Abstract
This article investigated the efficacy of the combination of antiepileptic drug therapy in protecting against soman-induced seizure severity, epileptogenesis and performance deficits. Adult male rats with implanted telemetry transmitters for continuous recording of electroencephalographic (EEG) activity were exposed to soman and treated with atropine sulfate and the oxime HI-6 one minute after soman exposure and with midazolam, ketamine and/or valproic acid 40 min after seizure onset. Rats exposed to soman and treated with medical countermeasures were evaluated for survival, seizure severity, the development of spontaneous recurrent seizure and performance deficits; combination anti-epileptic drug therapy was compared with midazolam monotherapy. Telemetry transmitters were used to record EEG activity, and a customized MATLAB algorithm was used to analyze the telemetry data. Survival data, EEG power integral data, spontaneous recurrent seizure data and behavioral data are illustrated in figures and included as raw data. In addition, edf files of one month telemetry recordings from soman-exposed rats treated with delayed midazolam are provided as supplementary materials. Data presented in this article are related to research articles “Rational Polytherapy in the Treatment of Cholinergic Seizures” [1] and “Early polytherapy for benzodiazepine-refractory status epilepticus [4].
Keywords: Soman, Ketamine, Midazolam, Valproic acid, Chemical warfare nerve agent, Epileptogenesis, Status epilepticus
Specifications Table
| Subject area | Pharmacology, Toxicology and Pharmaceutical Science |
| More specific subject area | Anti-epileptic drug efficacy against soman exposure |
| Type of data | Figures, spreadsheet with raw data |
| How data was acquired | Data Sciences International Telemetry Transmitters (F20-EET), Imaging System (Morris water maze) |
| Data format | Raw and analyzed data, and representative EEG files (edf) |
| Experimental factors | Rats were pre-implanted with telemetry transmitters 1–2 weeks prior to soman exposure and administration of post-exposure medical countermeasures. |
| Experimental features | Adult male rats with implanted telemetry transmitters for continuous recording of electroencephalographic (EEG) activity were exposed to soman and treated with atropine sulfate and the oxime HI-6 one min after soman exposure and with midazolam, ketamine and/or valproic acid 40 min after seizure onset. Rats exposed to soman and treated with medical countermeasures were evaluated for survival, seizure severity, the development of spontaneous recurrent seizure and performance deficits; combination anti-epileptic drug therapy was compared with midazolam monotherapy. |
| Data source location | Aberdeen Proving Ground, MD, USA, US Army Medical Research Institute of Chemical Defense |
| Data accessibility |
With this article, and in the following repository: Repository name: Mendeley Data Direct URL to data:https://doi.org/10.17632/zwcx948yjc.2 |
| Related research article | J. Niquet, L. Lumley, R. Baldwin, F. Rossetti, L. Suchomelova, D. Naylor, I.B.F. Estrada, C.G. Wasterlain Rational polytherapy in the treatment of cholinergic seizures, Neurobiology of Disease, Neurobiol. Dis. 2019.https://doi.org/10.1016/j.nbd.2019.104537 [1]. |
Value of the data
|
1. Data
The first set of data corresponds to a dose-response experiment of delayed treatment with midazolam in soman-exposed rats. Fig. 1A is a survival plot of soman-exposed rats treated with a dose range of midazolam at 40 min after seizure onset. Fig. 1B is a bar graph illustrating the EEG power integral at 1 h and 6 h after soman exposure in rats treated with midazolam. Fig. 1C and D illustrate the percentage of rats that developed SRS and the number of SRS, respectively. Corresponding raw data are contained in a supplementary excel file (Fig. 1A, MDZ Survival; Fig. 1B, MDZ Power; Fig. 1C, MDZ SRS; Fig. 1D, SRS onset and #).
Fig. 1.
Delayed midazolam increases survival but does not prevent status epilepticus or epileptogenesis. A) Midazolam administered 40 min after the onset of seizures induced by soman (GD) dose-dependently increased survival to GD: rats that received saline (GD/SAL; n = 10) or low midazolam (1 mg/kg; GD/MDZ1; n = 14) had poor survival (30% and 50% respectively), while those that received 3 mg/kg midazolam (GD/MDZ3; n = 13) or 9 mg/kg midazolam (GD/MDZ9; n = 13) had 85–90% survival. B) Soman exposure increased EEG power integral during status epilepticus. Treatment with midazolam (3 or 9 mg/kg) at 40 min after seizure onset reduced GD-induced seizure severity compared to saline treatment as shown by power integral during the 1 h period after treatment. Data shown are mean ± SEM. C) Following a latent period of 1–2 weeks, all of the surviving GD-exposed rats treated with saline or with 1 mg/kg midazolam developed SRS, while 57 and 70% of those treated with 3 mg/kg and 9 mg/kg midazolam, respectively developed SRS. D) Number of SRS is shown as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
Four representative telemetry recordings of over one month of continuous data from soman exposed rats treated with one of three doses (1, 3, 9 mg/kg; ip) of midazolam are provided in Mendeley Data Direct (URL to data: https://doi.org/10.17632/zwcx948yjc.2) as supplementary materials in a data repository. Each folder contains raw EEG data compressed into 7zip format split into 3–4 volumes. After uncompressing (we suggest using https://www.7-zip.org) the set of files for each animal, the recordings can be accessed using EDF (European Data Format)-compatible software and contain signals collected at 250 Hz from two EEG channels, one signal strength channel (used for assessing gross motor activity) and one body temperature channel, and include baseline and post-exposure to soman data. The animal identification and date/time of each recording were modified per IACUC suggestion with date set back to 2000. A05_GD_MDZ3.edf is from a GD-exposed rat treated with 3 mg/kg midazolam 40 min after seizure onset that had over 25 SRS; the modified date and time stamp has exposure on 1/5/2000 at 21:55 (actual 10:04 a.m., which is ∼2 hr after onset of the dark cycle). Since the exposure was performed in a hood in another room, there is an interruption of signal during this period (between 21:52–21:57), followed by seizure onset at 22:03 (with seizure latency of 8 min). Treatment time (40 min after seizure onset), characterized by a short signal interruption is 22:43. For A06_GD_MDZ1.edf from a GD exposed rat treated with 1 mg/kg midazolam that had over 30 SRS, the modified date and time stamp has exposure on 1/5/2000 at 22:47 with a data gap between 22:44 and 22:49 and seizure onset at 22:50 with seizure latency of 3 min. Treatment time (40 min after seizure onset), characterized by a short signal interruption is 23:30. For A08_GD_MDZ9.edf from a GD exposed rat treated with 9 mg/kg midazolam that had less than 5 SRS, the modified date and time stamp has exposure on 1/5/2000 at 22:11 with a data gap between 22:08 and 22:13 and seizure onset at 22:19 with seizure latency of 8 min. Treatment time (40 min after seizure onset), characterized by a short signal interruption was 22:58. A fourth animal A09_GD_MDZ3.edf from a soman exposed rat treated with 3 mg/kg midazolam is included to illustrate an animal with an extremely large number of SRS (over 100 SRS). The modified date and time stamp has exposure on1/5/2000 at 22:43 with a data gap between 22:40 and 22:45 and seizure onset at 22:49 with seizure latency of 7 min. Treatment time (40 min after seizure onset), characterized by a short signal interruption is 23:30.
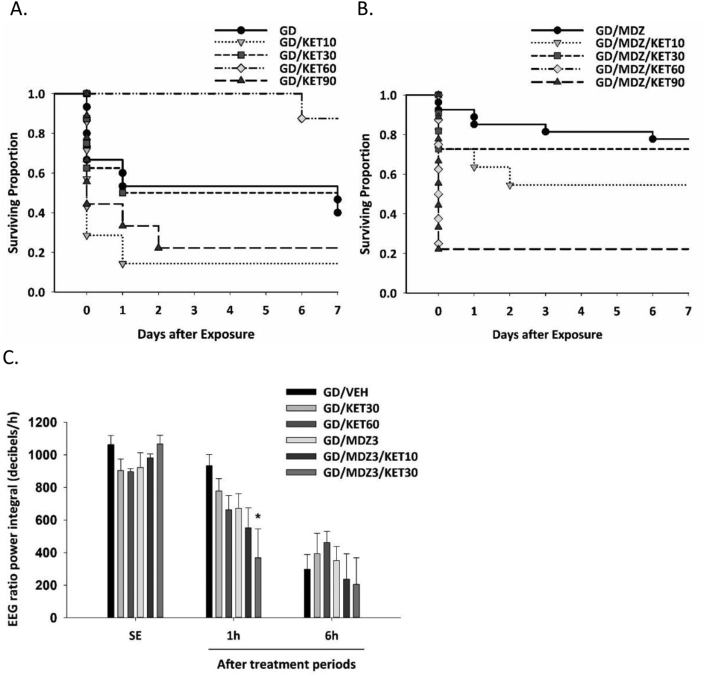
The second set of data corresponds to a dose-response study of ketamine monotherapy or as an adjunct to midazolam against soman-induced toxicity in rats. Survival plots are shown in Fig. 2A (ketamine monotherapy) and Fig. 2B (ketamine and midazolam combination). Fig. 2C is a bar graph illustrating the effect that midazolam and ketamine dual therapy had on EEG power integral (a measure of seizure severity) compared to vehicle or midazolam monotherapy at 1 h after treatment. Some of these data are captured in Fig. 2B in Niquet et al. [1], with additional groups shown here. The number of rats that develop SRS are in Fig. 3C in Niquet et al. [1], and in Fig. 2D in Niquet et al. [4]. Raw data for Fig. 2 is in a supplementary excel file titled “Data Combination Therapy Against Soman” (Fig. 2A, KET survival; Fig. 2B, MDZKET Survival; Fig. 2C, KET & MDZKET Power). We present data from a comparison of the effects of monotherapy and dual therapy on performance deficits that follow soman exposure. Latency to locate the platform in the Morris water maze is shown in Fig. 3E in Niquet et al. [1] and Fig. 2F, Niquet et al. [4] with raw data in the excel file “Morris Water Maze.” Additional measures of performance in the Morris water maze are shown in Fig. 3A (Training sessions percent time in target quadrant), 3B (Training sessions distance travelled), 3C (Training sessions thigmotaxis), and 3D (Probe trial Gallagher score) with raw data included in the supplementary excel file (Morris water maze). We also provide data from an evaluation of the efficacy of adding valproic acid to a combination therapy of ketamine and midazolam against soman-induced seizure severity [4]. Raw data for Fig. 1B in Niquet et al. [4] is included in the excel file labelled “MDZ_KET_VPA_Power.”
Fig. 2.
A dose range of ketamine (KET; 10, 30, 90 mg/kg; n = 7–11/group) with or without midazolam (MDZ) was administered 40 min after soman (GD)-induced seizure onset. A) GD-exposed rats treated with KET (60 mg/kg; GD/KET60) had a high survival rate, while those treated with 10 mg/kg (GD/KET10) or 90 mg/kg KET (GD/KET90) had a poor survival rate. B) GD-exposed rats administered KET (30 mg/kg) in combination with midazolam (MDZ; 3 mg/kg; GD/MDZ3/KET30) had a high survival rate, while those treated with 60 or 90 mg/kg of KET in combination with MDZ (GD/MDZ3/KET60 or GD/MDZ3/KET90, respectively) had poor survival. C) Combination of KET (30 mg/kg) and MDZ (3 mg/kg; GD/MDZ/KET30) significantly reduced the EEG ratio power integral 1 h after treatment compared to MDZ monotherapy (GD/MDZ3) or to vehicle (GD/VEH) or to KET (GD/KET30 or GD/KET60). Data shown are mean ± SEM. *p < 0.05.
Fig. 3.
Ketamine (KET; 30 mg/kg) with or without midazolam (MDZ; 3 mg/kg) was administered 40 min after soman (GD)-induced seizure onset and compared with no agent control mice (n = 10–11/group). Morris water maze testing was conducted one month after GD exposure. GD-exposed rats treated with combination therapy of KET and MDZ (GD/MDZ/KET) performed similarly to no-agent control (No GD) rats. Rats treated with MDZ monotherapy (GD/MDZ) or KET monotherapy (GD/KET) spent less time in the target quad (A), had greater distance travelled (B), spent more time in thigmotaxis (C), had greater cumulative distance in the probe trial compared to no-agent control rats (D), and had greater latency to locate the platform [[1], [4]]. Data shown are mean ± SEM. *p < 0.05.
2. Experimental design, materials, and methods
2.1. Animals
Male Sprague-Dawley rats (350–400 g; Charles River) individually housed and maintained on a reverse, 12 h light-dark cycle were implanted with F40-EET telemetry transmitters [Data Sciences International (DSI), Inc., St. Paul, MN, USA] for the continuous EEG monitoring. Rats were weighed daily, and treatment groups were counterbalanced according to pre-exposure weight. The experimental protocol was approved by the Animal Care and Use Committee at the United States Army Medical Research Institute of Chemical Defense, and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89–544), as amended.
2.2. Soman exposure and treatments
Rats were subcutaneously (s.c.) administered 1.2 LD50 soman (pinacolyl methylphosphonofluoridate; 132 μg/kg; 0.5 ml/kg) obtained from the U.S. Army Combat Capabilities Development Command Chemical Biological Center (Aberdeen Proving Ground, MD, USA) and 1 min later administered an intramuscular (i.m.) injection with an admix of atropine sulfate (2 mg/kg; Sigma Aldrich; St. Louis, MO, USA) and the oxime HI-6 dimethanesulfonate salt (118.5 mg/kg; Starkes Associates, Buffalo, NY, USA). Forty minutes after seizure onset, drugs were administered intraperitoneally (i.p.). Treatments included midazolam (1, 3, or 9 mg/kg; Hospira, Lake Forest, IL, USA), ketamine (10, 30, 60, or 90 mg/kg; Mylan, Canonsburg, PA, USA), and/or valproic acid (90 mg/kg; Sigma-Aldrich, St. Louis, MO, USA).
2.3. Seizure recording and analysis
Rats anesthetized with isoflurane were surgically implanted with F40-EET telemetry transmitters (DSI, Inc.) to record bi-hemispheric cortical EEG activity as previously described [5]. Following 7–10 days of recovery, rats were exposed to soman and monitored for seizure onset. For EEG activity recording, an RPC-1 physiotel receiver from DSI was placed under each rat's home cage for continuous data collection (24 h/day) using Dataquest ART Acquisition software (DSI, Inc.). The Dataquest EEG files were converted to European Data Format (edf) [6] using Neuroscore 1.1.1 (DSI), and the EEG channel with the best signal-to-noise ratio was chosen for each animal. Signals from each EEG channel were visually screened to identify the presence of artifacts, and the channel with the least number of artifacts was chosen for further analysis. In case several data sets were accumulated per animal, the datasets were linked to each other in MATLAB, allowing proper representation of the data. The signal was filtered using a Butterworth filter (pass band of 0.1–125 Hz; notch filter of 60 Hz [7]). Epileptiform activity was identified using Dataquest ART 4.1 (analysis software), Neuroscore 1.1.1 (DSI), and a customized MATLAB (MATLAB; Mathworks, 2008a) algorithm according to de Araujo Furtado et al. [7] and confirmed by visual screening. Converted edf files were analyzed to identify the time spent in status epilepticus and the development of SRS. The EEG ratio power integral was calculated by taking the average of power spectra of each hour period through a customized MATLAB algorithm and applying a formula [decibels = 10*(Log(Vˆ2sample/Vˆ2normal))]*60 min, resulting in decibels/h. The range of frequency analyzed was 0.1–100 Hz, and the data represent the full spectrum and the ratio of power of EEG signal in the first 24 h and in specific time periods of 1 and 6 hours after onset of status epilepticus.
2.4. Behavioral assessments
One month after soman exposure and treatment, rats were evaluated for spatial memory acquisition and retention in the Morris water maze (MWM) test. A hidden platform (10 × 10 cm) was placed in a fixed position 1.25 cm below the surface of a 170 cm diameter pool filled with paint-blackened water (26 ± 1 °C; for detailed methods, see Schultz et al. [5]). Briefly, rats received four 60 s trials per session, 2 training sessions per day and a 30 min rest period between sessions for a total of 8 trials/day. After three training days (6 training sessions total), the platform was removed, and two 60 s probe trials were conducted. A video tracking program (HVS Watermaze 2100, HVS Image, Cambridge, UK) was used to measure latency to escape, path length, speed, heading error, thigmotaxis, target quad time, Gallagher score [8], and number of platform passes (definitions listed below). Following the second probe test, a visual acuity test was conducted in which the latency to locate a visible platform was evaluated over four successive 60 s trials. Data are included in supplementary material (excel sheet 9) with corresponding labels listed below.
2.4.1. Latency to escape
Time in seconds from the start of the trial for the rat to reach the platform and end the trial. A maximum latency of 60 s is assigned if the rat fails to find the platform in 60 s. In the supplementary excel file, listed as S1Lat, S2Lat, etc.
2.4.2. Path length
The length of the path that the rat took from the starting location to its location at the end of the trial (meters). Listed in supplementary excel data file as S1Path, S2Path, etc.
2.4.3. Speed
Average speed over entire trial (meters per second), path length/latency. Listed in supplementary excel data file as S1Speed, S2Speed, etc.
2.4.4. Heading error
The absolute value of the angle between the platform, the rat's starting position, and the rat's position after traveling 20 cm. A smaller heading error indicates a more accurate initial direction of the rat navigating towards the platform. Listed in supplementary excel data file as S1Head, S2Head, etc. For probe trial, listed as P1Head, P2Head.
2.4.5. Thigmotaxis
The percent of trial time the rat spent in the perimeter of the water maze. Listed in supplementary excel data file as S1Thig, S2Thig, etc.
2.4.6. Target quadrant time
The percent of trial time the rat spent in the target quadrant. Listed in supplementary excel data file as S1TargetQuad, S2TargetQuad, etc.
2.4.7. Gallagher score (cumulative)
The Gallagher score is the distance between the rat and the platform at every second in the trial [8]. The cumulative version is the total Gallagher score for the entire trial. Listed in supplementary excel data file as S1GalCum, S2GalCum, etc. For probe trial, listed as P1Gal, P2Gal.
2.4.8. Platform passes in probe trial
Number of times during the probe trial that the rat passed through the platform location. Listed in supplementary excel data file as P1Pass, P2Pass.
2.5. Statistical analysis
Statistical analyses were performed using SPSS (IBM Inc, Armonk, NY), and graphs were compiled using SigmaPlot (Systat Software Inc., San Jose, CA). Survival across time and spontaneous recurrent seizure onset were analyzed with a Kaplan-Meier analysis, followed by a log-rank test to determine treatment effect on the distributions. EEG power integral were analyzed using an ANOVA. Significant interactions were followed up at each time point (baseline, 1 hr, 6 hr) followed by multiple comparisons of treatment groups. Measures in the Morris water maze were analyzed using a repeated measures ANOVA. For significant interactions between group and repeat (time or trial), a one-way ANOVA was used to compare groups at each repeat and to compare each group over time. Differences were considered statistically significant when p < 0.05.
Disclaimer
The views expressed are solely those of the authors and do not necessarily represent the official views of the CCRP, NIAID, NIH, HHS, USAMRICD or DoD.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS; grant U01 NS074926; CW). The authors appreciate the assistance of Ms. Amanda Furman, Dr. Linnzi Wright, Mr. Andrew Bourne, Ms. Caroline Schultz, and Mr. Michael Stone in data collection, data compilation, or agent exposures. Dr. Mark Schultz, Mr. Andrew Bourne and Ms. Amanda Furman were supported in part by an appointment to the Research Participation Program for the U.S. Army Medical Research and Development Command administered by the Oak Ridge Institute for Science and Education through an agreement between the U.S. Department of Energy and U.S. Army Medical Research and Materiel Command.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104629.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Niquet J., Lumley L., Baldwin R., Rossetti F., Suchomelova L., Naylor D., Estrada I.B.F., Schultz M., de Araujo Furtado M., Wasterlain C.G. Rational polytherapy in the treatment of cholinergic seizures. Neurobiol. Dis. 2019 doi: 10.1016/j.nbd.2019.104537. PMID: 31454548. E-Pub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 2.Chapman S., Yaakov G., Egoz I., Rabinovitz I., Raveh L., Kadar T., Gilat E., Grauer E. Sarin-induced brain damage in rats is attenuated by delayed administration of midazolam. Neurotoxicology. 2015;49:132–138. doi: 10.1016/j.neuro.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Niquet J., Baldwin R., Norman K., Suchomelova L., Lumley L., Wasterlain C.G. Midazolam-ketamine dual therapy stops cholinergic status epilepticus and reduces Morris water maze deficits. Epilepsia. 2016;57(9):1406–1415. doi: 10.1111/epi.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niquet J., Lumley L., Baldwin R., Rossetti F F., Schultz M., de Araujo Furtado M., Suchomelova L., Naylor D., Estrada I.B.F., Wasterlain C.G. Early polytherapy for benzodiazepine-refractory status epilepticus. Epilepsy Behav. 2015 doi: 10.1016/j.yebeh.2019.06.011. in press. [DOI] [PubMed] [Google Scholar]
- 5.Schultz M.K., Wright L.K., de Araujo Furtado M., Stone M.F., Moffett M.C., Kelley N.R., Bourne A.R., Lumeh W.Z., Schultz C.R., Schwartz J.E., Lumley L.A. Caramiphen edisylate as adjunct to standard therapy attenuates soman-induced seizures and cognitive deficits in rats. Neurotoxicol. Teratol. 2014;44:89–104. doi: 10.1016/j.ntt.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Kemp B., Varri A., Rosa A.C., Nielsen K.D., Gade J. A simple format for exchange of digitized polygraphic recordings. Electroencephalogr. Clin. Neurophysiol. 1992;82(5):391–393. doi: 10.1016/0013-4694(92)90009-7. [DOI] [PubMed] [Google Scholar]
- 7.de Araujo Furtado M., Zheng A., Sedigh-Sarvestani M., Lumley L., Lichtenstein S., Yourick D. Analyzing large data sets acquired through telemetry from rats exposed to organophosphorous compounds: an EEG study. J. Neurosci. Methods. 2009;184(1):176–183. doi: 10.1016/j.jneumeth.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher M., Burwell R., Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 2015;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.