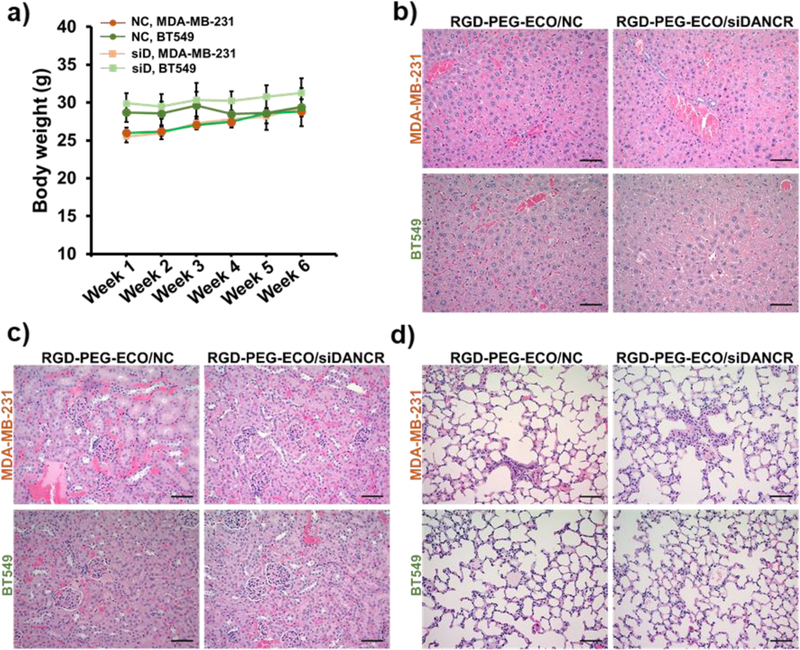
Systemic administrations of RGD-PEG-ECO/siDANCR nanoparticles do not cause evident side-effects in the treated mice. (a) Both siDANCR- and NC-treated mice show no changes in their general health and no significant differences in the body weights during the 6 weeks of nanoparticle injections. H&E staining of the tumor sections demonstrates no significant changes in the morphology of the vital organs in NC- and siDANCR-treated mice, with the (b) liver, (c) kidney, and (d) lungs showing normal hepatocyte, renal, and alveolar histology, respectively. (error bars denote s.e.m., scale bar = 100 μm).
Discussion
Formerly considered fragments of “junk DNA”, lncRNAs are contributors of pervasive transcription that simultaneously influence the transcriptome, proteome, and epigenome.10, 37–39 Onco-lncRNAs can function as decoys, scaffolds, sponges, and tethers in cis or trans through dynamic binding to their DNA, RNA, and protein partners,10, 37, 40 making them attractive candidates for highly efficient cancer therapy with minimal risk of toxic side-effects in healthy tissues. However, systemically targeting these “undruggable” macromolecules with conventional approaches remains a challenge, which can be circumvented through RNAi, a promising strategy for specifically downregulating aberrantly overexpressed onco-lncRNAs for systemic therapy. This work demonstrates that DANCR is highly overexpressed and acts as an oncogenic lncRNA in TNBC, and treatment with targeted RGD-PEG-ECO/siDANCR nanoparticles mediates robust DANCR silencing and therapeutic effects in TNBC, with concomitant gene expression changes in numerous cancer-driving pathways.
RGD-PEG-ECO/siDANCR nanoparticles were developed using multifunctional pH-sensitive amino lipid, ECO, for systemic targeted siRNA delivery. 23, 41–42 ECO self-assembles with siRNA to form stable ECO/siRNA nanoparticles, which possess unique features that protect the siRNA cargo during systemic delivery and facilitate pH-sensitive endosomal escape and reductive cytosolic siRNA release (PERC) in target cells.24, 43 RGD-PEG-ECO/siDANCR nanoparticles mediate robust silencing of DANCR in TNBC cells for at least a week. DANCR knockdown induces apoptotic/cytostatic effects and inhibits invasion, migration, and colony formation, which are important characteristics of tumorigenesis, proliferation, and aggression in TNBC.2 Consequently, weekly systemic injections of RGD-PEG-ECO/siDANCR nanoparticles at a low dose result in tumor suppression and regression in TNBC models. It can be speculated that the tumor growth arrest or cytostasis may, in part, be due to increased apoptosis, suppression of tumor-promoting proteins, and as yet unidentified epigenetic changes. The therapeutic efficacy of DANCR silencing can be attributed to the diverse gene expression changes we observed in the treated TNBC cells.
Although the overexpression of DANCR has been shown to be associated with poor prognosis and tumor progression in multiple cancers,14–15, 17, 19, 44 little is known about the pathways that upregulate DANCR in TNBC. During epidermal and osteoblast development, DANCR is required to maintain the progenitor cells in the undifferentiated state.11, 30 Since TNBC consists of many poorly differentiated cell types, and a high content of cancer stem cells, it is possible that the maintenance of self-renewal and cancer stemness or the transformation of well-differentiated mammary epithelial cells into the highly aggressive, mesenchymal and poorly differentiated phenotype requires or triggers the overexpression of DANCR.45 Irrespective of the underlying causes, we demonstrate that DANCR functions pleiotropically at epigenetic, transcriptional, translational, and post-translational levels of gene expression.9–10, 37 DANCR silencing results in inhibition of EZH2 and PRC2-mediated H3K27-trimethylation at the epigenetic level; downregulation of TFs β-catenin, ZEB1, and Stat proteins, and proteins N-cad and survivin; and decreased phosphorylation of Src, FAK, and other oncoproteins. These proteins are upregulated in TNBC; they play critical roles in disease progression, metastasis and drug resistance,2–3, 29, 46–47 and some of them are common targets for developing targeted therapies.3, 35, 48 Unfortunately, limited curative outcomes have been achieved with these targeted therapies individually. The pleiotropic effects of DANCR silencing with RGD-PEG-ECO/siDANCR may overcome the limitations of conventional approaches of targeting an individual pathway or protein to achieve better therapeutic outcomes. DANCR silencing and the consequent downregulation of the EZH2-SUZ12 axis of the PRC2 complex likely alters the recruitment or binding of EZH2 to its target genes or proteins, in turn impacting the transcriptional silencing of tumor promoters or activation of tumor suppressors. 14, 20, 30, 46 Interestingly, although both cell lines show reduced expression of phospho-AKT, the phosphorylated residues were different, e.g., S473 in BT549 vs T308 in MDA-MB-231, indicating that DANCR silencing impacts the cell lines differently, underscoring the broad therapeutic functions of the lncRNA. It can be hypothesized that DANCR downregulation enables collective repression of multiple TNBC-promoting pathways, including Wnt and EMT signaling, anti-apoptosis, phosphorylated FAK, Src, RSK1/2/3, AKT1/2/3, and other oncoproteins, resulting in efficacious therapeutic outcome. Thus, it can be speculated that the functional downregulation of a single onco-lncRNA can simultaneously impair several different tumor-promoting pathways, potentially circumventing the problem of compensatory mitogenic pathways arising from drug resistance.5, 48
This study demonstrates the promise of systemically silencing the oncogenic lncRNA DANCR with targeted RGD-PEG-ECO/siRNA nanoparticles for treating TNBC. Further studies are needed to evaluate the precise role of DANCR in the expression and modifications of multiple proteins at the same time, and to explore the role of DANCR in tumorigenesis, metastasis, and drug resistance. Because DANCR also promotes other types of cancers,22 this nanoparticle platform can be potentially expanded as a promising therapy for broader clinical applications. Although no overt toxicity was observed with systemic treatments of RGD-PEG-ECO/siDANCR, comprehensive studies on pharmacokinetics and toxicity of the nanoparticles are needed to assess potential off-target effects, activation of immune responses, and other shortcomings of lncRNA-targeted therapy49 for clinical translation.
In summary, we demonstrate that DANCR is overexpressed in TNBC and plays pleiotropic roles by regulating multiple molecular pathways. DANCR silencing mediates effective regulation of an array of oncogenic activities and inhibits invasion and proliferation of TNBC cells. Additionally, systemic treatment with targeted nanoparticles results in tumor suppression and regression in two independent TNBC models. This research opens new avenues for developing novel therapeutics by systemically regulating onco-lncRNAs to effectively treat TNBC and other aggressive cancers.
Materials and Methods
Cell lines and culture
Normal human mammary epithelial cells (HMECs), hormone receptor positive breast cancer cell lines MCF7 (ER+, PR+/−, Her2−) and ZR-75–1 (ER+, PR+/−, Her2+), and triple-negative breast cancer cell lines, namely, MDA-MB-231, Hs578T, and BT549 (claudin-low ER−, PR−, Her2−), were purchased from ATCC (Manassas, VA). HMECs were passaged in Mammary Epithelial Cell Basal Medium supplemented with components of the Mammary Epithelial Cell Growth Kit (Lonza, Allendale, NJ). MCF7 and ZR-75–1 cells were cultured in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS, 1% Penicillin/Streptomycin and 0.01 mg/mL recombinant human insulin (Sigma, St. Louis, MO). MDA-MB-231, Hs578T, and BT549 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin. All the cells were grown at 37°C and 5% CO2. The cell lines were tested for the absence of mycoplasma using the MycoAlert™ Mycoplasma Detection Kit (Lonza).
TCGA analysis
RNA-Seq data from TCGA database for DANCR transcript (ENST00000411630) was mined from the lncRNAtor website.50 Graphpad Prism was used to perform differential gene expression analysis between 104 normal and 790 breast cancer samples (comprising 80 TNBC, 584 ER+, 508 PR+, 128 Her2+ samples). Graphpad Prism was also used to construct dot plots and heat maps, and perform statistical analyses for the expression data.
cDNA array
Breast cancer cDNA Array IV (BCRT104) was purchased from Origene Technologies (Rockville, MD). DANCR expression was quantified by qRT-PCR using SyBr Green PCR Master Mix (Applied Biosystems, CA) and DANCR-specific primers, with 18S primers as controls. Gene expression was analyzed by the 2−ΔΔCt method with 18S expression as the control.
Nanoparticle formulation and transfections
ECO-PEG-RGD/siRNA nanoparticles were formulated as previously described.28 Briefly, the amino lipid ECO (5 mM stock in ethanol) was gently agitated with RGD-PEG-Mal (RGD-PEG/ECO=2.5 mol%) in nuclease-free ultra-pure water for 30 min at RT. This was followed by complexation with siDANCR or siLuc (as negative control NC) at a final siRNA concentration of 100 nM and N/P = 10 for an additional 30 min to enable self-assembly formation of ECO-PEG-RGD/siDANCR or ECO-PEG-RGD/NC nanoparticles, respectively. For transfections, the nanoparticle formulation was mixed with culture media and added on to plated cells for 24–48 h, depending on the relevant experiments. The siDANCR duplex 51 [sense 5ʹ-GGU CAU GAG AAA CGU GGA UUA CAdCdC-3ʹ and antisense 5ʹ-GGU GUA AUC CAC GUU UCU CAU GAC CUC-3ʹ] was purchased from IDT (Coralville, IA). The siLuc duplex [sense 5ʹ- CCU ACG CCG AGU ACU UCG AdTdT-3ʹ and antisense 5ʹ-dTdT GGA UGC GGC UCA UGA AGC U-3ʹ] was purchased from Dharmacon (Lafayette, CO).
Nanoparticle characterization
The nanoparticles were diluted in nuclease-free water and characterized for particle size and zeta potential on the LiteSizer™ 500 (Anton Paar), according to manufacturer’s instructions. The morphology of the nanoparticles was determined using Transmission electron microscopy (TEM), as described previously.26 In short, the nanoparticle suspension (20 μL) was pipetted onto a 300-mesh copper grid coated with a thin amorphous carbon film (20 nm). After blotting away excess sample with a filter paper, 3 μL of 2% uranyl acetate solution was added to stain the samples. After two rounds of staining, the samples were dried and imaged via TEM.
RNA extraction and analysis
Total RNA was extracted from cells and tissues using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD), according to manufacturer’s instructions. Reverse transcription was performed using the miScript II RT Kit (Qiagen) and qPCR was performed using the SyBr Green PCR Master Mix. Gene expression was analyzed by the 2−ΔΔCt method with 18S expression as the control. The following primer sequences were used- DANCR: Fwd 5ʹ-GCGCCACTATG TAGCGGGTT-3ʹ and Rev 5ʹ-TCAATGGCTTGTGCCTGTAGTT-3ʹ; 18S: Fwd 5ʹ-TCAAGAAC GAAAGTCGGAGG-3ʹ and Rev 5ʹ-GGACATCTAAGGGCATCACA-3ʹ; EZH2: Fwd 5ʹ-AGGA CGGCTCCTCTAACCAT-3ʹ and Rev 5ʹ-CTTGGTGTTGCACTGTGCTT-3ʹ; Survivin: Fwd 5ʹ- ATGGCCGAGGCTGGCTTCATC-3ʹ and Rev 5ʹ- ACGGCGCACTTTCTTCGCAGTT-3ʹ
Viability assay
MTT assay was performed according to manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA). Briefly, 5000 cells were plated on 96-well plates. After 24 h, the cells were transfected with therapeutic and negative control nanoparticles. After 48 h, MTT agent (5 mg/mL) was added into the wells and incubated for 4 h. The purple crystals were dissolved in SDS-HCl by incubation for 4 h. The absorbance was measured on SpectraMax microplate reader at 570 nm. Viability of control cells was normalized to 100%, for comparison to treated cells.
BrdU incorporation assay
BrdU incorporation was measured using the BrdU Cell Proliferation ELISA Kit from Abcam (Cambridge, MA), according to manufacturer’s instructions. Briefly, 5000 cells were plated on a 96-well plate for 24 h and then transfected with the nanoparticles. After 40 h, diluted BrdU reagent was added to the cells. After 8 h, the cells were fixed for 30 min and incubated with anti-BrdU antibody for 1 h. After 3 washes, the cells were then incubated with Peroxidase Goat Anti-mouse IgG conjugate for 30 min. Finally, TMB Peroxidase Substrate was added for color development (30 min), followed by the Stop Solution. Absorbance was measured at 450 nm using SpectraMax Microplate reader. Viability of control cells was normalized to 100%, for comparison to treated cells.
Scratch wound and Transwell migration assays
For the scratch wound assay, approximately 5 × 105 TNBC cells were plated on 6-well plates for 24 h. A single straight scratch was made in the monolayer with a small pipet tip. After washing the detached cells with PBS, the cells were transfected with therapeutic and control nanoparticles and monitored for up to 48 h, or until the scratch wounds were closed. Images were taken using the Moticam T2 camera.
For Transwell migration assays, 1–2 × 105 cells transfected with nanoparticles were plated on Transwell inserts (VWR, Radnor, PA) coated with 0.28 mg/mL Corning™ Matrigel™ Membrane Matrix (Corning, NY). The next day, the un-migrated cells in the inserts were removed by swabbing with Q-tips. The migrated cells were fixed by treating the inserts with 10% formalin for 10 min. After washing with PBS three times, the inserts were placed in 0.05% crystal violet stain for 20 min to stain the migrated cells. The inserts were washed under running water to remove excess stain and set to dry overnight. Images of the purple migrated cells were taken using the Moticam T2 camera. The cells in the images were counted using the ImageJ software.
3D tumor spheroid growth
For the Matrigel growth assay, 3–4 × 105 TNBC cells were transfected with the nanoparticles, suspended in 5% Matrigel™-containing media and plated on to 24-well plates coated with a thick layer of Corning™ Matrigel™ Membrane Matrix. The ability of the cells to form tumor spheroids in the 3D Matrix was monitored and photographed for up to 7 days using the Moticam T2 camera.
Western blot
Total cellular protein was extracted as previously described.52–53 Protein extracts (40 μg) were separated by SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with primary antibodies overnight. The following primary antibodies were purchased from Cell Signaling Technology (Danvers, MA)-: anti-β-catenin, anti-ZEB1, anti-N-cadherin, anti-EZH2, anti-SUZ12, anti-me3-H3K7, and anti-survivin. After secondary antibody incubation, the membranes were developed using ChemiDoc™ XRS+ Imager (Biorad, Hercules, CA). β-Actin was used as the loading control.
RNA Immunoprecipitation (RIP)
Magna RIP kit and DZNep were purchased from Millipore Sigma (Burlington, MA). RIP was performed according to manufacturer’s instructions. Briefly, cell lysates were incubated with antibody-bead mix overnight at 4°C. Following this, proteins were degraded and the RNA was isolated by the phenol-chloroform extraction method. The RNA was precipitated using Kit components and ethanol at −80°C overnight. For EZH2 inhibition, cells were treated with 500 μM DZNep for 72 h before harvesting for RIP assay.
Phosphokinase array
The differential phosphorylation status of 43 different kinases and 2 whole proteins with and without DANCR silencing was detected using the Proteome Profiler Human Phospho-Kinase Array Kit (RnD Systems, Minneapolis, MN). MDA-MB-231 and BT549 cells were transfected with RGD-PEG-ECO/NC and RGD-PEG-ECO/siDANCR nanoparticles. After 48 h, the cells were lysed and the lysates were analyzed for the relative phosphorylation profiles, according to the manufacturer’s instructions. The expression was quantified by subtracting the background signal from the mean pixel intensity of the duplicate spots and plotted as bar graphs.
Animal models
Nude athymic mice (6-week-old nu/nu females) were purchased from the Athymic Animal and Preclinical Therapeutic Facility of the Case Comprehensive Cancer Center and housed in the Case Center for Imaging Research at CWRU. All animal experiments were performed according to the protocol and guidelines laid down by the IACUC and ARC of CWRU. For tumor xenografts, 2 × 106 MDA-MB-231 cells and 4 × 106 BT549 cells suspended in Matrigel-PBS (6 mg/mL) were injected into the mammary fat pads of each nude mouse. Tumor volumes were monitored and measured once a week using a Vernier caliper. When the average tumor volumes reached 70–90 mm3, mice were randomized into control and treatment groups (n = 6 for MDA-MB-231 and n = 4 for BT549).
Tumor xenograft treatments
For in vivo therapy, nanoparticles were formulated as follows: ECO (50 mM stock) was conjugated to RGD-PEG-Mal (RGD-PEG/ECO=2.5 mol%) for 30 min, followed by complexation with siDANCR or siLuc (siRNA dose=1 mg/kg, [siRNA]=0.3 mg/mL) at N/P=8 for 30 more min, to obtain ECO-PEG-RGD/siDANCR or ECO-PEG-RGD/NC nanoparticles, respectively. The formulations were made in nuclease-free ultra-pure water and injected into the tail vein at siRNA dose of 1.0 mg/kg (100 μL per mouse) once a week for 6 weeks. Tumor volumes and body weights of mice were monitored once a week for 6 weeks. At the end of the experiment, the animals were euthanized, and the tumors and vital organs were harvested for analysis of morphology, histology, and metastasis. Portions of the tissues were fixed in 10% neutral buffered formalin, followed by paraffin embedding, sectioning, and H&E staining. Staining and IHC services were provided by the Tissue Resources Core Facility of CWRU. All the slides were reviewed by a certified pathologist.
Statistical analyses
All the experiments were independently replicated at least 3 times (n = 3), unless otherwise stated. Data are represented as mean ± s.e.m. Statistical analysis was performed using Graphpad Prism. Data between two groups was compared using unpaired Student’s t-test. Data between three groups was compared using One-way ANOVA. p < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgements
This research was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number R01 CA194518 and R01 CA235152. ZRL is M. Frank Rudy and Margaret Domiter Rudy Professor of Biomedical Engineering.
Footnotes
Competing Interests
AMV and ZRL are co-inventors on a patent application. ZRL is a co-founder of Cleveland Theranostics, LLC, a startup company with a focus on developing nonviral gene therapies based on the multifunctional pH-sensitive amino lipids.
Supporting information
Raw data of colony assay, heat map of phospho-kinase profiles and combination plot depicting individual tumor volumes of mice treated with nanoparticle therapy
References
- 1.Metzger-Filho O; Tutt A; de Azambuja E; Saini KS; Viale G; Loi S; Bradbury I; Bliss JM; Azim HA Jr.; Ellis P; et al. (2012) Dissecting the heterogeneity of triple-negative breast cancer. J. Clin. Oncol 30, 1879–1887. [DOI] [PubMed] [Google Scholar]
- 2.Kalimutho M; Parsons K; Mittal D; Lopez JA; Srihari S; Khanna KK (2015) Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol. Sci 36, 822–846. [DOI] [PubMed] [Google Scholar]
- 3.Arnedos M; Bihan C; Delaloge S; Andre F. (2012) Triple-negative breast cancer: are we making headway at least? Ther. Adv. Med. Oncol 4, 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Du F; Eckhardt BL; Lim B; Litton JK; Moulder S; Meric-Bernstam F; Gonzalez-Angulo AM; Ueno NT (2015) Is the future of personalized therapy in triple-negative breast cancer based on molecular subtype? Oncotarget 6, 12890–12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eccles SA; Aboagye EO; Ali S; Anderson AS; Armes J; Berditchevski F; Blaydes JP; Brennan K; Brown NJ; Bryant HE; et al. (2013) Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res 15, R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooding AJ; Zhang B; Jahanbani FK; Gilmore HL; Chang JC; Valadkhan S; Schiemann WP (2017) The lncRNA BORG Drives Breast Cancer Metastasis and Disease Recurrence. Sci. Rep 7, 12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YL; Overstreet AM; Chen MS; Wang J; Zhao HJ; Ho PC; Smith M; Wang SC (2015) Combined inhibition of EGFR and c-ABL suppresses the growth of triple-negative breast cancer growth through inhibition of HOTAIR. Oncotarget 6, 11150–11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathy NW; Chen XM (2017) Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J. Biol. Chem 292, 12375–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercer TR; Dinger ME; Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat. Rev. Genet 10, 155–159. [DOI] [PubMed] [Google Scholar]
- 10.Dey BK; Mueller AC; Dutta A. (2014) Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription 5, e944014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kretz M; Webster DE; Flockhart RJ; Lee CS; Zehnder A; Lopez-Pajares V; Qu K; Zheng GX; Chow J; Kim GE; et al. (2012) Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev 26, 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J; Zhou L. (2018) Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/beta-catenin signaling. Biomed. Pharmacother 102, 602–607. [DOI] [PubMed] [Google Scholar]
- 13.Yang JX; Sun Y; Gao L; Meng Q; Yang BY (2018) Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma 65, 790–798. [DOI] [PubMed] [Google Scholar]
- 14.Jia J; Li F; Tang XS; Xu S; Gao Y; Shi Q; Guo W; Wang X; He D; Guo P. (2016) Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget 7, 37868–37881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y; Zhang M; Liang L; Li J; Chen YX (2015) Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol 8, 11480–11484. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y; Lu Z; Wang N; Feng J; Zhang J; Luan L; Zhao W; Zeng X. (2018) Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp. Mol. Med 50, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao YP; Qiu JH; Zhang DB; Yu CG (2017) Long non-coding RNA DANCR, a prognostic indicator, promotes cell growth and tumorigenicity in gastric cancer. Tumour Biol 39, 1010428317699798. [DOI] [PubMed] [Google Scholar]
- 18.Ma X; Wang X; Yang C; Wang Z; Han B; Wu L; Zhuang L. (2016) DANCR Acts as a Diagnostic Biomarker and Promotes Tumor Growth and Metastasis in Hepatocellular Carcinoma. Anticancer Res 36, 6389–6398. [DOI] [PubMed] [Google Scholar]
- 19.Yuan SX; Wang J; Yang F; Tao QF; Zhang J; Wang LL; Yang Y; Liu H; Wang ZG; Xu QG; et al. (2016) Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology 63, 499–511. [DOI] [PubMed] [Google Scholar]
- 20.Sha S; Yuan D; Liu Y; Han B; Zhong N. (2017) Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol. Open 6, 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y; Hu Z; Mangala LS; Stine ZE; Hu X; Jiang D; Xiang Y; Zhang Y; Pradeep S; Rodriguez-Aguayo C; et al. (2018) MYC Targeted Long Noncoding RNA DANCR Promotes Cancer in Part by Reducing p21 Levels. Cancer research 78, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thin KZ; Liu X; Feng X; Raveendran S; Tu JC (2018) LncRNA-DANCR: A valuable cancer related long non-coding RNA for human cancers. Pathol. Res. Pract 214, 801–805. [DOI] [PubMed] [Google Scholar]
- 23.Gujrati M; Vaidya A; Lu ZR (2016) Multifunctional pH-Sensitive Amino Lipids for siRNA Delivery. Bioconjug. Chem 27, 19–35. [DOI] [PubMed] [Google Scholar]
- 24.Gujrati M; Malamas A; Shin T; Jin E; Sun Y; Lu ZR (2014) Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol. Pharm 11, 2734–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvani JG; Gujrati MD; Mack MA; Schiemann WP; Lu ZR (2015) Silencing beta3 Integrin by Targeted ECO/siRNA Nanoparticles Inhibits EMT and Metastasis of Triple-Negative Breast Cancer. Cancer research 75, 2316–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun D; Sahu B; Gao S; Schur RM; Vaidya AM; Maeda A; Palczewski K; Lu ZR (2017) Targeted Multifunctional Lipid ECO Plasmid DNA Nanoparticles as Efficient Non-viral Gene Therapy for Leber’s Congenital Amaurosis. Mol. Ther. Nucleic Acids 7, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun D; Sun Z; Jiang H; Vaidya A; Xin R; Ayat N; Schilb A; Qiao P; Han Z; Naderi, et al. (2018) Synthesis and Evaluation of pH-Sensitive Multifunctional Lipids for Efficient Delivery of CRISPR/Cas9 in Gene Editing. Bioconjug Chem doi: 10.1021/acs.bioconjchem.8b00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gujrati M; Vaidya AM; Mack M; Snyder D; Malamas A; Lu ZR (2016) Targeted Dual pH-Sensitive Lipid ECO/siRNA Self-Assembly Nanoparticles Facilitate In Vivo Cytosolic sieIF4E Delivery and Overcome Paclitaxel Resistance in Breast Cancer Therapy. Adv. Healthc. Mater 5, 2882–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holliday DL; Speirs V. (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res 13, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu L; Xu PC, (2013) Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem. Biophys. Res. Commun 432, 612–617. [DOI] [PubMed] [Google Scholar]
- 31.Hock H. (2012) A complex Polycomb issue: the two faces of EZH2 in cancer. Genes Dev 26, 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X; Haider Ali MSS; Moran M. (2017) The role of interactions of long non-coding RNAs and heterogeneous nuclear ribonucleoproteins in regulating cellular functions. Biochem. J 474, 2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuenca-Lopez MD; Montero JC; Morales JC; Prat A; Pandiella A; Ocana A. (2014) Phospho-kinase profile of triple negative breast cancer and androgen receptor signaling. BMC Cancer 14, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer IA; Abramson VG; Lehmann BD; Pietenpol JA (2014) New strategies for triple-negative breast cancer--deciphering the heterogeneity. Clin. Cancer Res 20, 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolos V; Gasent JM; Lopez-Tarruella S; Grande E. 92010) The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco. Targets Ther 3, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gwinn DM; Shackelford DB; Egan DF; Mihaylova MM; Mery A; Vasquez DS; Turk BE; Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutschner T; Diederichs S. (2012) The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 9, 703–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anastasiadou E; Faggioni A; Trivedi P; Slack FJ (2018) The Nefarious Nexus of Noncoding RNAs in Cancer. Int. J. Mol. Sci 19 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidovich C; Cech TR (2015) The recruitment of chromatin modifiers by long noncoding RNAs: lessons from PRC2. RNA 21, 2007–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liz J; Esteller M. (2016) lncRNAs and microRNAs with a role in cancer development. Biochim. Biophys. Acta 1859, 169–176. [DOI] [PubMed] [Google Scholar]
- 41.Malamas AS; Gujrati M; Kummitha CM; Xu R; Lu ZR (2013) Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J. Control. Release 171, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang XL; Ramusovic S; Nguyen T; Lu ZR (2007) Novel polymerizable surfactants with pH-sensitive amphiphilicity and cell membrane disruption for efficient siRNA delivery. Bioconjug. Chem 18, 2169–2177. [DOI] [PubMed] [Google Scholar]
- 43.Sahay G; Alakhova DY; Kabanov AV (2010) Endocytosis of nanomedicines. J. Control. Release 145, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan L; Liang W; Gu J; Zang X; Huang Z; Shi H; Chen J; Fu M; Zhang P; Xiao X; et al. (2018) Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget 9, 1915–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howard CB; McDowell R; Feleke K; Deer E; Stamps S; Thames E; Singh V; Pervin S. (2016) Chemotherapeutic Vulnerability of Triple-negative Breast Cancer Cell-derived Tumors to Pretreatment with Vernonia amygdalina Aqueous Extracts. Anticancer Res 36, 3933–3943. [PMC free article] [PubMed] [Google Scholar]
- 46.Gan L; Yang Y; Li Q; Feng Y; Liu T; Guo W. (2018) Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. Biomark. Res 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamagishi M; Uchimaru K. (2017) Targeting EZH2 in cancer therapy. Curr. Opin. Oncol 29, 375–381. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Aranda M; Redondo M. (2017) Protein Kinase Targets in Breast Cancer. Int. J. Mol. Sci 18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavorgna G; Vago R; Sarmini M; Montorsi F; Salonia A; Bellone M. (2016) Long non-coding RNAs as novel therapeutic targets in cancer. Pharmacol. Res 110, 131–138. [DOI] [PubMed] [Google Scholar]
- 50.Park C; Yu N; Choi I; Kim W; Lee S. (2014) lncRNAtor: a comprehensive resource for functional investigation of long non-coding RNAs. Bioinformatics 30, 2480–2485. [DOI] [PubMed] [Google Scholar]
- 51.Lennox KA; Behlke MA (2016) Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res 44, 863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaidya A; Mao Z; Tian X; Spencer B; Seluanov A; Gorbunova V. (2014) Knock-in reporter mice demonstrate that DNA repair by non-homologous end joining declines with age. PLoS Genet 10, e1004511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seluanov A; Danek J; Hause N; Gorbunova V. (2007) Changes in the level and distribution of Ku proteins during cellular senescence. DNA Repair (Amst) 6, 1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.