Abstract
The data described below is related to the manuscript “Late life maintenance and enhancement of functional exercise capacity in low and high responding rats after low intensity treadmill training” [1]. Rodents exhibit age-related declines in skeletal muscle function that is associated with muscle denervation and cellular senescence. Exercise training is a proven method to delay or even reverse some aging phenotypes, thus improving healthspan in the elderly. The beneficial effects of exercise to preserve muscle may be reliant on an individual's innate ability to adapt to aerobic training. To examine this question, we assessed aged rats that were selectively bred to be either minimally or highly responsive to aerobic exercise training. We specifically asked whether mild treadmill training initiated late in life would be beneficial to preserve muscle function in high response and low response trainer rats. We examined gene expression data on markers of denervation and senescence. We also evaluated measures of aerobic training and neuromuscular muscle function through work capacity, contractile properties, and endplate fragmentation for further analysis of the aging phenotype in older rodents.
Keywords: High response trainers, Nonresponders, Aging, Skeletal muscle, Adaptive exercise capacity, Healthspan
Specifications Table
| Subject area | Biology |
| More specific subject area | Skeletal muscle, aging, aerobic treadmill training |
| Type of data | Graphs, images, tables |
| How data was acquired | Treadmill distance, contractile force, endplate fragmentation, RT-PCR |
| Data format | Raw and analyzed |
| Experimental factors | Twenty month old female rats were randomly selected to remain sedentary or participate in 4 months of aerobic exercise training. Eleven month old female rats served as an age control. |
| Experimental features | All rats were selectively bred to either elicit a low or high response to training for ≥ 20 generations. |
| Data source location | University of Michigan, Ann Arbor, Michigan |
| Data accessibility | All data are provided with this article |
| Related research article | Brown, L. A., Macpherson, P. C., Koch, L. G., Qi, N., Britton, S. L., & Brooks, S. V. (2019). Late life maintenance and enhancement of functional exercise capacity in low and high responding rats after low intensity treadmill training. Experimental Gerontology,https://doi.org/10.1016/j.exger.2019.110657. |
Value of the Data
|
1. Data
1.1. Work capacity
The work capacity in aged LRT and HRT female rats had a similar pattern to exercise capacity as previously reported [1]. By 22 months of age, baseline work capacity was 60% greater for HRT compared to LRT rats (Fig. 1A). Work capacity dropped roughly the same rate (39–42%) between 22 and 26 months for both LRT and HRT rats that remained sedentary (SED) (p < 0.001; Fig. 1A). Exercise training allowed LRT rats to maintain their work capacity unlike the LRT-SED group (Fig. 1A–B). After four months of training the aged HRT rats were able to increase their work capacity by 54% compared to their pre-training distance (p < 0.001; Fig. 1B).
Fig. 1.
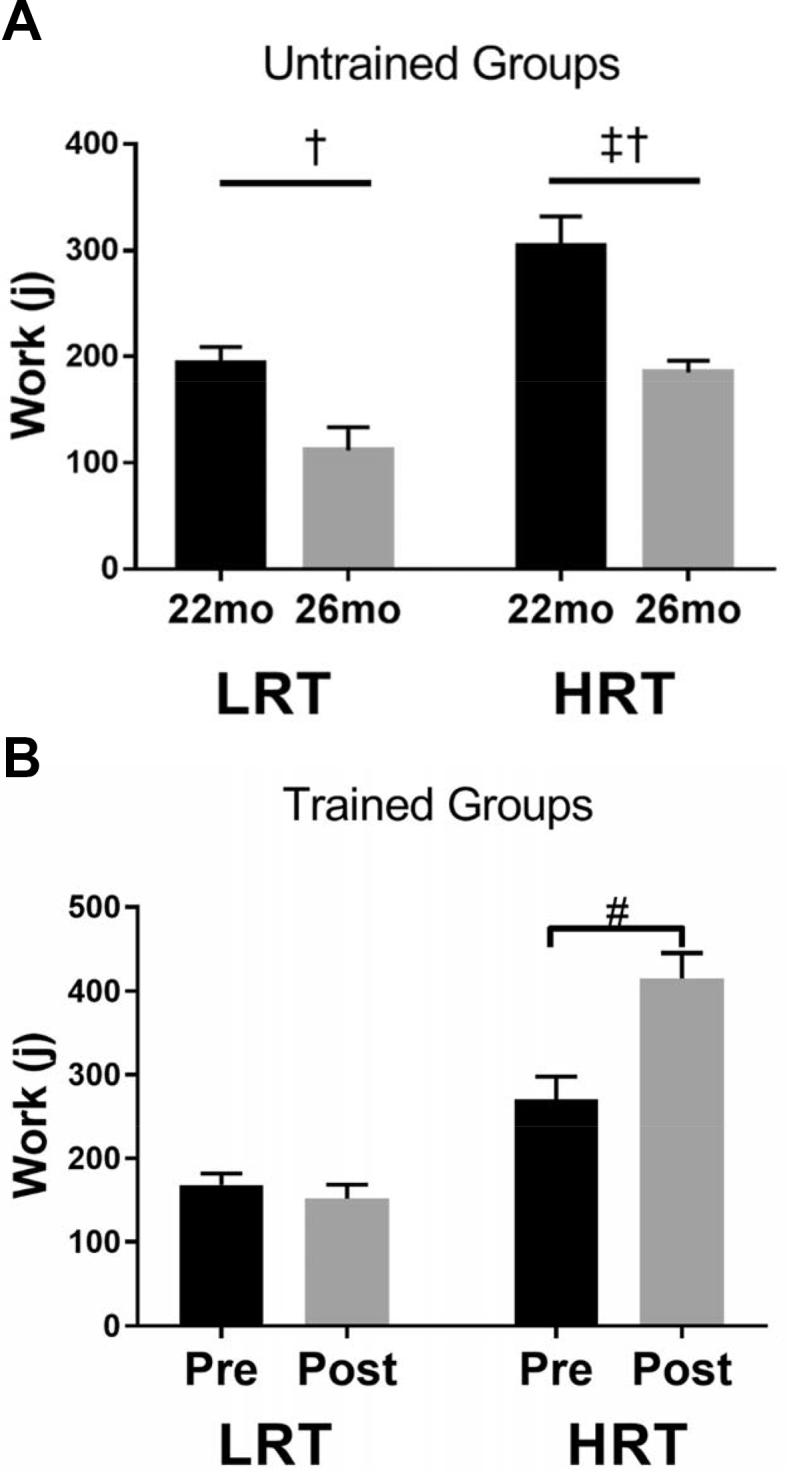
Total work capacity of aged low response trained (LRT) and high response trained (HRT) sedentary (SED) and trained (EXER) rats (n = 7–9). Data are shown for (A) 22 mo (black bars) and 26 mo (gray bars) rats that remained sedentary (B) rats before (Pre) and after (Post) four months of treadmill training. Work capacity of both LRT and HRT rats that remained sedentary decreased with age, † and HRT rats had a higher work capacity than LRT rats, ‡. Training increased work capacity in HRT but not LRT rats, #. Work capacity was calculated as force by distance. In all cases, p ≤ 0.05.
1.2. Contractile properties
EDL muscles of aged HRT rat generated 8% greater maximum isometric force compared to the LRT rats (p < 0.033; Fig. 2A). Specific force normalized for total muscle fiber cross-sectional area was also greater for aged HRT than for LRT rats (p < 0.050; Fig. 2B). Raw data can be found in the supplement material documents.
Fig. 2.
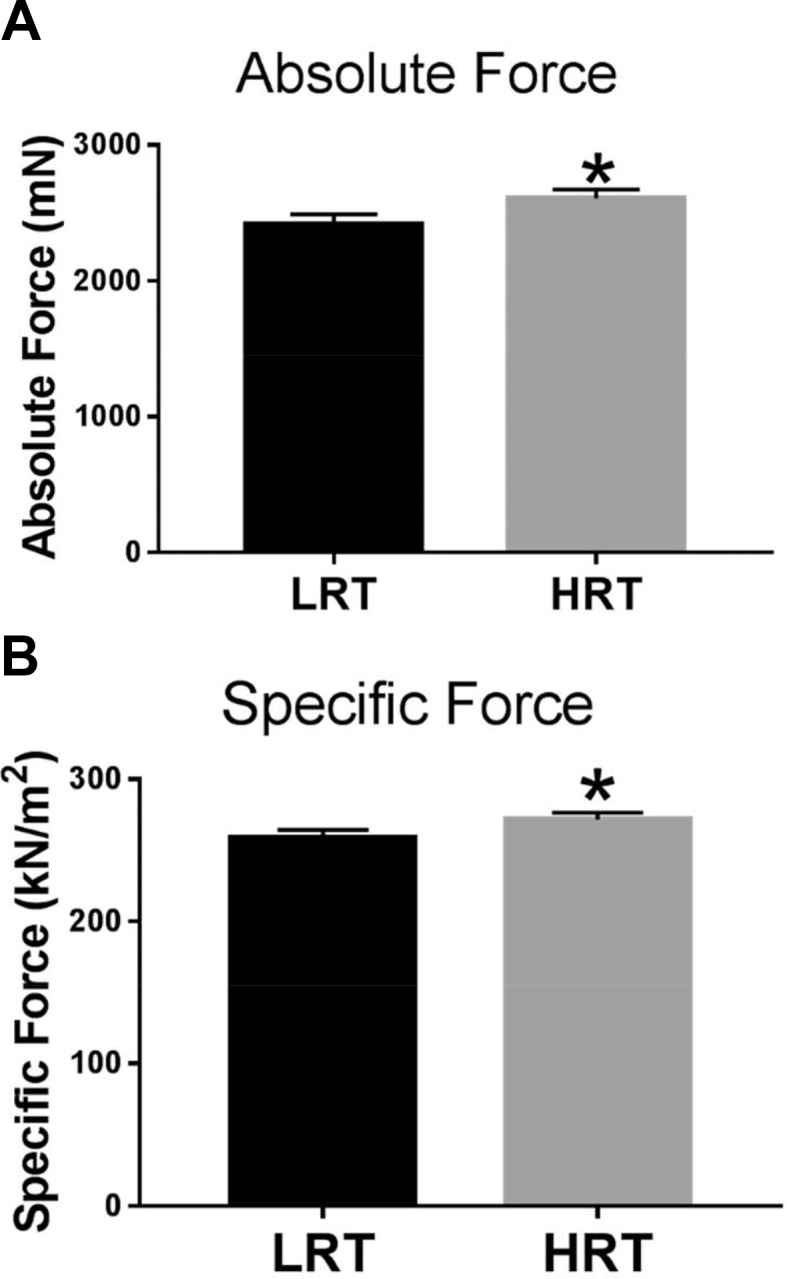
Absolute and specific force measures of aged LRT and HRT rats (n = 7–8). Data are shown for (A) absolute force and (B) specific force of pooled LRT and pooled HRT rats. HRT rats had greater absolute and specific force, *. p ≤ 0.05.
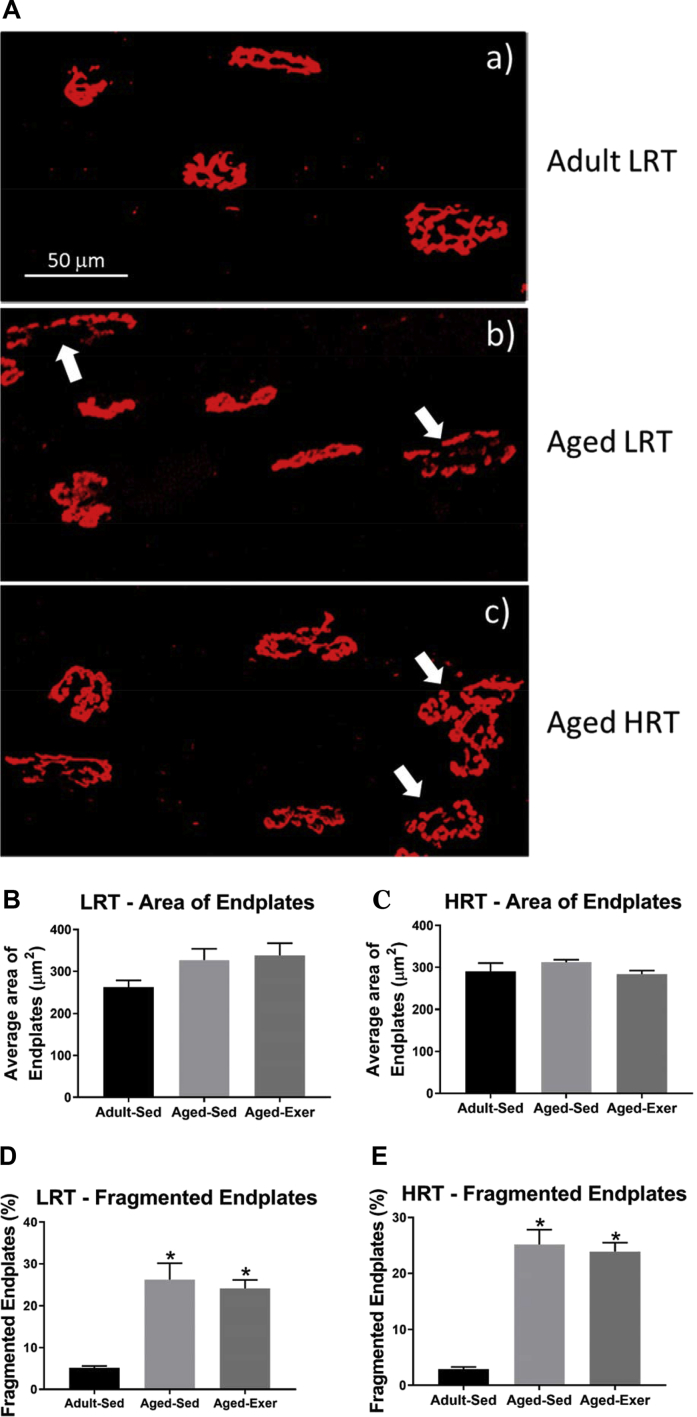
1.3. Assessment of motor endplates
There were no differences in area of endplates in the aged LRT-SED or aged LRT-EXER rats compared to the young LRT-SED controls (p = 0.112; Fig. 3B). Likewise, there were no differences in area of endplates in the aged HRT-SED or aged HRT-EXER rats compared to the young HRT-SED (p = 332; Fig. 3C). The percentage of endplate fragmentation in the aged LRT-SED was 5-fold greater compared to the young LRT-SED group (p < 0.001; Fig. 3D), while the percentage of endplate fragmentation in the aged LRT-EXER was similarly increased (p < 0.002; Fig. 3D). Age-related increases in endplate fragmentation were also observed in the HRT rats with 9-fold more fragmented endplates in the aged HRT-SED compared to the young HRT-SED group (p < 0.001; Figs. 3E) and 8-fold more in the aged HRT-EXER group (p < 0.001; Fig. 3E). Despite age-related increases in endplate fragmentation, the ultimate degree of fragmentation was similar in each group and exercise did not improve the morphology of the endplates in either LRT (p = 827; Fig. 3D) or HRT rats HRT rats (p = 870; Fig. 3E).
Fig. 3.
Motor endplate morphology and measures of area and fragmentation in extensor digitum longus (EDL) muscles of LRT and HRT rats (n = 4). Immunofluorescent stains are shown for (A) motor endplates of (a) adult LRT rats, (b) aged LRT rats, (c) aged HRT rats. Data are shown for average area of endplates of (B) LRT and (C) HRT rats and fragmentation of endplates of (D) LRT and (E) HRT rats. Fragmentation percentage calculated by five or more acetylcholine receptor segments. Fragmentation was greater in aged SED and EXER rats compared to adult SED, *. p ≤ 0.05.
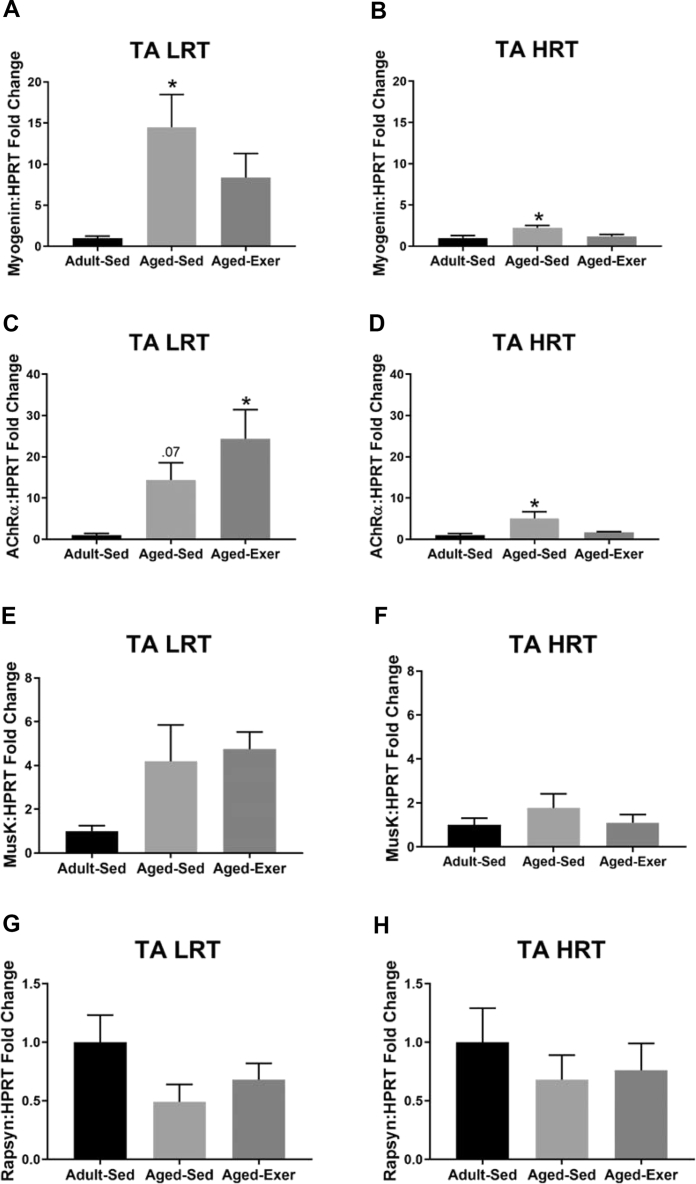
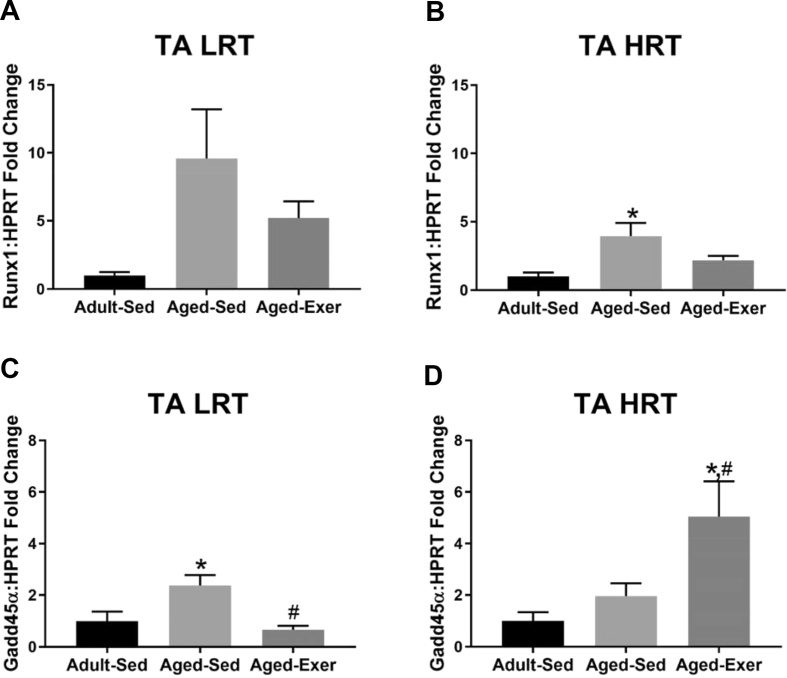
1.4. Markers of denervation
Fig. 4, Fig. 5, Fig. 6, Fig. 7 contain the gene expression data for select markers of denervation examined in the TA and GTN muscles of LRT and HRT rats. Raw and analyzed data can be found in the supplement material documents.
Fig. 4.
Gene expression of denervation associated markers in adult and aged LRT and HRT rats (n = 4–7) in the tibialis anterior (TA) muscles. (A) TA LRT Myogenin gene expression, (B) TA HRT Myogenin gene expression, (C) TA LRT AChRα gene expression, (D) TA HRT AChRα gene expression, (E) TA LRT MuSK gene expression, (F) TA HRT MuSK gene expression, (G) TA LRT Rapsyn gene expression, and (H) TA HRT Rapsyn gene expression. Age-related upregulation of genes associated with denervation were observed in aged sedentary (SED) and exercise (EXER) rats, *. p ≤ 0.05.
Fig. 5.
Gene expression of atrophy related denervation markers in adult and aged LRT and HRT rats (n = 4–7) in the TA muscles. (A) TA LRT Runx1 gene expression, (B) TA HRT Runx1 gene expression, (C) TA LRT GADD45α gene expression, and (D) TA HRT GADD45α gene expression. Age-related upregulation of gene associated with denervation were observed in aged sedentary (SED) and exercise (EXER) rats, *. Exercise-induced gene expression changes in GADD45α were observed in aged rats, #. p ≤ 0.05.
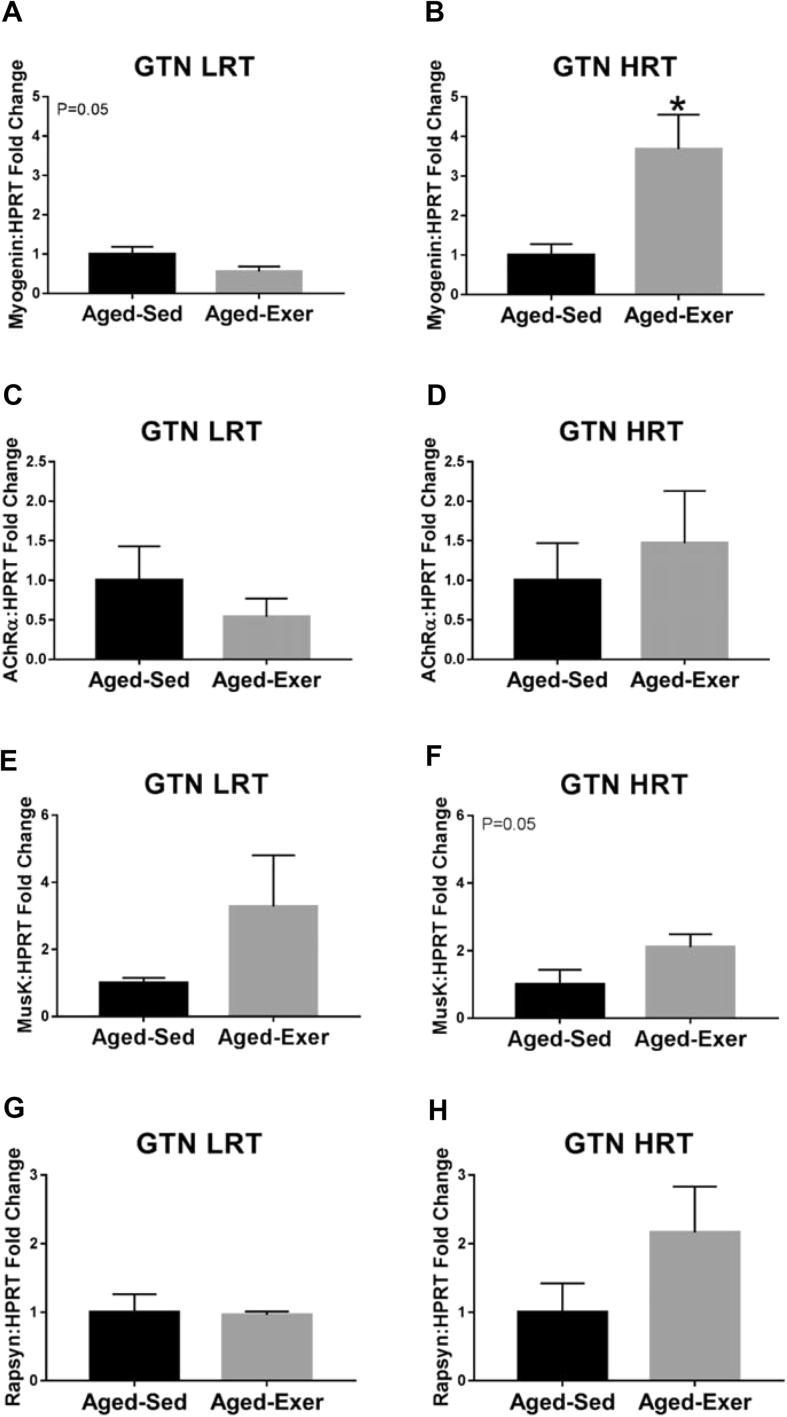
Fig. 6.
Gene expression of denervation associated markers in aged LRT and HRT rats (n = 3–6) in the gastrocnemius (GTN) muscles. (A) GTN LRT Myogenin gene expression, (B) GTN HRT Myogenin gene expression, (C) GTN LRT AChRα gene expression, (D) GTN HRT AChRα gene expression, (E) GTN LRT MuSK gene expression, (F) GTN HRT MuSK gene expression, (G) GTN LRT Rapsyn gene expression, and (H) GTN HRT Rapsyn gene expression. Exercise-induced upregulation of Myogenin was observed in aged HRT rats, *. p ≤ 0.05.
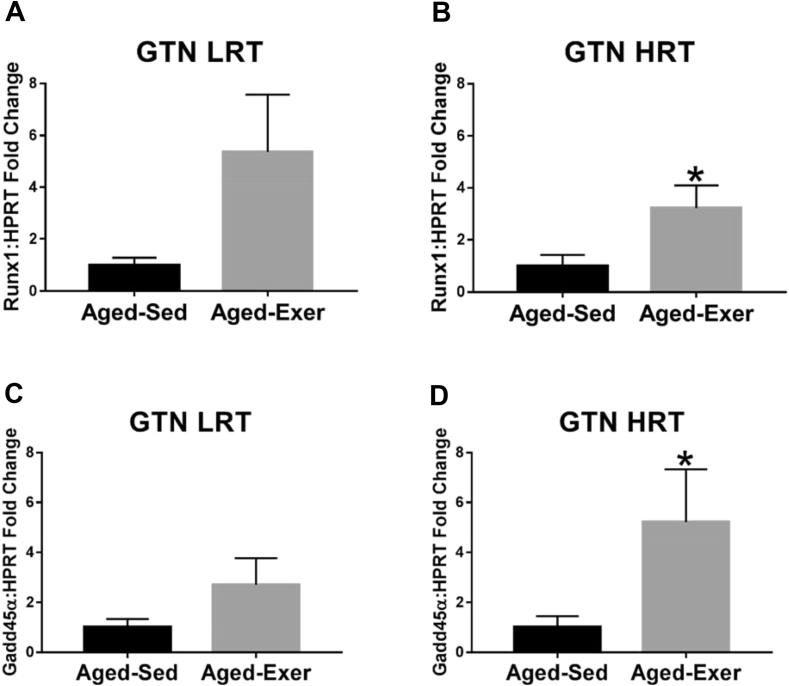
Fig. 7.
Gene expression of atrophy-related denervation markers in adult and aged LRT and HRT rats (n = 4–6) in the GTN muscles. (A) GTN LRT Runx1 gene expression, (B) GTN HRT Runx1 gene expression, (C) GTN LRT GADD45α gene expression, and (D) GTN HRT GADD45α gene expression. Exercise-induced upregulation of Runx1 and GADD45α was observed in aged HRT rats, *. p ≤ 0.05.
1.5. Markers of cell senescence
Fig. 8, Fig. 9 contain the gene expression data for markers of cellular senescence examined in the TA and GTN muscles of LRT and HRT rats. Raw and analyzed data can be found in the supplement material documents.
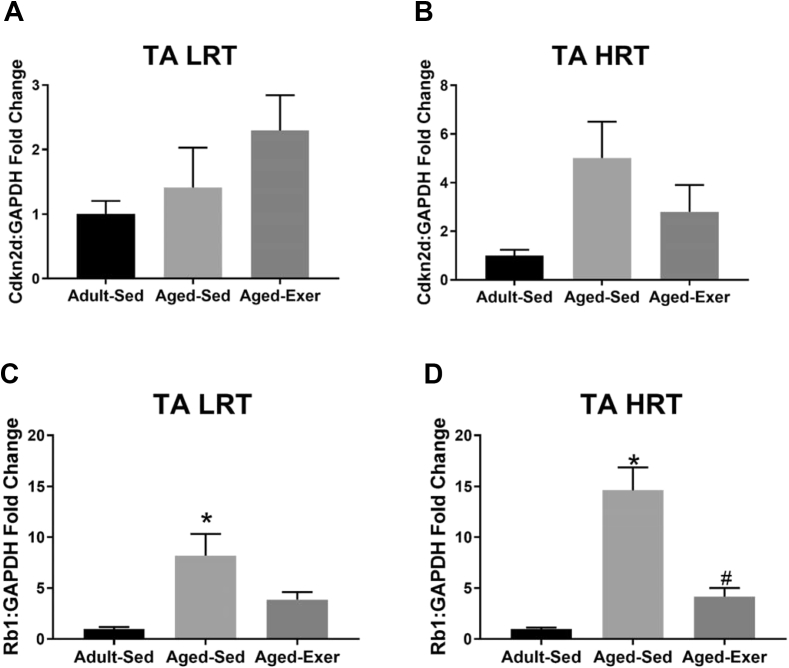
Fig. 8.
Gene expression of cellular senescence associated markers in adult and aged LRT and HRT rats (n = 5–7) in the TA muscles. (A) TA LRT Cdkn2d gene expression, (B) TA HRT Cdkn2d gene expression, (C) TA LRT Rb1 gene expression, and (D) TA HRT Rb1 gene expression. Age-related upregulation of Cdkn2d was observed in aged SED rats, *. Exercise-induced downregulation of Cdkn2d was observed in aged HRT rats, #. p ≤ 0.05.
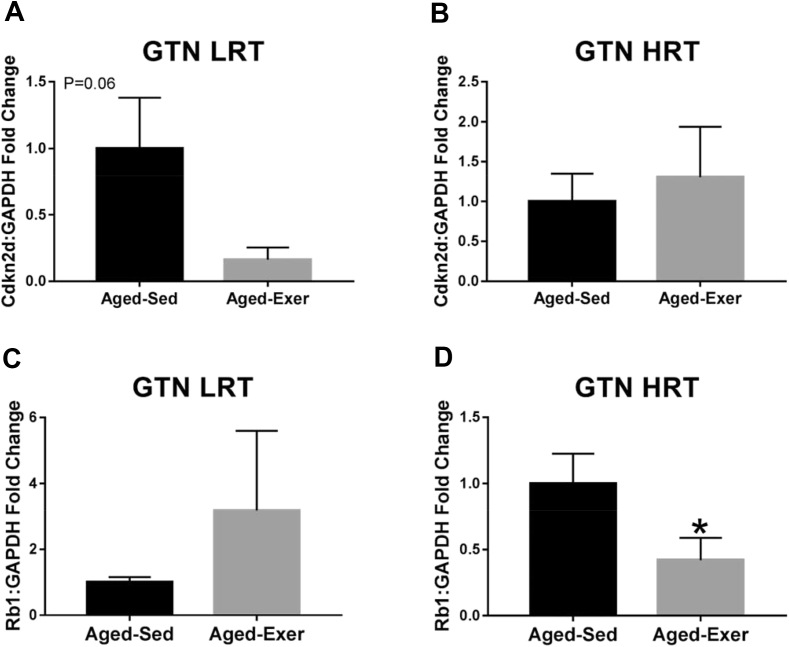
Fig. 9.
Gene expression of cellular senescence associated markers in aged LRT and HRT rats (n = 3–6) in the GTN muscles. (A) GTN LRT Cdkn2d gene expression, (B) GTN HRT Cdkn2d gene expression, (C) GTN LRT Rb1 gene expression, and (D) GTN HRT Rb1 gene expression. Exercise-induced downregulation of Rb1 was observed in aged HRT rats, *. p ≤ 0.05.
2. Experimental design, materials, and methods
2.1. Experimental rats
Selectively bred low response trainer (LRT) and high response trainer (HRT) rats were obtained from the Koch and Britton laboratory [2]. Thirty-one aged female rats (22 months) from the 20th generation of selection, 15 LRT and 16 HRT, and 12 adult female rats (11 months) from the 23rd generation of selection, 6 LRT and 6 HRT, were studied [1]. Animals were housed in the Unit for Laboratory Animal Medicine at the University of Michigan and all procedures conducted were approved by the University Institutional Animal Care and Use Committee.
2.2. Exercise training
LRT and HRT rats that were trained as previously described [[1], [2], [3]]. Sedentary (SED) and exercise (EXER) groups were introduced to running on a motor driven treadmill (Columbus Inst. Columbus, OH) for one week and tested for maximal treadmill running capacity on the following week as previously described [2,3]. After the testing period, rats in the EXER group trained 2–3 times a week, at 60% of their maximum tested running speed and duration for 16 weeks (37 total trials). SED rats performed maximal running distance tests for exercise capacity and were placed in non-moving treadmills during the training period.
2.3. Post-training
At 26 months of age in both SED and EXER groups, maximal treadmill running distance was measured as previously described as an indicator of exercise capacity [1,2]. Briefly, rats ran on a motor driven treadmill set at a constant grade of 15° and an initial speed of 10 m/min. Speed was progressively increased 1 m/min every 2 min until exhaustion. Exhaustion was operationally defined as the third time a rat remained on the shock grid for 2 s. The LRT and HRT rats that participated in treadmill training were sacrificed two days after their last exercise session.
2.4. Contractile force
Contractile properties of the left EDL muscles were collected as previously described [1,[4], [5], [6]]. The EDL muscles were then removed from the rat hindlimb and immediately placed in a bath containing Krebs mammalian Ringer solution supplemented with 11 mM glucose and 0.3 mM tubocurarine chloride. The bath was maintained at 25 °C and bubbled with 95% O2 and 5% CO2 to maintain a pH of 7.4. Custom designed software (LabVIEW, National Instruments, Austin, TX, USA) controlled pulse properties and servomotor activity and recorded data from the force transducer. The voltage of pulses was incrementally increased, and subsequently muscle length was increased or decreased to provide the length (Lo) that results in maximal twitch force (Pt). Muscles were held at Lo and stimulated with pulse trains of 300 ms at steadily increasing frequencies to generate isometric contractions.
2.5. Endplate fragmentation
The area and fragmentation of motor endplates were obtained as previously described [7,8]. Proximal or distal ends of the EDL muscles were sectioned at 10 μm using the CryoStar NX50 cryostat (Thermofisher Scientific, Waltham, MA). Sections were incubated with Alexa-594 conjugated alpha-Bungarotoxin (#B13423, Molecular Probes, Eugene, OR) for 24 hours in PBS at 4 °C. Sections were then washed for 3 hours in PBS. Motor end plate slides were imaged on a Nikon A1 confocal microscope at 20× magnification (Nikon, Tokyo, Japan), and analyzed with ImageJ (NIH, Washington D.C.). Age-related fragmentation was defined as an endplate with five or more AChR segments. Approximately 100 endplates were analyzed per muscle (n = 4 per group).
2.6. Polymerase chain reaction
cDNA was reverse transcribed from 1 μg of total RNA as previously described [1,[9], [10], [11], [12]]. Real-time PCR was performed, and results were analyzed by using the CFX Real-Time PCR detection system (Bio-Rad). cDNA was amplified in a 25 μL reaction containing appropriate primer pairs or probes and SYBR Green (Bio-Rad) or TaqMan Universal Mastermix (Applied Biosystems) primer pairs used for RT-PCR were designed as previously described [10] and are listed on Table 1. Fluorescence labeled probes for Chrna1 (Rn01278033_m1, FAM dye), Gadd45α (Rn01425130_g1, FAM dye), MusK (Rn00579211_m1, FAM dye), Myog (Rn01490689_g1, FAM dye), Rapsyn (Rn01486207_m1, FAM dye), Runx1 (Rn01645281_m1, FAM dye) and HPRT (Rn01527840_m1, FAM dye) were purchased from Applied Biosystems and quantified with TaqMan Universal mastermix. Cycle threshold (Ct) was determined, and the ΔCt value was calculated as the difference between the Ct value and the 18S Ct value. Final quantification of gene expression was calculated using the ΔΔCT method Ct = [ΔCt (calibrator) – ΔCt (sample)]. Relative quantification was then calculated as 2^-ΔΔCt.
Table 1.
Forward and reverse primer sequences of markers of senescence and housekeeping gene.
| Gene | Primer sequences 5′-3′ |
|
|---|---|---|
| Forward | Reverse | |
| Rb1 | CAGCGGAGTCCAAATTCCA | CCATGAGACACGAGTCAGGT |
| Cdkn2d | CTGAACCGCTTTGGCAAGAC | CCAGAGGCATCTTGGACGTT |
| Gapdh | AGTGCCAGCCTCGTCTCATA | GAGAAGGCAGCCCTGGTAAC |
2.7. Statistical analyses
All data was analyzed using GraphPad (Prism version 7.0, La Jolla, CA). Results are reported as mean ± SEM. Comparisons between the SED and EXER rats or pooled samples of the LRT and HRT rats were conducted using a Student's t-tests. In the analysis of adult and aged LRT and HRT rats, a one-way ANOVA was performed to analyze dependent variables. A two-way ANOVA was performed for exercise capacity to analyze the main effects of age and strain and if there were any interactions between dependent variables. When significant results were detected in either ANOVA analysis, differences among individual means were assessed with Tukey post-hoc analysis. Statistical significance was set at P ≤ 0.05.
Acknowledgments
The authors would like to thank Carol Davis for technical assistance collecting force data; Austin Qasawa, Eunice Lim, and Ragad Alsaeed for tissue preparation and collecting PCR data; and the Unit for Laboratory Animal Medicine (ULAM) for animal care. For technical contributions the authors thank the Microscopy and Image Analysis Laboratory (MIL) and laboratory staff. The work was supported by AG-051442 (to SVB) and Glenn Foundation (2015 Glenn Award to LGK). JLJ was supported by GM111725. Contact LGK (Lauren.Koch2@UToledo.edu) or SLB (brittons@umich.edu) for information on the LRT and HRT rats: these rat models are maintained as part of an Exercise Rat Resource for Researcher (ER3) at the Center for Hypertension and Precision Medicine, The University of Toledo, Toledo, Ohio.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104570.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships the could have appeared to influence the work reported in this paper.
Appendix ASupplementary data
The following are the Supplementary data to this article:
References
- 1.Brown L.A. Late life maintenance and enhancement of functional exercise capacity in low and high responding rats after low intensity treadmill training. Exp. Gerontol. 2019 doi: 10.1016/j.exger.2019.110657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch L.G., Pollott G.E., Britton S.L. Selectively bred rat model system for low and high response to exercise training. Physiol. Genom. 2013;45(14):606–614. doi: 10.1152/physiolgenomics.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessard S.J. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes. 2013;62(8):2717–2727. doi: 10.2337/db13-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wurtzel C.N. Pharmacological inhibition of myostatin protects against skeletal muscle atrophy and weakness after anterior cruciate ligament tear. J. Orthop. Res. 2017;35(11):2499–2505. doi: 10.1002/jor.23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal S.S., Faulkner J.A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am. J. Physiol. Cell Physiol. 1985;248(3):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- 6.Mendias C.L. Changes in skeletal muscle and tendon structure and function following genetic inactivation of myostatin in rats. J. Physiol. 2015;593(8):2037–2052. doi: 10.1113/jphysiol.2014.287144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdez G. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. U. S. A. 2010;107(33):14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang Y.C. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24(5):1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown L.A. Diet-induced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol. 2015;215(1):46–57. doi: 10.1111/apha.12537. [DOI] [PubMed] [Google Scholar]
- 10.Greene N.P. Mitochondrial quality control, promoted by PGC-1α, is dysregulated by Western diet-induced obesity and partially restored by moderate physical activity in mice. Phys. Rep. 2015;3(7) doi: 10.14814/phy2.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washington T.A. Monocarboxylate transporter expression at the onset of skeletal muscle regeneration. Physiolog. Rep. 2013;1(4):e00075. doi: 10.1002/phy2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloboda D.D., Brown L.A., Brooks S.V. The Journals of Gerontology: Series A; 2018. Myeloid Cell Responses to Contraction-Induced Injury Differ in Muscles of Young and Old Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.