Abstract
Dendrimers are hyperbranched nanoparticle structures along with its surface modifications can to be used in dental biomaterials for biomimetic remineralisation of enamel and dentin. The review highlights the therapeutic applications of dendrimers in the field of dentistry. It addresses the possible mechanisms of enhancement of mechanical properties of adhesives and resins structure. Dendrimers due to its unique construction of possessing inner hydrophobic and outer hydrophilic structure can act as drug carrier for delivery of antimicrobial drugs for treatment of periodontal diseases and at peripheral dental implant areas. Dendrimers due to its hyperbranched structures can provides a unique drug delivery vehicle for delivery of a drug at specific site for sustained release for therapeutic effects. Thus, dendrimers can be one of the most important constituents which can be incorporated in dental biomaterials for better outcomes in dentistry.
Keywords: Dentistry, Materials science, Dental plaque, Antimicrobial, Dendrimers, Adhesives, Biomimetic, Periodontal
1. Introduction
Oral cavity is a doorway to all the systems of human body. Dental biofilms comprising of polymicrobial species are the main source of dental disease [1]. Mechanical removal of these leads to prevention of spread of dental caries and periodontal diseases [2]. Antimicrobials have also been used as adjunct to mechanical aids, but these biofilms preserve their structural integrity and develops resistance against antimicrobial agents [3]. Dental diseases due to biofilms remains a major concern. Dental caries is a prevalent finding in the oral cavity caused by the acid producing bacteria. These acids disturb the acid–base equilibrium on the tooth surface. Due to this, the physiological equilibrium between demineralization and remineralization of the tooth structure shifts towards demineralization, which removes the hydroxyapatite crystals from the tooth causing caries [4].
Remineralization, which is a natural process can only compensate a certain amount of this acid challenge. When acid challenge becomes severe, natural process of remineralization is incapable to prevent caries progression [5]. Different materials, such as newer adhesive systems and composite resins [6, 7] are used to treat dental caries. However, they still have some disadvantages to their use. Secondary caries occurs under these materials, high occlusal stress can lead to their fracture and even microleakage leading to hypersensitivity. Therefore, the in-situ remineralization or regeneration of Hydroxyapatite (HA) under normal physiological conditions as an alternate restorative material is the need of the hour. Various types of nanoparticles are incorporated to improve of the shortcomings of these restorative materials. The need is to formulate a strategy which will simulate the roles of organic matrices to initiate process of biomineralisation on the surface of enamel and dentin.
The 20th century has observed some striking innovations in synthesis of polymers and improvements in the design of biodegradable macromolecules. Dendrimers is one of the results of these advances in the field of polymer science. For the first time, dendrimers were synthesized by 1970–1990 with unique architecture with suitable surface groups which could be fine-tuned [8, 9]. Dendrimer which is a type of nanoparticle is made up of unimolecular micelles having an interior hydrophobic and exterior hydrophilic shells acting as a carrier for drug. It arises from Greek word dendron which means ““tree” or “branch” [10, 11, 12, 13]. Additional names of dendrimers are “cascade polymers.” or “arboroles”. It has three parts, a central core, building blocks and multiple functional groups at periphery. The core at the centre encapsulates chemical species while the interior layers with repeating units in the building blocks is structured in a geometrical pattern leading to concentric layers named as “generations”.
2. Theory
2.1. Dendrimer generations
Dendrimers have many branches constituted of duplication elements having at least one branch connection, whose duplication is organized in a geometrical progression that outcomes in a series of radially concentric layers called “generations”. Dendrimers are produced in an iterative arrangement of reaction steps, in which each added iteration lead to a higher generation dendrimer. Each new layer creates a new 'generation', with double the number of active sites (called end groups) and around doubles the molecular weight of the former generation [14, 15, 16]. The increase in the number of dendrimer terminal groups is consistent with the geometric progression:
| Z = nc.nmG |
where z = molecular weight, nc = the branch-juncture multiplicity, nm = the core multiplicity, G = the number of generations. As dendrimers are synthesized by step-by-step growth method, the branching elements are shown by generation number. The core molecule is demonstrated by generation 0 (G0) whereas succeeding integration of branching units leads to generations G1, G2, G3, G4 and so on. Dendrimers of lower generations (G0, G1, and G2) have highly asymmetric shape and hold more open structures as compared to higher generation dendrimers [17]. As the chains grow from the core, molecule becomes longer and more branched (in G4 and higher generations) dendrimers accept a globular structure. Dendrimers become densely packed as they extend out to the periphery, which forms a closed membrane-like structure. When a critical branched state is reached dendrimers cannot raise because of a lack of space. This is termed the ‘starburst effect’ [18, 19, 20, 21].
Amongst the existing polymeric nanocarriers, dendrimer is the most extensively explored polymeric nanocarriers [10, 16, 22, 23]. The multivalent surface at the periphery with numerous functionalities interact with the outer environment to give dendrimers its macroscopic properties [18, 24, 25, 26, 27, 28, 29]. The polypropylene imine (PPI) dendrimers established by Meijer and Mulhaupt groups [21, 30, 31, 32] and polyamidoamine dendrimers (PAMAM) developed by Tomalia et al [28, 33, 34, 35] are extensively studied in field of medicine. Dendrimers can act as biomimics as their globular structure with diameters less than 10nm makes them with uniform shapes and sizes, that can be altered into different generations of dendrimers [23, 36, 37, 38]. These hyperbranched structures with peripheral functional groups provides with opportunity for having specific drug molecules on the surface in multivalent manner [39, 40, 41] Also interior of dendrimers with hydrophilic or hydrophobic shells helps them to encapsulate specific drug molecules [20, 42, 43, 44, 45]. Lastly, due to their low polydispersity, guarantees the reproducibility of biodistribution of polymeric agents utilizing them as scaffolds [46]. Dendrimers finds wide applications in transdermal, oral, ocular, pulmonary and target specific drug deliveries, microvascular extravasation, gene deliveries [8, 28, 47]. The shape and size of HA can be controlled by dendrimers in various concentrations, different generations and peripheral group modifications. This review aims to discuss the application of dendrimers dentistry in relation to the remineralisation of tooth structure, reduction in progression of periodontal disease, surface alterations of dental implant surfaces to enhance osseointegration and various other applications for improvement in properties of dental biomaterials used in dentistry.
3. Experimental
3.1. Applications of dendrimers in dentistry (Table 1)
Table 1.
Dendritic approach in the delivery of therapeutics in dentistry.
| Source | Type of Dendrimer | Generation | Objective | Methodical approach | Outcome | Source |
|---|---|---|---|---|---|---|
| (Gardiner et al., 2008) | PAMAM | G3 | Incorporation of triclosan in dendrimer to enhance solubility | π-π stacking between G3 dendrimer and the amino acid, phenylalanine to enhance solubility |
|
[95] |
| (Kim et al., 2010) | PAMAM | G5 | Modified G5 dendrimer could bind to dental pulp cell to increase odontogenic potential | G5 dendrimer with RGD ligand |
|
[108] |
| (Dodiuk-Kenig et al., 2004) | PAMAM | - | To check the adhesive properties of hyper-branched and dendritic polymers in acrylate-based dental composite | Commercial hyper-branched polyesteramide, two dendripolyamides and PAMAM dendrimer |
|
[78] |
| (Kawaguchi et al., 2011) | Commercial dendrimer | - | Evaluation of mechanical properties of methacrylated dendrimer crosslinked denture resin | Methylmethacrylate and crosslinker ethyleneglycol dimethacrylate or crosslinker dendrimer |
|
[107] |
| (Eichler et al., 2011) | PAMAM | G5 | PAMAM dendrimer could modify adsorption/desorption behaviour of human saliva compared to self-assembled monolayers grafted surface | Surface of the periodontitis model grafted with PAMAM-NH2 |
|
[112] |
| (Li et al., 2013) | PAMAM | G3 and G4 | Restorative substitution with PAMAM in human hard tissues to mimic the functions of noncollagenous proteins to promote mineralization | Carboxyl (-COOH) terminated G3 and G4 PAMAM dendrimers to substitute noncollagenous proteins on dentine surface |
|
[113] |
| (Bengazi et al., 2014) | Methyl methacrylate dendrimer | - | To investigate the degree of utilization of methyl methacrylate monomers in different dendrimer conjugated resins | The commercial dendrimers of different methyl methacrylate units (12 in D12 and 24 in D24) were incorporated in dental resin |
|
[105] |
| (Wu et al., 2013) | PAMAM | - | Investigated to mimic organic matrices tempted biomineralization procedure to develop tooth enamel with increased binding strength at the interface of remineralization | Hydroxyapatite anchored PAMAM-COOH-alendronate conjugate (ALN-PAMAM-COOH) for in situ mineralization approach |
|
[59] |
| (Paul et al., 2006) | Methyl methacrylate dendrimer | - | To enhance the composite properties in dental additive | Highly branched, globular 2,3-dihydroxybenzyl motif to achieve multi-methacrylate dendritic additive |
|
[80] |
| (Dung, Do and Yoo, 2013) | PAMAM | G5 | To obtain sustained release profile of the model antibacterial/antiprotozoal agent | G5-pluronic F127 (G5-PF127) nanofilms loaded with metronidazole |
|
[96] |
| (Chen et al., 2014) | PAMAM | G4 | Investigation of phosphate-terminated PAMAM dendrimer in the remineralization procedure of acid-etched human tooth enamel | G4 PAMAM dendrimer is converted to phosphate terminated PAMAM dendrimer using dimethyl phosphate to simulate amelogenin |
|
[61] |
| (Galli et al., 2014) | Poly(epsilon-lysine) dendron | G3 | Dendritic approach to titanium surfaces could improve differentiation of osteoblastic cells and the activation of Wnt/b-catenin signalling | Phosphoserine-tethered poly(epsilon-lysine) dendrons in endosseous implants |
|
[106] |
| (Ge et al., 2017) | PAMAM | G3 | The anti-caries effect and mechanical properties of the modified adhesive in biofilm regulation and remineralization capabilities | PAMAM and dimethylaminododecyl methacrylate in biofilm adhesive |
|
[77] |
| (Xiao et al., 2017) | PAMAM | G3 | Development of bioactive multifunctional composite (BMC) via nanoparticles of amorphous calcium phosphate, 2-methacryloyl-oxyethyl phosphoryl-choline, dimethylamino-hexadecyl methacrylate and silver nanoparticles for class V restoration Investigation of BMC with PAMAM dendrimer on remineralization of demineralized root dentin in a cyclic artificial saliva/lactic acid environment for the first time |
BMC complex mixture with nanoparticles of amorphous calcium phosphate, 2-methacryloyl-oxyethyl phosphoryl-choline, dimethylamino-hexadecyl methacrylate and silver nanoparticles And BMC with PAMAM dendrimer |
|
[76] |
| (Tao et al., 2017) | PAMAM | G4 | Determination of dentin remineralization extent with PAMAM dendrimer | PAMAM-OH, PAMAM-COOH, PAMAM-NH2 coated dentin |
|
[75] |
| (Lin et al., 2017) | PAMAM | - | Application of dendrimer functionalized with nano-hydroxyapatite in dentin tubule occlusion | Modification of nano-hydroxyapatite with COOH-terminated PAMAM dendrimer |
|
[74] |
| (Liang et al., 2017) | PAMAM | G3 | Remineralization of dentin in acidic environment (devoid of calcium/phosphate ion) with modified nanocomposite | Modified nanocomposite of PAMAM and nanoparticle of amorphous calcium phosphate |
|
[73] |
| (Gao et al., 2017) | PAMAM | G4 | Evaluation of PAMAM effectiveness and stability on dental tubule occlusion | Amine-terminated-G4-PAMAM in dental permeability |
|
[72] |
| (XIE et al., 2016) | PAMAM | G3.5 | Evaluation of the effects COOH-terminated PAMAM on dentinal tubules occlusion and biomineralization on human demineralized dentin | COOH-terminated PAMA |
|
[71] |
| (Zhang et al., 2015) | PAMAM | G4 | Dendrimer based dentine restoration | Phosphate-terminated PAMAM dendrimer |
|
[68] |
| (Xie et al., 2015) | PAMAM | Determination of remineralization effect of calcium hydroxide pre-treated with carboxyl modified PAMAM dendrimer | Carboxyl-modified PAMAM and pre-treated with Ca(OH)2 solution |
|
[70] | |
| (Wang et al., 2015) | PAMAM | G3 | Determination of the remineralisation effect of phosphorylated PAMAM dendrimers in demineralized dentin | Phosphate groups in PAMAM dendrimer through Mannich-type reaction |
|
[66] |
| (Liang et al., 2015) | PAMAM | G3 | Investigate the remineralization effect of amine terminated PAMAM on demineralized dentin |
Amine terminated PAMAM on remineralization |
|
[64] |
| (Zhou et al., 2014) | PAMAM | G4 | Evaluation of the effect of triclosan loaded carboxyl terminated PAMAM- dendrimer on the human dental caries. | Triclosan laded carboxyl terminated PAMAM |
|
[63] |
| (Backlund et al., 2014) | PAMAM | G1 | Investigate the bactericidal efficacy of propylene oxide modified PAMAM by nitric oxide releasing against cariogenic bacteria | Nitric oxide releasing scaffold propylene oxide modified PAMAM |
|
[100] |
| (Jia et al., 2014) | PAMAM | G4 | Evaluate the biological mineralization of the PAMAM dendrimer on the demineralized dentinal tubules | PAMAM dendrimer and peptide bond condensing agent |
|
[62] |
3.1.1. Biomimetic mineralisation: need of the hour
The concept of biomimetic mineralization has been applied in recent years in repair of damaged tooth. This process involves induction of in-situ remineralization of enamel and dentin by various biomaterials by an ‘amorphous precursor pathway’ that is mediated by non-collagenous proteins (NCPs) [48, 49, 50]. The key factor for this biomimetic mineralisation is to have proteins which can provide template for formation of inorganic components [51, 52]. As it is challenging for extraction of NCPs, the need is to formulate a macromolecular assembly which will simulate the structure and function of NCPs. The key feature in formulation of NCPs analog is to have precise steric assembly which can efficiently control biomineralisation. Research has shown that various materials have been tested to simulate organic matrices in regeneration of enamel and dentin by stimulation of HA remineralization. For example, an anionic peptide developed to produce scaffolds on lesions similar to on enamel under conditions similar to intraoral environment resulted in HA formation [53, 54].
Amelogenin protein and their supramolecular structures have been applied for regeneration of HA crystals on etched enamel [55]. Glycerine-enriched gel having fluorine and phosphate ions and mixture of nanoapatite particles and glutamic acid have depicted the formation of structures resembling enamel on the tooth surface. In addition, a glycerine-enrich gelatin gel having fluoride and phosphate ions [56], and mixture of nanoapatite particles and glumatic acid [57] have both shown to have the capacity of initiating enamel-like mineral on dental surface. Nonetheless, many of these trials have some drawbacks like challenges in formulating peptide structures, excessive use of various ions and difficulty in clinical application. Thus, its required to develop a concept which is simple and functionally mimics process of biomimetic mineralization on tooth surface. Dendrimer, frequently discussed as ‘artificial proteins’, has a well-defined structure, monodispersity similar to proteins and biomimetic properties [58] makes it an ideal candidate to simulate NCPs. These dendrimers can provide a biomimetic effect on the process of crystal formation on enamel and dentin hard tissues. Thus, dendrimers can be one of the most important proteins to be incorporated in dental biomaterials to initiate the natural process of remineralization.
3.1.1.1. Effect of dendrimers on remineralization of tooth
3.1.1.1.1. Remineralization of enamel
Tooth enamel possess HA crystals organized into a prism to form the core unit. Damage to this enamel can be caused by bacterial plaque, mechanical force and external beverages. Remineralisation of this damaged enamel is the key in restoration of natural structure. Dendrimer has shown the potential to initiate in-situ remineralisation of damaged enamel.
Wu et al assessed the prospects of carboxyl terminated PAMAM alendronate conjugate as a biomaterial for in situ remineralizing agent for the human enamel. In the in-vitro part of this investigation, the etched enamel slices were exposed to artificially synthesized carboxyl terminated PAMAM alendronate conjugate, which was the test group of the investigation whereas the control group consisted of etched enamel slices that were exposed to carboxyl terminated PAMAM dendrimer and etched enamel slices without any dendrimer treatment. All the groups were submerged in artificial saliva for 4 weeks which acted as a biomineralizing agent and subjected to SEM and Knoop microhardness analysis. For the in-vivo part, all three group discs were fixed on the inner part of the rats’ cheek for 4 weeks and then investigated by scanning electron microscopy (SEM). The carboxyl terminated PAMAM alendronate conjugate showed very low cytotoxicity and more adsorption on HA as compared to that of the carboxyl terminated PAMAM dendrimer and this adsorption could resist desorption by water. The ATR-IR spectra analysis showed tight binding capability of the carboxyl PAMAM alendronate conjugate on to the enamel surface inspite of washing the samples with PBS solution which is supposed to have stronger washing off power than deionized water. With the increase in time span, the remineralization of the test group increased compared to the acid etched enamel without any treatment or treated with carboxyl terminated PAMAM dendrimer and the morphology of this newly regenerated mineral closely resembled that of the normal tissue. The x-ray diffraction (XRD) study observed that the group exposed to dendrimer group maintained the orientation of the new crystals coating. The Knoop micro hardness analysis showed both the dendrimer treated acid etched enamel surfaces recovered the surface hardness as compared to the group without any dendrimer treatment. The test group showed highest recovery of the hardness and was 95.5% after 4 weeks which was close to that of the enamel of natural tooth. At the end of the 4th week, when all the three group samples of the in vivo study were subjected to SEM, the results were as follows: all three groups induced regeneration of HA, but the test group HA crystals closely matched to that of the natural tooth. All groups showed surface cracks due to the mechanical activity of the rats like chewing or grinding, however, the test group had a better surface morphology. The Alendronate (ALN) has a specific chemical binding with HA, thus acts as the HA attached functional unit, but due to the large steric and rigid structure of dendrimer the adsorption capacity of carboxyl terminated PAMAM alendronate conjugate on HA crystal surface was limited. The acid etched enamel offers additional adsorption owing to its increased surface area.
The desorption of carboxyl terminated PAMAM dendrimer is faster than the carboxyl terminated PAMAM alendronate conjugate and the free carboxyl terminated PAMAM dendrimer itself restrain the biomineralisation of the mineral crystals. The strong binding between the test sample and tooth enamel lead to few free dendrimer in the artificial saliva causing superior results [59, 60].
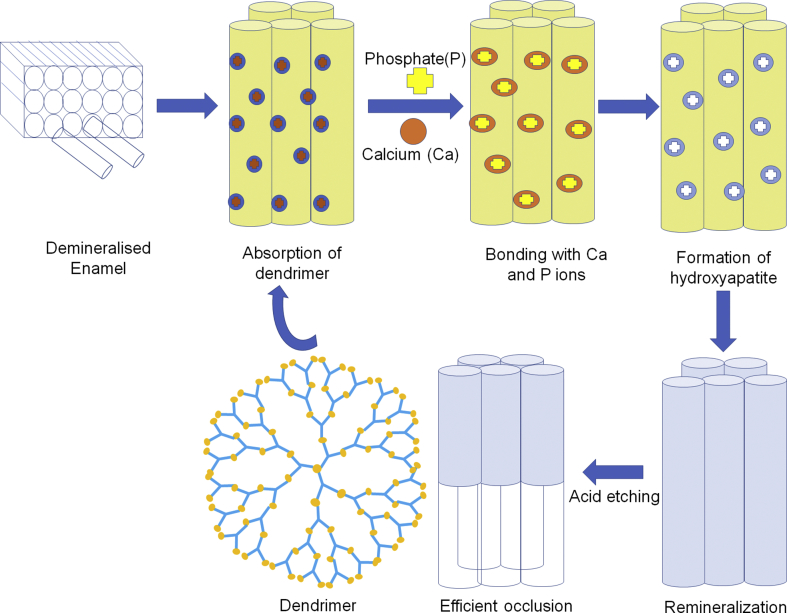
Chen et al. analysed remineralisation potential of phosphate-terminated dendrimer (PAMAM-PO3H2) as restorative material for tooth enamel. It was in-vivo study carried out in rats. Amelogenin is an extracellular matrix protein which plays a key role in formation of enamel. PAMAM-PO3H2 has a very high affinity towards calcium ions compared to carboxyl group and can have a strong binding ability with HA. (PAMAM-PO3H2) is dimensionally and functionally almost similar to amelogenin, hence its remineralisation capacity was assessed by XRD and SEM. The treatment of acid-etched tooth enamel with (PAMAM-PO3H2) depicted a newly formed HA layer (11.23 μm) compared to PAMAM-COOH (6.02μm). It was also observed that (PAMAM-PO3H2) can get strongly adsorbed on to the enamel by adsorption tests. The newly formed enamel with prism like structures were found to be analogous to human tooth enamel. Thus it was observed that (PAMAM-PO3H2) has pronounced potential as a biomimetic material for remineralisation of enamel [61]. Dendrimer by adsorption onto the enamel rods can form HA crystals resulting in remineralisation of enamel rods thereby preventing further damage (Fig. 1).
Fig. 1.
Remineralisation of demineralised enamel by dendrimers.
3.1.1.1.2. Remineralization of dentin
Li et al assessed the potential of 4th generation dendrimer for intrafibrillar mineralization of the dentin and thus its possible role as a substitute or restorative material. Six dentin disks were etched for 15 secs with 37% phosphoric acid and other 6 disks for 30 mins with 0.5M EDTA followed by immersion in guanidine chloride. Both the group disks were then exposed to 10,000 ppm concentration artificially synthesized 4th generation dendrimer solution whereas the controlled group was given treatment of deionized water and later immersed in artificial saliva and incubated followed by field emission scanning electron microscope examination. The dendrimer treated dentine disks showed both types of remineralisation (intra and interfibrillar on the surface as well as in the dentinal tubules. The intrafibrillar remineralization been the characteristic of 4th generation dendrimer and lacked by small molecule remineralization agents like sodium fluoride (NaF).
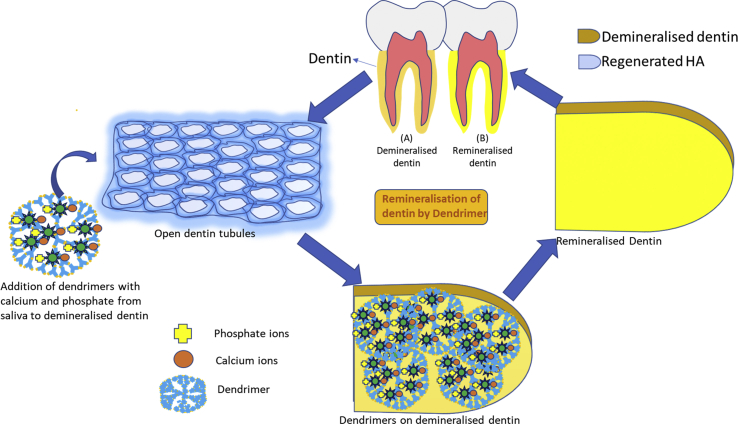
In vivo experiment also revealed regeneration of mineral crystals analogous to HA (Ca/P 1.67) of natural dentin. Non-collagenous proteins like dendrimers provides template to form nanoprecursors arranged in an orderly manner leading to intrafibrillar and interfibrillar mineralization. This being a process happening in 4 steps of binding of the dendrimer to the collagen fibrils at a specific site followed by attraction and stabilization of amorphous calcium phosphate nanoprecursors, these nanoprecursors slows the crystallization process to be converted into apatite crystals, which is vital for the purpose of intrafibrillar mineralization (Fig. 2). The nitrogen moiety acts as a complexation site of the nanoprecursors.
Fig. 2.
Remineralisation of demineralised dentin by dendrimers.
Jia et al studied the effectiveness of 4th generation PAMAM in the occlusion of open dentinal tubule by the process of biomimetic mineralization thus evaluating its potential in the treatment of dentinal hypersensitivity. In this controlled in-vitro study, the test group having 36 dentinal disc samples were immersed in the mixture of the 4th generation PAMAM, peptide bond condensing agent, energy dispersive spectrometer (EDC), and N-hydroxy succinimide for 30 mins whereas in control group, the dentinal disc specimen was immersed in deionized water for half an hour. All the specimens were then incubated in artificial saliva and observed for 2,4,6 upto 8 weeks. The 4th generation dendrimer has the potential to form a three-dimensional semi rigid structure which initiates the development of mineralized template on the dendrimer treated dentine thereby reducing the diameter and the density of the open dentinal tubules through the process of biomineralisation. But the present research did not observe the total blockage of the dentinal tubules and relation of the newly formed hydroxyapatite crystal to the collagen [62].
Zohu et al assessed the in-situ remineralization potential of triclosanloaded carboxyl-terminated PAMAM dendrimer on the etched human dentine thus evaluating its potential as a restorative material for treating damaged dentine. 3000 ppm and 6000 ppm triclosan loaded dendrimer formulations were prepared by adding and processing the Solid triclosan powder to the aqueous solution of 4th generation dendrimer.
The encapsulated triclosan loaded dendrimer showed strong binding property to the dentine surface and low cell cytotoxicity. With increase in time, the remineralization of the exposed dentine also progressed, with all the dentin tubules getting remineralized after 4 weeks. The 6000 ppm concentration showed more triclosan release in the incubated artificial saliva, higher degree of remineralization and more adsorption of the triclosan on the treated dentine compared to that of 3000 ppm concentration. The Ca/P ratio with 6000 ppm concentration (1.63) was similar to the HA in natural dentin (1.67). The 4th generation dendrimer had the potential to act as a carrier for triclosan molecule by binding to the dentine surface by electrostatic interaction as well as a controlled release delivery system thus inducing in situ remineralization of the etched (infected) dentine. Thus this biomaterial has the potential as a restorative material for repairing the carious dentine by means of amorphous precursor pathway [63].
Liang et al evaluated the potential of third generation dendrimer (amine terminated PMAMAM dendrimer) as a template for inducing dentin remineralization. Demineralized dentin discs were covered with fluorescein isothiocyanate (FITC) labelled third generation dendrimer. FITC labelling was done to observe the binding of the dendrimer to the demineralized dentin (test group). The control group specimens were covered with free FITC solution. Both group specimens were incubated in artificial saliva for 4 weeks. Third generation dendrimer had a good binding ability with the demineralized dentin which was observed by the yellow green fluorescence of the free FITC. The SEM study of the test group revealed progressive remineralization form 2 weeks–4 weeks with the dentinal tubules getting completely occlude by denser rod like crystal after 4 weeks. The energy dispersive spectroscopy revealed that the test group had Ca/P ratio (1.62) very similar to HA (1.67) in natural dentin unlike the control group. The XRD pattern confirmed the finding that the newly regenerated minerals in test group were HA. The test group exhibited a good capacity towards acid resistance, with all the tubules still occluded by the mineralized crystals. The mineral precipitation from in the control group resulted in a smooth surface compared to the baseline as evaluated by AFM ultra-morphology analysis.
The amine terminated PMAMAM dendrimer macromolecules has shown to have a firm binding with the demineralized dentinal collagen fibrils, and act as an ideal template to attract calcium ions and start the process of nucleation by virtue of NH2 and C=O. The regenerated hydroxyapatite itself acts as a new nucleation template to attract more phosphate and calcium ions leading to precipitation of needle like crystals on and within the tubules, but within the limits of the study no mineralization has been observed [64].
In another study, Liang et al, 2016 PAMAM+NACP lead to remineralization of dentin in an acid challenge environment compared to the other groups, thus proving the potential of PAMAM+NACP composite in inhibiting dental caries [65].
Wang et al in an in vitro study, used phosphorylated polyamidoamine (P-PAMAM) dendrimer along with a stabilizing agent amorphous calcium phosphate and polyacrylic acid as the potential dentine remineralizing agent on the demineralized dentine discs. Dentine disc were prepared from extracted caries free third molar and demineralized with 0.5M neutral EDTA. These dentine discs were immersed first into P-PAMAM for phosphorylation and then 16 of these discs were immersed into the biomimetic remineralization solution composed of stabilizing agent - amorphous calcium phosphate and polyacrylic acid for one week with the solution being changed every 2 days. The dentine discs immersed in the calcium phosphate solution without P-PAMAM dendrimer were considered as negative control.
Attenuated total reflection Fourier transfer infrared spectroscopy (ATR-FTIR) revealed that the dentine discs treated with P-PAMAM dendrimer had these functional groups introduced to collage molecules. Further these discs showed almost complete occlusion of the dentinal tubules to a depth of approximately 5μm. XRD pattern of these discs was observed to be similar to the sound dentin but the EDS revealed the presence of calcium deficient mineral crystals. P-PAMAM dendrimer had lesser cytotoxic effects on human dental pulp stem cells in comparison to the unmodified counterparts.
However, P-PAMAM dendrimer acted as a promoter of apatite nucleation/growth at lesser concentration (less than 100mL-1) but an inhibitor at higher concentration (more than 500mL-1). The P-PAMAM dendrimer which are regarded as artificial proteins have the ability to perform dual functions of induction of mineral nucleation binding to the collagen by virtue of its phosphate group along with the properties of excellent biocompatibility and low cytotoxicity. The biomineralisation potential can also be attributed to the amorphous calcium phosphate stabilizing potential of P-PAMAM dendrimer [66, 67].
Zhang et al investigated the in-vitro (in artificial saliva) and in-vivo (rat's oral cavity) effect of the 4th generation phosphate-terminated PAMAM dendrimer (G4-PO3H2) in repair of etched human dentin. The apparent remineralization in the experimental group (demineralised dentin discs treated with G4-PO3H2)) appeared after the 1-week immersion in the artificial saliva which increased over a period of time, and after 4th week the disks were fully covered with HA as that of the intact dentin.
The important aspect of this group was the intrafibrillar mineralization as the mechanical properties of the dentin are dependent on it thus demonstrating 95% of the surface microhardness recovery (SMHR) as compared to that of the control group (demineralised dentin discs treated with G4-COOH) (74% SHMR). The in-vivo results of the experimental group were in accordance with the in-vitro findings demonstrating relatively well arranged, compact, intact and dense mineral layer after 28 days with the Ca/P ratio (1.588) similar to the HA in human dentin (1.667). Whereas the control group showed limited, thin and loose mineral deposits.
The significance of superior results in the experimental group can be attributed to the encapsulation capability of (G4-PO3H2) produced by one-step modification along with it acting as an dentin phosphophoryn (DPP) analog to get attached to the exposed collagen matrix, attracting and stabilizing of the phosphate group leading to formation of apatite amorphous nanoprecursor, directing these nanocrystals along the microfibrils causing intrafibrillar mineralization and guiding the other apatite nanocrystals in orientation forming huge mesocrystals which further grow into large apatite sheets along and within the surface of the collagen fibrils [68, 69].
Xie F et al. evaluated remineralization potential of demineralized dentin by group [PAMAM + Ca(OH)2]: Ca(OH)2 pre-treated by carboxyl modified PAMAM. Remineralization effect of this group was compared with other groups like, PAMAM group treated with carboxyl-modified PAMAM, Ca(OH)2 group pre-treated with Ca(OH)2 solution, no treatment (control group). Off all the groups [PAMAM + Ca(OH)2] displayed the highest remineralization potential as per SEM. The XRD and EDS observation were positive for HA. Thus, it was concluded that PAMAM dendrimer with carboxyl modification and Ca(OH)2 pre-treatment has the potential for remineralisation [70]. In 2016, they also evaluated in-vivo biomineralisation potential of carboxyl-terminated PAMAM dendrimer (PAMAM-COOH) on demineralized dentin and its ability to occlude dentinal tubules. Test models of demineralization dentin treated with PAMAM-COOH and control without the treatment were placed and sutured towards the inside of the cheeks of the rats and were incubated in the saliva.
New HA crystals were observed in the test groups whereas the control group did not depict any growth. Newly formed HA showed similar characteristics to that of natural dentin. Also, the microhardenss was higher with them control to the controls. Thus, it was concluded that application of PAMAM-COOH possess the capability to induce biomineralisation and can find an application in dentin hypersensitivity due to its blocking action of dentin tubules [71]. They conducted another in-vivo investigation on the biomineralisation and ability of occlusion of dentinal tubules by (PAMAM-COOH) at various time points. The investigations were done on male Sprague-Dawley (SD) rats. Random assignment was performed with dentin specimens into control group (category A) and experimental group (category B). Microhardness of the dentin specimen treated with PAMAM-COOH was significantly higher than the control group (p < 0.001). XRD analysis of for PAMAM-COOH showed exhibited sharp diffraction peaks (characteristic of HA) while control specimens didn't exhibit any diffraction peaks. EDS test exhibited complete obliteration of tubules for test group while incomplete for control group. Significance is that there are two mechanisms projected for crystal formation stimulated by G3.5 PAMAM-COOH. First is dendrimer serving as nucleation site because of supramolecular aggregation and the second is the binding of calcium at the carboxylic group on the surface of the dendrimer which may function as a nucleus. PAMAM-COOH also has high bonding strength with demineralized dentin. G3.5 PAMAM-COOH causes biomimetic remineralisation of human dentin leading to increased surface microhardness [71].
Gao Y et al investigated the in-vitro effect and stability of the fourth generation amine-terminated PAMAM on occlusion of dentinal tubule in relation to dentin permeability. Demineralized dentine samples were coated with PAMAM, NaF solutions and distilled water and soaked in artificial saliva at 37 °C at immediate, one week, two weeks and four weeks. After four weeks, samples of both groups were equally divided into brushing challenge group and an acid challenge group. Fluid filtration system was used to measure dentine permeability of every sample. In brushing and acid etch challenge the blocking rate between PAMAM and NaF did not differ significantly (p > 0.1). Pre-challenge SEM micrographs showed that the tubules of PAMAM groups had needle-like crystals while in NaF group, minerals precipitation inside the tubules was only few microns underneath the surface. After acid etch challenge PAMAM yielded a significantly lower permeability than sodium fluoride because the PAMAM group had more crystals in the dentinal tubules as shown in the SEM [72].
Liang K et al investigated a novel technique of dentin remineralization by combination of PAMAM with a composite of nanoparticles of amorphous calcium phosphate and hardness in an acidic solution lacking Ca and P ions. Demineralized dentin samples were separated into four groups: dentin samples, dentin samples layered with PAMAM, dentin samples coated with NACP nanocomposite, dentin samples coated with PAMAM+NACP composite. . Ca and P peaks (p < 0.05) were highest for PAMAM + NACP compared with other groups in terms of chemical composition in remineralized dentin. NACP group had greater dentin hardness compared to PAMAM and control groups (p < 0.05) while PAMAM + NACP caused the maximum increase in dentin hardness. The significance is that two strategies worked together to promote dentin remineralization when using PAMAM + NACP viz. presentation of nucleation templates and creating a high concentration Ca and P ions locally. The PAMAM and NACP composite combination achieved dual benefits of promotion of remineralization and prevention of demineralization thereby leading to increase in hardness [73].
Lin X et al. investigated effect of reformed nano-hydroxyapatite (n-HAP) with PAMAM-COOH on occlusion of dentin tubules. In-vitro tests showed that dendrimer-functionalized n-HAP could effectively occlude the dentinal tubules by forming across linkage with collagen fibres compared to other groups. In addition, this modified n-HAP with PAMAM-COOH also showed potential to form HAP crystals which can seal off the tubules. Thus, these can have a clinical application of dentin hypersensitivity [74]. Tao et al in an invitro study assessed the extent of quantitative remineralization of the demineralized dentin using dendrimer with –OH, –COOH and –NH2 terminal groups. Using EDTA, the uniform thickness dentin disks were demineralized and treated with distilled water as a control and with dendrimer with –OH, –COOH and –NH2 terminal groups. All the treated dentin specimen were incubated in artificial saliva for four weeks and examined with transverse microradiography (TMR), SEM, EDS and Vicker's hardness testing. SEM revealed that the tubule occlusion was similar in PAMAM-NH2 and PAMAM-COOH and was noticeable than PAMAM-OH group. The tubule blocking rate of PAMAM-COOH and PAMAM-NH2 groups were significantly higher than PAMAM-OH group, and that of PAMAM-OH group was significantly higher than control group. The atomic percentages of Ca and P in dendrimer treated groups were significantly higher than the control group. The was the first of its kind in which quantitative remineralization of the demineralized dentin using dendrimer with –OH, –COOH and –NH2 terminal groups was evaluated. Significance here is that dendrimer acts as a template for remineralization. More charged –NH2 and –COOH terminal dendrimer cause more molecules binding to the collagen fibrils than the –OH group. Similarly charged –NH2 and –COOH terminal dendrimer has better ability to attract calcium phosphate that –OH terminal dendrimer resulting in better mineralization [75].
Xiao et al conducted a study to develop a bioactive multifunctional composite (BMC) with nanoparticles of amorphous 2-methacryloyloxyethyl phosphorylcholine (MPC), dimethylaminohexadecyl methacrylate (DMAHDM), calcium phosphate (NACP) and silver nanoparticles (NAg) and assess the effect of combined BMC + third generation dendrimer (PAMAM) on remineralization potential of the demineralized root dentin in an environment having lactic acid and artificial saliva. Two types of composites were prepared in this study- (1) Remineralizing and antibacterial and composite (2) Control (without antibacterial agents. Two commercially available composites served as control for mechanical testing like calculating the flexure strength and elastic modulus. BMC + PAMAM group showed greatest gain in the dentin hardness and degree of remineralization due to highest mineral regeneration in this group. Combination of MPC, DMAHDM, NACP, NAg and PAMAM could act as a composite (with protein repellent action) to repel bacteria attachment and reduce biofilm growth by virtue of presence of MPC polymer in it. The quaternary amines also acted as antibacterial due to their contact inhibition property along with Nag which is toxic to a variety of microorganisms. The NACP nanocomposite induced remineralization and release of P and Ca ions at low pH and PAMAM attracted Ca ions by Ca complexes with larger numbers of amine groups on the outer surface and amide groups in its branches. The combination of BMC + PAMAM showed the highest mineral regeneration and root dentin hardness almost resembling that of the healthy dentin due to the triple advantages of provision of nucleation templates, increased Ca and P ion concentrations and neutralization of acid. Thus this combination of BMC+PAMAM has a potential use in treatment of root caries to promote remineralization [76].
Yang et al. investigated the dentine bonding properties and biofilm regulating and remineralization potential of a novel adhesive containing dendrimer (PAMAM) and di methylaminododecyl methacrylate (DMADDM). The composite resin disks were coated with four bonding systems. SEM showed 1% PAMAM and 1% PAMAM + 5% DMADDM for the presence of newly formed minerals in dentinal tubules. The strong antibacterial effect of 5% DMADDM group and 1% PAMAM + 5% DMADDM group can be attributed to the property of contact killing mechanism and triggering the programmed cell death of DMADDM, thus attributing the anti caries effect and combination of PAMAM and DMADDM regulated the biofilm to a healthy condition. PAMAM has the property of biomineralisation established on particle based crystallization concept and DMADDM had no counteractive effect of PAMAM. Thus the novel adhesive with dual anti caries action having PAMAM and DMADDM could be a solution for various dental applications [77].
3.1.1.2. Effect of dendrimers on composite resins and adhesive systems
Dental composites are made up of inert filler and hydrophobic resin. Resin cure is achieved by polymerization stimulated by activators and or light. The cured composite possesses excellent wear resistance, flexural and compressive strength. (1) They have excellent aesthetic properties. However, they have a high shrinkage (2.5–4%) which can be of disadvantage in relation to microfractures. (2–4) Addition of fillers like silica, quartz, silica glass decreases the polymerization shrinkage and enhances the mechanical properties of composites. Hyper-branched (HB) and dendrimer polymers including polyethers, polyurethanes, polyesters and polyamides. Main characteristic of these is the low viscosity compared to the other additives. Dental materials encompassing dendrimer and HB polymers along with monomers used in dental composite resins can be a favourable technique for enhancing the properties of composite resin system.
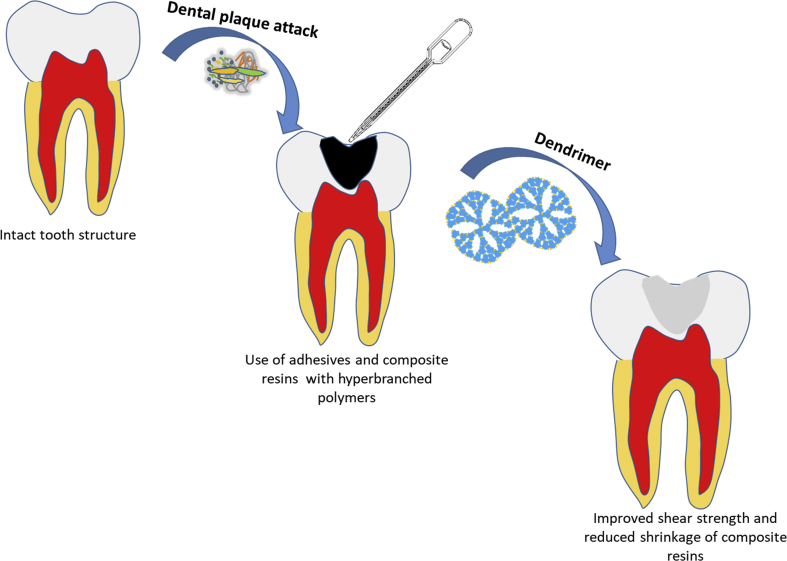
H. Dodiuk-Kenig et al. evaluated the effect of dendrimer and HB polymers on shrinkage and strength of dental composites and bond strengths of adhesive systems. In this experiment, HB (possessing hydroxyl or amide group) or dendrimer (carboxylate end groups) was used to replace a part of Bis-GMA [2,2-bis-[p-(2-hydroxy-3-methacryloyloxypropoxy)-phenyl] propane]. Four HB polymers were tested namely two dendripolyamides, PAMAM, and HB polyesteramide. It was observed that there was significant improvement in mechanical properties and decrease polymerization shrinkage of dental composites and improved shear strength and bond durability of adhesive systems due to addition of hyper-branched (HB) polyesteramide. This can serve as a guide in developing newer novel materials with inclusions of HB components for better and long-term durable properties of a dental restorative material [78, 79].(Fig. 3).
Fig. 3.
Addition of HB Polymers to adhesives and composite resins improves the mechanical properties of restorative material.
Paul N et al. investigated influence of addition of dendrimers on mechanical properties of dental composite materials. Incorporation of multi-methacrylate dendritic additive (0.5%) enhanced the flexural strength (21–35%) in comparison to control. Addition of dendrimers with higher molecular weights increased the flexural strength of composites. The acetone extractable product was reduced by 85% after incorporation of low concentrations of dendritic additives. Thus it was observed that at cross-linking, highly globular and even low concentrations of dendrimer additions also can improve properties of dental composite resins [80].
Viljanen EK et al. determined the degree of double bond conversion (DC) and thermal properties resins having dendritic copolymers. The test composites were the ones with filler particles along with dendrimer monomer, AAEM (acetoacetoxyethyl methacrylate) and MMA (methyl methacrylate) along with added BDDMA (difunctional methacrylate, 1,4-butanediol dimethacrylate) and compared to unfilled resins. It's important to study the DC as it influences the strength and properties of the polymers. It can prevent the leaching out of monomers preventing toxic effects or any allergic reactions [81].
3.1.1.3. Effect of dendrimers in periodontology
Periodontitis is an infectious-inflammatory disease elicited by oral bacteria organized in biofilms, causing loss of bone support and, in numerous cases, in tooth loss [82, 83]. Efficient removal of plaque and calculus is very effective in many cases [84], it has limitations in advanced periodontal disease conditions and aggressive periodontitis where the tissue invasive organisms can repopulate and cause recurrence of the disease condition. Hence adjunctive treatment like systemic antimicrobials can be advocated to present the recurrence and spread of the condition. There is robust evidence to support the use of systemic antibiotics as adjuncts to scaling and root planning (SRP) in the management of severe periodontitis [85, 86, 87, 88].
Over the years, various antimicrobials have been used along with SRP, combination of amoxicillin (AMX) and metronidazole (MTZ) has been shown to be very much effective in management of severe periodontitis [87, 89]. MTZ acts by inhibition of DNA synthesis. Most of the teeth surfaces are covered with biofilm. The organisms harbouring these biofilms are bacteria, fungi, viruses and protozoa. Dental plaque is a community of various microflora embedded in matrix of the bacterial polymers growing onto the teeth surfaces as a biofilm [90]. The bacteria present can cause periodontal disease and dental caries. Hence, most of the mouthwash and toothpastes include an antiplaque agent to prevent plaque formation. For effective action of any antibacterial agent, it should be released in sustained manner over a period of time to have a long-lasting effect. The use of drug delivery system can be useful to enhance drug release and retention for suitable mode of action. PMAMA dendrimers can find a vast application as drug delivery carriers in dentistry. The active molecules can either be conjugated onto the surfaces or incorporated into the dendrimer architecture [91, 92]. PAMAM have been used in various applications for delivery of active agents [93, 94]. The cationic PAMAM binds well to the anionic mucin displaying mucoadhesive properties for its use in the oral cavity.
J. Gardiner et al. evaluated various concentrations of PAMAM dendrimers (1–5mM) for their potential for solubilisation of Triclosan (TCN). The observations of the study were that, at lower pH, dendrimer was not able to maintain the previous observations of increased solubility of TCN. This highlights that solubilisation effect could be a result of TCN ionization instead of dendrimer encapsulation. Modification of the dendrimer was performed by incorporation of aromatic phenylalanine groups to the terminal amine groups. This was done to enhance the affinity of dendrimer with TCN through stacking interactions. However, these modifications didn't show any enhancement in solubility of TCN in comparison to buffer solutions. A different approach is being studied to observe conjugation of PAMAM with TCN via hydrolysable linkage [95].
Dung TH. studied the use of PAMAM dendrimer G5-pluronic F127 nanofilm as a suitable matrix for or sustained release of metronidazole as an antibacterial drug. Dendrimer nanofilm was loaded with different concentrations of metronidazole coated with and without gelatin. The erosion rate and drug release profiles were reduced in acidic conditions. Gelatin coating helped in prolonged release of metronidazole. G5-PF127 with 20% gelatin coatings depicted the best release of metronidazole. Thus, it was concluded that, PAMAM G5-pluronic F127 can serve as suitable carrier for delivery of antibacterial drugs at the required site. Thus, it can find a relevant application in field of periodontology to act as an adjunct for mechanical therapy [96].
Yuan Lu. investigated effect of Nitrous oxide (NO) releasing amphiphilic dendrimers (PAMAM) with exterior hydrophobicity (propylene oxide (PO), 1,2-epoxy-9-decene (ED), or a ratio of the two i.e. PO/ED) to eliminate biofilm bacteria and minimum cytotoxicity. Equal PO/ED modification showed highest efficacy in eliminating P. aeruginosa biofilms with minimum effect on viability of L929 mouse fibroblast. The exterior hydrophobicity had significant influence on bactericidal action and minimal cytotoxic effect. The optimal PO/ED observed were 7:3 and 5:5. Thus, study demonstrated that size and exterior properties of dendrimer can determine its bactericidal efficacy along with maintenance of its biocompatible properties [97].
Backlund et al. assessed antimicrobial efficiency of generation 1 (G1) polyamidoamine (PAMAM) (propylene oxide (PO)-modified) and NO-releasing 3-methylaminopropyltrimethoxysilane silica particles (MAP3) against Streptococcus Sanguis (S.sanguis)), Porphyromonas gingivalis (P.g) and Aggregatibacter actinomycetemcomitans (A.a). These are caries and periodontal disease-causing bacteria. It was observed that even though comparable amounts of NO were released by both, NO-releasing PAMAM showed superior antimicrobial efficacy in comparison to silica. The effect was more pronounced for P. g and A.a while Streptococcus mutans (S.mutans) and (S. sanguis) were less sensitive. It could be explained that, these cariogenic bacteria utilises nitrite reductase in conversion of nitrite to NO to enable survival in nitrite-rich acidic medium [98]. Another reason presented as per Gusarov and Nudler findings is that NO mediates protection against oxidative stress [99]. PAMAM dendrimers and silica based NO release displayed considerably less toxicity for human gingival fibroblasts at the levels required to kill periodontal pathogens (2 and 4 mg/mL, respectively) compared to clinical concentrations of chlorhexidine (0.12 and 0.20% (w/w). Thus, this observation shows that dendrimers or silica can act as potential scaffolds for release of NO for efficient eradication of A.a and P.g. These results advocate the possible use of novel material (macromolecular NO-release scaffolds) for the development of therapeutics for elimination of periodontal diseases [100].
3.1.1.4. Effects of dendrimers in implant dentistry
Titanium (Ti) is choice of material in dental implants. Inspite of being successful, lack of osseointegration leading to failure of implants is a concern. To mimic the biological situation and decrease the chances of infection and inflammation around implants, various surface modifications in terms of coatings, roughness, thread geometry have been used to enhance the long-term success. The ultimate aim is to improve the osseointegration and prevent the bacterial adhesion in relation to the implant surfaces [101]. Recently, nanotechnology has been applied to modify implant surface to enhance osseointegration. One of them is the implant surface modification by fluoride implant surface which showed better bone-to-implant (improved by 13%) in comparison with conventional rough implant surfaces [102]. Also, hydrophilic modified SLA surface depicted better BIC compared to the hydrophobic SLA surfaces [103]. Alternative approach could be to enhance and improve osseointegration is by dendrimer coatings on implant surface.
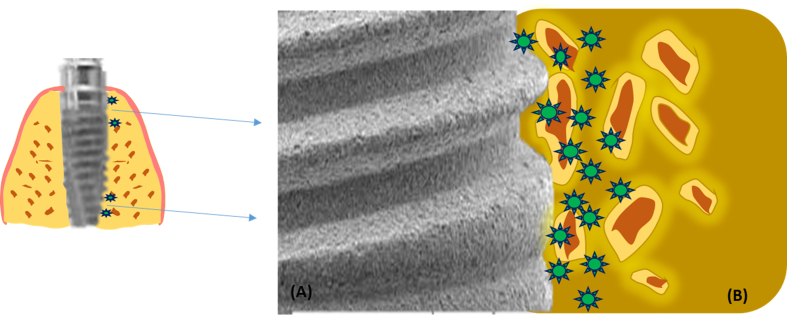
Scheideler et al. assessed in vitro the influence of different PAMAM dendrimers like PAMAM-NH2 and PAMAM-pyridinium as well as PAMAM-COOH on adhesion of early colonisers. It was observed that dendrimer coatings were efficient in reducing the S.g adhesion in vitro situations. However, they can also be utilised for in-vivo models in preventing the attachment of similar plaque bacteria onto the implant surface. Thus, it can be hypothesized that addition of PAMAM coatings can prevent peri-implant infections and enhance the success of implant survival [104] (Fig. 4).
Fig. 4.
Implant surface (A) coated with dendrimer. Localised delivery of these cations possess antibacterial action and also enhances osseointegration with the bone (B).
Bengazi F et al. compared the osseointegration of implants coated with and without coatings of dendrimers. It was an in-vivo study done in beagle dogs. The comparison was between zirconia sand blasted, acid etched (ZirTi) surface and dendrimer modified ZirTi by phosphoserine and polylysine installed in mandible of beagle dogs. It was observed that percentage of bone to implant contact (BIC%) was higher for ZirTi (74%) in comparison to dendrimer surface (65%) which was statistically significant. This study observed that dendrimer coating did not enhance osseointegration the surface coating of implants compared to conventional surface [105].Various surface functionalization approaches are being developed to mimic biomimetic environment to enable tissue repair and promote biomineralisation around implants. One of the method is the use of phosphatidylserine films on titanium implant surfaces to enhance rapid biomineralisation.
Galli et al. tested phosphoserine-tethered generation 3 poly(epsilon-lysine) [G3 PSL-PL] dendrons as film applications as implant coatings to improve the osteoblasts differentiation and Wnt/b-catenin signalling. MC3T3 osteoblastic cells derived from murine model along with mesenchymal, undifferentiated C2C12 cells and bone marrow cells were utilised to test titanium disks with and without dendron coatings. Reporter assay was used to test for Wnt signalling pathway and Real Time PCR for analysis of gene expression. Test results showed that there were increased expression osteoblastic markers namely osteocalcin and alkaline phosphatase. These were positive for primary bone marrow cells as well as MC3T3 cells. Expression of Wnt genes, mRNA levels of Wisp-2 and of b-catenin and osteoprotegerin were also noticeably high in cells growing on dendron coated surfaces. The signalling pathway activation in cells growing on dendron films was established by utilisation of TCF/b-catenin reporter system in the C2C12 cell line. To conclude, findings of this study shows that G3 PSL-PL can act as stimulation for initiation of osteogenic signalling cascades in-vitro and stimulate differentiation of mesenchymal cells into osteoblasts. Thus, in vivo testing might help to use these films as novel coatings to enhance the process of osseointegration and prevent implant failures [106].
3.1.1.5. Effects of dendrimers on denture materials
Kawaguchi et al investigated the effect of methacrylated dendrimers (DD1) on flexural modulus, flexural strength and hardness of resin matrix of denture base resins. There was a statistically significant difference incorporation of quantity of cross linkers on flexural strength (p < 0.05), however it was not significant for flexural modulus. Incorporation of dendrimer showed a significant result on flexural modulus (p = 0.0196) and on microhardness in comparison to EGDMA (p < 0.001). Addition of DD1 had a high influence on mechanical properties of polymer. Resin cross-linked with DD1 was stiffer and high in hardness compared to those of EGDMA. The reason for this is that DD1 resists extreme movements by providing anchoring points. Incorporation of small quantities of cross linkers increased modulus and flexural strength however they decreased with addition of large quantities of the cross linkers. This can explain the formation of IPN (interpenetrating polymer network) layer which may vary with larger quantities of cross linkers and gradually influenced the flexural properties. Thus from clinical view point, addition of dendrimer as cross linker for denture base resins can be useful to enhance flexural properties of the material [107].
3.1.1.6. Effect of dendrimers on dental pulp cell differentiation
Kim et al studied the effect of 5 PAMAM dendrimers (G5) on binding and differentiation capacity of dental pulp cells using western blot. Dental pulp cells showed strong binding and uptake with this conjugate dendrimer and demonstrated increase in the gene expression of Dentin matrix protein (DMP-1), dentin sialoprotein (DSPP), vascular endothelial growth factor (VEGF) and matrix extracellular phosphoglycoprotein (MEPE). Treatment of dental pulp cells with the conjugate dendrimer led to increased mineralization (measured by Von Kossa assay) suggests that PAMAM dendrimers conjugates with cyclic RGD peptides by targeting the integrins of the dental pulp cells can increase the odontogenic potential of these cells [108].
3.1.1.7. Effect of dendrimers on cancer
Yan Y et al [109], conducted a therapeutic investigation by developing a unique carboxyl-terminated dendrimer as a therapeutic agent for delivery of drug at a specific target. The carboxyl-terminated dendrimer enveloped with platinum nanoparticle exhibits selective action at the osteolytic areas of tumors without affecting the healthy tissues. Due to higher density of carboxyl-terminated dendrimer provides the polymer with effective binding with the host bone. It also exhibits good biocompatibility. Thus, this type of dendrimer can serve as a potential agent for delivery of a drug for treatment of bony lesions in relation to malignant tumors.
Liu X et al [110] experimented in vitro the effect of PAMAM -mediated short hairpin RNA (shRNA) against Human telomerase reverse transcriptase (hTERT) in oral cancer. It was observed that PAMAM mediated shRNA proficiently silenced the hTERT which prevented the cellular multiplication and resulted in apoptosis. This cancer in which hTERT is widely observed, dendrimer mediate silencing can provide a effective option for treating oral cancers. Ma et al [111] investigated use of PAMAM dendrimers for delivery of sulfamethoxazole as antibacterial agent. They experimented effect of PAMAM dendrimer (G2-G4) on solubility and in vitro effect on release of sulfamethoxazole along with PAMAM. The G3 PAMAM (10 mg/ml) exhibited 40-fold rise in solubility of sulfamethoxazole in comparison to double distil water at around 37 °C. with advancement from lower to higher generation, there was a proportional increase in solubility of sulfamethoxazole. Thus, PAMAM dendrimers were able to increase the solubility of sulfamethoxazole to enhance its use for clinical applications. Furthermore, G3 PAMAM along with sulfamethoxazole depicted 4 or 8 –fold surge in antibacterial action compared to sulfamethoxazole along with NaOH solution. Thus under appropriate environment, PAMAM can be considered an effective drug carrier for sustained release of sulfonamides to achieve the desired results.
4. Conclusion
PAMAM dendrimer has shown to have marked prospective to be used as biomimetic biomaterial for remineralisation of enamel. It has been tested various modifications like OH, –COOH and –Nh2 terminal groups. It induces regeneration of HA crystals which has shown to increase the hardness of acid etched enamel. This is similar to that of hardness of natural tooth enamel. The phosphate-terminated PAMAM which is functionally similar to amelogenin, has a very strong affinity for calcium ions in comparison to carboxyl group and it can also have a high binding capability with HA. Hence, PAMAM dendrimer with structural modifications can serve as an excellent biomaterial for remineralization of demineralised enamel due to acids produced by cariogenic bacteria [59, 61].
Majority of problems associated with dentin are due to its demineralisation and increase in the permeability of dentin tubules. Dendrimers have depicted double benefits by stimulation of remineralization and prevention of demineralization. Application of dendrimers on etched dentin has shown to have stable bond with demineralised collagen fibrils and serves as a three-dimensional template to attract calcium ions to initiate the process of nucleation of formation of HA. These nanoprecursors serves foci for attraction of more phosphate and calcium ions leading to intrafibrillar and interfibrillar mineralisation. This process of biomineralisation reduces diameter of dentin tubules. Also, newly produced HA structure is similar in properties to that of natural dentin. The other mechanism is that these dendrimers forms a firm bond with demineralised dentin which resists the salivary flow action before it can accomplish the formation of HA crystals [66]. Thus, it has capability to induce biomineralisation and can find an application in dentin hypersensitivity due to its blocking action of dentin tubules, incorporation of dendrimers in dental materials can repair carious dentin by remineralization of the loss tooth structure.
Addition of dendrimers has shown significant enhancement of mechanical properties of adhesive systems and reduction in polymerization shrinkage of dental composites. It also causes improvement in shear strength and better bonding durability of adhesive systems [78, 80].
Due to its improved mucoadhesive property and its hyperbranched structure with inner core, it can serve as a unique scaffold for release of reactive oxygen species (eg.NO) to eradicate tissue invasive organisms like A.a and Pg [100]. Thus, it offers the possibility for development of materials with antimicrobial potential in management of periodontal disease [63, 96]. They can be used as antibacterial coating on titanium implant surfaces to prevent peri-implant infection and enhance osseointegration. Thus, can act as surface modifiers in implantology to improve the longevity of implant treatment [106]. Incorporation of dendrimer as cross linking agent in denture base resins is beneficial in improving of the flexural properties of the material [107]. Dendrimers possess the property of increasing the odontogenic potential of pulp cells [108].
Thus, it can be concluded that due to triple action of dendrimers of neutralization of acids, nucleation precursors of HA crystals and high phosphate and calcium ions concentrations, it has the capacity to stimulate biomineralisation and can serve a biocompatible agent in treatment of carious lesions, dentin sensitivity. Also, due its high bond strength, hyperbranched structure and mucoadhesive property it can serve as superior biomaterial for dental restorations, surface coating of dental implants and treatment of periodontal diseases.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Allaker R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010;89:1175–1186. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 2.Baehni P.C., Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003;9(Suppl 1):23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 3.Jenkinson H.F., Lamont R.J. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13:589–595. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Geddes D.A. Acids produced by human dental plaque metabolism in situ. Caries Res. 1975;9:98–109. doi: 10.1159/000260149. [DOI] [PubMed] [Google Scholar]
- 5.Featherstone J.D.B. Remineralization, the natural caries repair process--the need for new approaches. Adv. Dent. Res. 2009;21:4–7. doi: 10.1177/0895937409335590. [DOI] [PubMed] [Google Scholar]
- 6.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 7.Drummond J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bapat R.A., Chaubal T.V., Joshi C.P., Bapat P.R., Choudhury H., Pandey M. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C. 2018 doi: 10.1016/j.msec.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 9.Jain K., Kesharwani P., Gupta U., Jain N.K. Dendrimer toxicity: let’s meet the challenge. Int. J. Pharm. 2010;394:122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Mansuri S., Kesharwani P., Tekade R.K., Jain N.K. Lyophilized mucoadhesive-dendrimer enclosed matrix tablet for extended oral delivery of albendazole. Eur. J. Pharm. Biopharm. 2016;102:202–213. doi: 10.1016/j.ejpb.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Jain S., Kesharwani P., Tekade R.K., Jain N.K. One platform comparison of solubilization potential of dendrimer with some solubilizing agents. Drug Dev. Ind. Pharm. 2014 doi: 10.3109/03639045.2014.900077. [DOI] [PubMed] [Google Scholar]
- 12.Gothwal A., Kesharwani P., Gupta U., Khan I., Iqbal Mohd Amin M.C., Banerjee S. Dendrimers as an effective nanocarrier in cardiovascular disease. Curr. Pharmaceut. Des. 2015;21:4519–4526. doi: 10.2174/1381612820666150827094341. http://www.ncbi.nlm.nih.gov/pubmed/26311317 [DOI] [PubMed] [Google Scholar]
- 13.Bakshi H.A., Mishra V., Satija S., Mehta M., Hakkim F.L., Kesharwani P. 2019. Dynamics of Prolyl Hydroxylases Levels during Disease Progression in Experimental Colitis, Inflammation; pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesharwani P., Tekade R.K., Jain N.K. Generation dependent cancer targeting potential of poly(propyleneimine) dendrimer. Biomaterials. 2014;35:5539–5548. doi: 10.1016/j.biomaterials.2014.03.064. [DOI] [PubMed] [Google Scholar]
- 15.Kesharwani P. 2015. Effect of Generation G on Cancer Targeting Propensity of PPI Dendrimer.https://www.morebooks.de/store/gb/book/effect-of-generation-g-on-cancer-targeting-propensity-of-ppi-dendrimer/isbn/978-3-8443-9285-2 [Google Scholar]
- 16.Choudhury H., Gorain B., Pandey M., Khurana R.K., Kesharwani P. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int. J. Pharm. 2019;565:509–522. doi: 10.1016/j.ijpharm.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Kesharwani P., Tekade R.K., Jain N.K. Dendrimer generational nomenclature: the need to harmonize. Drug Discov. Today. 2015 doi: 10.1016/j.drudis.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Thakur S., Kesharwani P., Tekade R.K., Jain N.K. Impact of pegylation on biopharmaceutical properties of dendrimers. Polymer (Guildf) 2015;59:67–92. [Google Scholar]
- 19.Thakur S., Kesharwani P., Tekade R.K., Jain N.K. Impact of pegylation on biopharmaceutical properties of dendrimers. Polym. (United Kingdom) 2015;59 [Google Scholar]
- 20.Kesharwani P., Gajbhiye V., Jain N.K. A review of nanocarriers for the delivery of small interfering RNA. Biomaterials. 2012;33:7138–7150. doi: 10.1016/j.biomaterials.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury H., Pandey M., Yin T.H., Kaur T., Jia G.W., Tan S.Q.L. Rising horizon in circumventing multidrug resistance in chemotherapy with nanotechnology. Mater. Sci. Eng. C. 2019;101:596–613. doi: 10.1016/j.msec.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Kesharwani P., Xie L., Banerjee S., Mao G., Padhye S., Sarkar F.H. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surfaces B Biointerfaces. 2015;136:413–423. doi: 10.1016/j.colsurfb.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi P.K., Gorain B., Choudhury H., Srivastava A., Kesharwani P. Dendrimer entrapped microsponge gel of dithranol for effective topical treatment. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomalia D.A. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005;30:294–324. [Google Scholar]
- 25.Kesharwani P., Mishra V., Jain N.K. Generation dependent hemolytic profile of folate engineered poly(propyleneimine) dendrimer. J. Drug Deliv. Sci. Technol. 2015;28 [Google Scholar]
- 26.Luong D., Kesharwani P., Deshmukh R., Mohd Amin M.C.I., Gupta U., Greish K. PEGylated PAMAM dendrimers: enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Patel H.K., Gajbhiye V., Kesharwani P., Jain N.K. Ligand anchored poly(propyleneimine) dendrimers for brain targeting: comparative in vitro and in vivo assessment. J. Colloid Interface Sci. 2016;482:142–150. doi: 10.1016/j.jcis.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 28.Kesharwani P., Jain K., Jain N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014;39:268–307. [Google Scholar]
- 29.Zeeshan F., Tabbassum M., Kesharwani P. Investigation on secondary structure alterations of protein drugs as an indicator of their biological activity upon thermal exposure. Protein J. 2019:1–14. doi: 10.1007/s10930-019-09837-4. [DOI] [PubMed] [Google Scholar]
- 30.Wörner C., Mülhaupt R. Polynitrile- and polyamine-functional poly(trimethylene imine) dendrimers. Angew Chem. Int. Ed. Engl. 1993;32:1306–1308. [Google Scholar]
- 31.de Brabander-van den Berg E.M.M., Meijer E.W. Poly(propylene imine) dendrimers: large-scale synthesis by hetereogeneously catalyzed hydrogenations. Angew Chem. Int. Ed. Engl. 1993;32:1308–1311. [Google Scholar]
- 32.Gorain B., Choudhury H., Pandey M., Kokare C., Khurana R.K., Sehdev A. Polyester, polyhydroxyalkanoate nanoparticles as a promising tool for anticancer therapeutics. Polym. Nanoparticles Promis. Tool Anti Cancer Ther. 2019:101–121. [Google Scholar]
- 33.Tomalia D.A., Baker H., Dewald J., Hall M., Kallos G., Martin S. A new class of polymers: starburst-dendritic macromolecules. Polym. J. 1985;17:117–132. [Google Scholar]
- 34.Kesharwani P., Choudhury H., Meher J.G., Pandey M., Gorain B. Dendrimer-entrapped gold nanoparticles as promising nanocarriers for anticancer therapeutics and imaging. Prog. Mater. Sci. 2019;103:484–508. [Google Scholar]
- 35.Shukla R., Handa M., Lokesh S.B., Ruwali M., Kohli K. Conclusion and future prospective of polymeric nanoparticles for cancer therapy, polym. Nanoparticles as a promis. Tool Anti-Cancer Ther. 2019:389–408. [Google Scholar]
- 36.Esfand R., Tomalia D.A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. http://www.ncbi.nlm.nih.gov/pubmed/11301287 [DOI] [PubMed] [Google Scholar]
- 37.Kesharwani P., Banerjee S., Gupta U., Mohd Amin M.C.I., Padhye S., Sarkar F.H. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today. 2015 [Google Scholar]
- 38.Gorain B., Choudhury H., Pandey M., Nair A.B., Iqbal Mohd Amin M.C., Molugulu N. Dendrimer-based nanocarriers in lung cancer therapy, nanotechnology-based target. Drug Deliv. Syst. Lung Cancer. 2019:161–192. [Google Scholar]
- 39.Kolhe P., Khandare J., Pillai O., Kannan S., Lieh-Lai M., Kannan R.M. Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials. 2006;27:660–669. doi: 10.1016/j.biomaterials.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Gurdag S., Khandare J., Stapels S., Matherly L.H., Kannan R.M. Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and -resistant cell lines. Bioconjug. Chem. 2006;17:275–283. doi: 10.1021/bc0501855. [DOI] [PubMed] [Google Scholar]
- 41.Choudhury H., Pandey M., Gorain B., Chatterjee B., Madheswaran T., Md S. Nanoemulsions as effective carriers for the treatment of lung cancer, nanotechnology-based target. Drug Deliv. Syst. Lung Cancer. 2019:217–247. [Google Scholar]
- 42.Gupta U., Agashe H.B., Asthana A., Jain N.K., Dendrimers Novel polymeric nanoarchitectures for solubility enhancement. Biomacromolecules. 2006;7:649–658. doi: 10.1021/bm050802s. [DOI] [PubMed] [Google Scholar]
- 43.Boas U., Heegaard P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004;33:43–63. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- 44.Dwivedi N., Shah J., Mishra V., Mohd Amin M.C.I., Iyer A.K., Tekade R.K. Dendrimer-mediated approaches for the treatment of brain tumor. J. Biomater. Sci. Polym. Ed. 2016;27:557–580. doi: 10.1080/09205063.2015.1133155. [DOI] [PubMed] [Google Scholar]
- 45.Gorain B., Bhattamishra S.K., Choudhury H., Nandi U., Pandey M. Overexpressed receptors and proteins in lung cancer, nanotechnology-based target. Drug Deliv. Syst. Lung Cancer. 2019:39–75. [Google Scholar]
- 46.Gillies E., Frechet J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 47.Dwivedi N., Shah J., Mishra V., Tambuwala M., Kesharwani P. Nanoneuromedicine for management of neurodegenerative disorder. J. Drug Deliv. Sci. Technol. 2019;49:477–490. [Google Scholar]
- 48.Tay F.R., Pashley D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127–1137. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Gorain B., Choudhury H., Pandey M., Kesharwani P. Paclitaxel loaded vitamin E-TPGS nanoparticles for cancer therapy. Mater. Sci. Eng. C. 2018 doi: 10.1016/j.msec.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 50.Gorain B., Choudhury H., Pandey M., Mohd Amin M.C.I., Singh B. Dendrimers as effective carriers for the treatment of brain tumor, nanotechnology-based target. Drug Deliv. Syst. Brain Tumors. 2018:267–305. [Google Scholar]
- 51.Mann S., Heywood B.R., Rajam S., Birchall J.D. Controlled crystallization of CaCO3 under stearic acid monolayers. Nature. 1988;334:692–695. [Google Scholar]
- 52.Singh G., Kesharwani P., Srivastava A.K. Tuberculosis treated by multiple drugs: an overview. Curr. Drug Deliv. 2018;15:312–320. doi: 10.2174/1567201814666171120125916. [DOI] [PubMed] [Google Scholar]
- 53.Kirkham J., Firth A., Vernals D., Boden N., Robinson C., Shore R.C. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007;86:426–430. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- 54.Md S., Bhattmisra S.K., Zeeshan F., Shahzad N., Mujtaba M.A., Srikanth Meka V. Nano-carrier enabled drug delivery systems for nose to brain targeting for the treatment of neurodegenerative disorders. J. Drug Deliv. Sci. Technol. 2018;43 [Google Scholar]
- 55.Fan Y., Nelson J.R., Alvarez J.R., Hagan J., Berrier A., Xu X. Amelogenin-assisted ex vivo remineralization of human enamel: effects of supersaturation degree and fluoride concentration. Acta Biomater. 2011;7:2293–2302. doi: 10.1016/j.actbio.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busch S. Regeneration of human tooth enamel. Angew. Chem. Int. Ed. 2004;43:1428–1431. doi: 10.1002/anie.200352183. [DOI] [PubMed] [Google Scholar]
- 57.Li L., Mao C., Wang J., Xu X., Pan H., Deng Y. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: an approach to biomaterials with optimal characteristics. Adv. Mater. 2011;23:4695–4701. doi: 10.1002/adma.201102773. [DOI] [PubMed] [Google Scholar]
- 58.SVENSON S., TOMALIA D. Dendrimers in biomedical applications—reflections on the field. Adv. Drug Deliv. Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Wu D., Yang J., Li J., Chen L., Tang B., Chen X. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials. 2013;34:5036–5047. doi: 10.1016/j.biomaterials.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 60.Kesharwani P., Gothwal A., Iyer A.K., Jain K., Chourasia M.K., Gupta U. Dendrimer nanohybrid carrier systems: an expanding horizon for targeted drug and gene delivery. Drug Discov. Today. 2017 doi: 10.1016/j.drudis.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Chen M., Yang J., Li J., Liang K., He L., Lin Z. Modulated regeneration of acid-etched human tooth enamel by a functionalized dendrimer that is an analog of amelogenin. Acta Biomater. 2014;10:4437–4446. doi: 10.1016/j.actbio.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Jia R., Lu Y., Yang C.-W., Luo X., Han Y. Effect of generation 4.0 polyamidoamine dendrimer on the mineralization of demineralized dentinal tubules in vitro. Arch. Oral Biol. 2014;59:1085–1093. doi: 10.1016/j.archoralbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Y., Yang J., Lin Z., Li J., Liang K., Yuan H. Triclosan-loaded poly(amido amine) dendrimer for simultaneous treatment and remineralization of human dentine. Colloids Surfaces B Biointerfaces. 2014;115:237–243. doi: 10.1016/j.colsurfb.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 64.Liang K., Yuan H., Li J., Yang J., Zhou X., He L. Remineralization of demineralized dentin induced by amine-terminated PAMAM dendrimer. Macromol. Mater. Eng. 2015;300:107–117. [Google Scholar]
- 65.Liang K., Weir M.D., Xie X., Wang L., Reynolds M.A., Li J. Dentin remineralization in acid challenge environment via PAMAM and calcium phosphate composite. Dent. Mater. 2016;32:1429–1440. doi: 10.1016/j.dental.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 66.Wang T., Yang S., Wang L., Feng H. Use of multifunctional phosphorylated PAMAM dendrimers for dentin biomimetic remineralization and dentinal tubule occlusion. RSC Adv. 2015;5:11136–11144. [Google Scholar]
- 67.Soni N., Tekade M., Kesharwani P., Bhattacharya P., Maheshwari R., Dua K. Recent advances in oncological submissions of dendrimer. Curr. Pharmaceut. Des. 2017;23:1. doi: 10.2174/1381612823666170329150201. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H., Yang J., Liang K., Li J., He L., Yang X. Effective dentin restorative material based on phosphate-terminated dendrimer as artificial protein. Colloids Surfaces B Biointerfaces. 2015;128:304–314. doi: 10.1016/j.colsurfb.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 69.Luong D., Sau S., Kesharwani P., Iyer A.K. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules. 2017;18 doi: 10.1021/acs.biomac.6b01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie F., Li Q., Wei X., Zhou T. Effect of Ca(OH)2 pre-treated on remineralization of the demineralized dentin induced by polyamidoamine dendrime. Zhonghua Kou Qiang Yi Xue Za Zhi. 2015;50:244–247. [PubMed] [Google Scholar]
- 71.Xie F., Wei X., Li Q., Zhou T. In vivo analyses of the effects of polyamidoamine dendrimer on dentin biomineralization and dentinal tubules occlusion. Dent. Mater. J. 2016;35:104–111. doi: 10.4012/dmj.2015-209. [DOI] [PubMed] [Google Scholar]
- 72.Gao Y., Liang K., Li J., Yuan H., Liu H., Duan X. Effect and stability of poly(amido amine)-induced biomineralization on dentinal tubule occlusion. Mater. (Basel, Switzerland) 2017;10:384. doi: 10.3390/ma10040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang K., Zhou H., Weir M.D., Bao C., Reynolds M.A., Zhou X. Poly(amido amine) and calcium phosphate nanocomposite remineralization of dentin in acidic solution without calcium phosphate ions. Dent. Mater. 2017;33:818–829. doi: 10.1016/j.dental.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Lin X., Xie F., Ma X., Hao Y., Qin H., Long J. Fabrication and characterization of dendrimer-functionalized nano-hydroxyapatite and its application in dentin tubule occlusion. J. Biomater. Sci. Polym. Ed. 2017;28:846–863. doi: 10.1080/09205063.2017.1308654. [DOI] [PubMed] [Google Scholar]
- 75.Tao S., Fan M., Xu H.H.K., Li J., He L., Zhou X. The remineralization effectiveness of PAMAM dendrimer with different terminal groups on demineralized dentin in vitro. RSC Adv. 2017;7:54947–54955. [Google Scholar]
- 76.Xiao S., Liang K., Weir M.D., Cheng L., Liu H., Zhou X. Combining bioactive multifunctional dental composite with PAMAM for root dentin remineralization. Mater. (Basel, Switzerland) 2017;10:89. doi: 10.3390/ma10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ge Y., Ren B., Zhou X., Xu H.H.K., Wang S., Li M. Novel dental adhesive with biofilm-regulating and remineralization capabilities. Mater. (Basel, Switzerland) 2017;10:26. doi: 10.3390/ma10010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dodiuk-Kenig H., Lizenboim K., Eppelbaum I., Zalsman B., Kenig S. The effect of hyper-branched polymers on the properties of dental composites and adhesives. J. Adhes. Sci. Technol. 2004;18:1723–1737. [Google Scholar]
- 79.Amin M.C.I.M., Giri N., Jain A., Gajbhiye V. Dendrimers in targeting and delivery of drugs, nanotechnology-based approaches target. Deliv. Drugs Genes. 2017:363–388. [Google Scholar]
- 80.Paul N.M., Bader S.J., Schricker S.R., Parquette J.R. 2,3-Branching benzyl ether dendrimers for the enhancement of dental composites. React. Funct. Polym. 2006;66:1684–1695. [Google Scholar]
- 81.Viljanen E.K., Skrifvars M., Vallittu P.K. Dendritic copolymers and particulate filler composites for dental applications: degree of conversion and thermal properties. Dent. Mater. 2007;23:1420–1427. doi: 10.1016/j.dental.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 82.Marsh P.D., Moter A., Devine D.A. Dental plaque biofilms: communities, conflict and control. Periodontol. 2000. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 83.Cekici A., Kantarci A., Hasturk H., Van Dyke T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drisko C.L. Periodontal debridement: still the treatment of choice. J. Evid. Based Dent. Pract. 2014;14:33–41.e1. doi: 10.1016/j.jebdp.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Feres M., Figueiredo L.C., Soares G.M.S., Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol. 2000. 2015;67:131–186. doi: 10.1111/prd.12075. [DOI] [PubMed] [Google Scholar]
- 86.Sgolastra F., Gatto R., Petrucci A., Monaco A. Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysis. J. Periodontol. 2012;83:1257–1269. doi: 10.1902/jop.2012.110625. [DOI] [PubMed] [Google Scholar]
- 87.Sgolastra F., Petrucci A., Gatto R., Monaco A. Effectiveness of systemic amoxicillin/metronidazole as an adjunctive therapy to full-mouth scaling and root planing in the treatment of aggressive periodontitis: a systematic review and meta-analysis. J. Periodontol. 2012;83:731–743. doi: 10.1902/jop.2011.110432. [DOI] [PubMed] [Google Scholar]
- 88.Zandbergen D., Slot D.E., Niederman R., Van der Weijden F.A. The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planing alone in treating periodontitis: =a systematic review= BMC Oral Health. 2016;16:27. doi: 10.1186/s12903-015-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cionca N., Giannopoulou C., Ugolotti G., Mombelli A. Microbiologic testing and outcomes of full-mouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis. J. Periodontol. 2010;81:15–23. doi: 10.1902/jop.2009.090390. [DOI] [PubMed] [Google Scholar]
- 90.Marsh P.D. Plaque as a biofilm: pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 2003;9(Suppl 1):16–22. doi: 10.1034/j.1601-0825.9.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 91.D’Emanuele A., Attwood D. Dendrimer-drug interactions. Adv. Drug Deliv. Rev. 2005;57:2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 92.Moorefield C.N., Newkome G.R. Unimolecular micelles: supramolecular use of dendritic constructs to create versatile molecular containers. Compt. Rendus Chem. 2003;6:715–724. [Google Scholar]
- 93.Fréchet J.M.J., Tomalia D.A. Wiley; 2001. Dendrimers and Other Dendritic Polymers. [Google Scholar]
- 94.Kopecek J., Kopecková P., Minko T., Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 95.Gardiner J., Freeman S., Leach M., Green A., Alcock J., D’Emanuele A. PAMAM dendrimers for the delivery of the antibacterial Triclosan. J. Enzym. Inhib. Med. Chem. 2008;23:623–628. doi: 10.1080/14756360802205257. [DOI] [PubMed] [Google Scholar]
- 96.Dung T.H., Do L.T., Yoo H. PAMAM dendrimer generation 5-pluronic F127 nanofilm as a matrix for local metronidazole release. J. Biomed. Nanotechnol. 2013;9:1286–1292. doi: 10.1166/jbn.2013.1534. [DOI] [PubMed] [Google Scholar]
- 97.Lu Y., Slomberg D.L., Shah A., Schoenfisch M.H. Nitric oxide-releasing amphiphilic poly(amidoamine) (PAMAM) dendrimers as antibacterial agents. Biomacromolecules. 2013;14:3589–3598. doi: 10.1021/bm400961r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choudhury T., Sato E.F., Inoue M. Nitrite reductase in Streptoccocus mutans plays a critical role in the survival of this pathogen in oral cavity. Oral Microbiol. Immunol. 2007;22:384–389. doi: 10.1111/j.1399-302X.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 99.Gusarov I., Nudler E. NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13855–13860. doi: 10.1073/pnas.0504307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Backlund C.J., Sergesketter A.R., Offenbacher S., Schoenfisch M.H. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J. Dent. Res. 2014;93:1089–1094. doi: 10.1177/0022034514529974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Damiati L., Eales M.G., Nobbs A.H., Su B., Tsimbouri P.M., Salmeron-Sanchez M. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018;9 doi: 10.1177/2041731418790694. 204173141879069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berglundh T., Abrahamsson I., Albouy J.-P., Lindhe J. Bone healing at implants with a fluoride-modified surface: an experimental study in dogs. Clin. Oral Implant. Res. 2007;18:147–152. doi: 10.1111/j.1600-0501.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- 103.Lang N.P., Salvi G.E., Huynh-Ba G., Ivanovski S., Donos N., Bosshardt D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011;22:349–356. doi: 10.1111/j.1600-0501.2011.02172.x. [DOI] [PubMed] [Google Scholar]
- 104.Lutz Scheideler J.G.-G., Eichler Mirjam, Katzur Verena, Mller R., Rupp Frank. Influ. Dendrimer Surf. Coatings Bact. Adhes. 2011. Conference: IADR general session 2011. [Google Scholar]
- 105.Bengazi F., Lang N.P., Canciani E., Viganò P., Velez J.U., Botticelli D. Osseointegration of implants with dendrimers surface characteristics installed conventionally or with Piezosurgery®. A comparative study in the dog. Clin. Oral Implant. Res. 2014;25:10–15. doi: 10.1111/clr.12082. [DOI] [PubMed] [Google Scholar]
- 106.Galli C., Piemontese M., Meikle S.T., Santin M., Macaluso G.M., Passeri G. Biomimetic coating with phosphoserine-tethered poly(epsilon-lysine) dendrons on titanium surfaces enhances Wnt and osteoblastic differentiation. Clin. Oral Implant. Res. 2014;25:e133–e139. doi: 10.1111/clr.12075. [DOI] [PubMed] [Google Scholar]
- 107.Kawaguchi T., Lassila L.V.J., Vallittu P.K., Takahashi Y. Mechanical properties of denture base resin cross-linked with methacrylated dendrimer. Dent. Mater. 2011;27:755–761. doi: 10.1016/j.dental.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 108.Kim J.K., Shukla R., Casagrande L., Sedgley C., Nör J.E., Baker J.R. Differentiating dental pulp cells via RGD-dendrimer conjugates. J. Dent. Res. 2010;89:1433–1438. doi: 10.1177/0022034510384870. [DOI] [PubMed] [Google Scholar]
- 109.Yan Y., Gao X., Zhang S., Wang Y., Zhou Z., Xiao J. A carboxyl-terminated dendrimer enables osteolytic lesion targeting and photothermal ablation of malignant bone tumors. ACS Appl. Mater. Interfaces. 2019;11:160–168. doi: 10.1021/acsami.8b15827. [DOI] [PubMed] [Google Scholar]
- 110.Liu X., Huang H., Wang J., Wang C., Wang M., Zhang B. Dendrimers-delivered short hairpin RNA targeting hTERT inhibits oral cancer cell growth in vitro and in vivo. Biochem. Pharmacol. 2011;82:17–23. doi: 10.1016/j.bcp.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 111.Ma M., Cheng Y., Xu Z., Xu P., Qu H., Fang Y. Evaluation of polyamidoamine (PAMAM) dendrimers as drug carriers of anti-bacterial drugs using sulfamethoxazole (SMZ) as a model drug. Eur. J. Med. Chem. 2007;42:93–98. doi: 10.1016/j.ejmech.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 112.Eichler M., Katzur V., Scheideler L., Haupt M., Geis-Gerstorfer J., Schmalz G. The impact of dendrimer-grafted modifications to model silicon surfaces on protein adsorption and bacterial adhesion. Biomaterials. 2011;32:9168–9179. doi: 10.1016/j.biomaterials.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 113.Li J., Yang J., Li J., Chen L., Liang K., Wu W. Bioinspired intrafibrillar mineralization of human dentine by PAMAM dendrimer. Biomaterials. 2013;34:6738–6747. doi: 10.1016/j.biomaterials.2013.05.046. [DOI] [PubMed] [Google Scholar]