Abstract
Carbon nanotubes (CNTs) hold tremendous potential due to their unique and modifiable properties. Their robust biological applications necessitate minimizing their cytotoxicity and increasing the solubilization. In the present manuscript, we have functionalized multiwalled carbon nanotubes (MWCNTs) using defect functionalization methodology to covalently bind carboxy and amino groups on their walls. This functionalization was reassured through fourier-transform infrared spectroscopy (FTIR), energy dispersive x-ray analysis (EDX), elemental and field emission scanning electron microscopy (FE-SEM) analysis. The observations demonstrated that addition of carboxy as well as amino groups on MWCNTs, besides enabling MWCNTs solubilization also significantly ameliorated the cytotoxicity and the oxidative stress in comparison to pristine MWCNTs. It is envisaged that changes in agglomeration of the functionalized MWCNTs and the acquired surface charge is the reason for the reduction of cytotoxicity. Zebra fish embryo model test system employed for in vivo analysis of the MWCNTs showed no significant toxicity on account of any nanoparticle tested pointing towards intrinsic mechanisms in place for deterring the damage in complex organisms. Overall, the observations besides pointing towards functionalized MWCNTs effectiveness towards weakening the toxicity of pristine MWCNTs also caution for extrapolating in vitro data to in vivo observations. The observations further lend credibility for exploiting the zebra fish as a model system for analyzing the effects of MWCNTs functionalization.
Keywords: Materials science, Chemistry, Environmental science, Biological sciences, Carbon nanotubes (CNTs), Multi walled carbon nanotubes (MWCNTs), Functionalized MWCNTs, MWCNT cytotoxicity
Materials science; Chemistry; Environmental science; Biological sciences; Carbon nanotubes (CNTs); Multi walled carbon nanotubes (MWCNTs); Functionalized MWCNTs; MWCNT cytotoxicity
1. Introduction
Following the accidental discovery of carbon nanotubes (CNTs), by Iijima in 1991, there has been a surge of technological advancements in the field of nanotechnology [1]. CNTs exist in two basic variants depending upon the number of layers, namely single walled carbon nanotubes (SWCNT) and multiwalled carbon nanotubes (MWCNT) and accordingly each of these types show a variation in their properties. From the physical perspective, CNTs can be visualized as rolled up graphene cylinders where the type of rolling and the number of concentric layers determine the properties of a particular CNT. For example, depending upon the axis of their rolling/chirality, SWCNTs are classified as having zigzag or arm chair configuration, wherein armchair CNTs display a metallic nature while the zigzag CNTs may display either metallic or semiconductor behaviour [2]. The number of concentric shells also influence the properties of different types of CNTs. For example, MWCNTs which have multiple rolled graphene layers show more mechanical strength than others, thus making it an ideal candidate for novel diagnostic applications.
Recent research in the field of bioengineering has placed this nanomolecule on the list of most promising material for development of bio-inspired composite materials e.g. CNTs show better entrapment efficiency and superior drug release compared to liposomes, dendrimers, etc [3]. However, their insolubility as pristine CNTs in aqueous solvents and/or culture medium (for in vitro cell culture) supposedly posed a major hurdle for their application in the biomedical sciences. Nonetheless, the strong intermolecular Van der Waals interactions between CNT molecules, stemming from π-π interactions of the sp2 hybridized carbon atoms can be altered by covalent functionalization making them compatible towards aqueous and biological medium [4]. Although other methods are indeed available such as molecular adsorption on the side walls and encapsulation of molecules in the CNT cavity, yet covalent functionalization proves better due to its robustness and controllability. This method involves inducing rehybridization of structured CNT carbons to sp3 hybridization, thereby disrupting its hydrophobic character and promoting solubility. The addition of charged groups on the CNT surface further supports solubility in aqueous solvents due to increased solvation energy of the functionalized CNT and also provides active sites of interactions with biological compounds [5, 6].
Functionalized CNTs have already been shown to be useful in promoting cell growth and directed differentiation of stem cells [7, 8]. Many such studies were carried out using different cell types wherein their ability to easily bind to the extracellular matrix (ECM) proteins and thus direct cellular behaviour has placed these molecules among the top tissue engineering aids/constructs [9, 10].
However, with their popularity, grew the numerous health concerns stemming from various toxicology studies. With several of the reports claiming functionalization as a means to reduce cytotoxicity of MWCNTs, while others concluded functionalization being the aggravator of MWCNT cytotoxicity [11, 12, 13, 14]. The conundrum about their biocompatibility thus necessitates a more comprehensive research to get a better perspective of CNTs safety before being further used in varied applications. It is known that chemical modifications of CNTs help influence its interactions with its microenvironment thereby guiding cell growth and differentiation. Moreover, the available reports individually discuss the effect of functionalization (COOH and NH2) but a comparative study on the two remains sparse specifically in relation to cytotoxicity and cellular prooxidant state.
Therefore, we have devised a study wherein dual-covalent modifications of CNTs with carboxy and amino groups were utilized to understand the effect of surface functionalization on the cytotoxicity profile of CNTs. Human embryonic kidney cells (HEK 293 cell line) were chosen for this study to elucidate the mechanism of interaction of CNTs at the cellular level and for the knowhow on the toxicity issues. It was found that functionalization of MWCNTs in general had a positive effect on the viability of HEK 293 cells. Further, the MWCNT-COOH showed promising results with respect to the associated cellular viability and reactive oxygen species (ROS) generation profiles taken against the varied concentrations of MWCNTs tested.
In order to further assess the toxicity of these nanoparticles zebra fish was chosen as the model organism since cell lines do not give the organ level of complexity because of which it is difficult to extrapolate these results to higher mammals. Zebra fish embryos are: -transparent, have high fecundity, and have easy and cheap handling techniques which enable them to be potentially robust organisms to analyze toxicity. Unlike 2-dimensional cell culture, the zebra fish embryos remained refractory to highest tested concentrations of different MWCNTs suggesting variations between in vitro cell culture system and in vivo zebra fish model organisms with respect to different functionalized MWCNTs. Together, these observations, besides characterizing functionalization of MWCNTs demonstrated difference in the toxicity under in vitro versus in vivo condition.
2. Materials and methods
The MWCNTs (purity >98 %) produced by chemical vapor deposition method were purchased from Sigma-Aldrich. The average length of these MWCNTs was 10 μm with an average diameter of 12 nm. Ethylenediamine (EDA), o-(benzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate (HBTU), 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and trypan blue solution were also purchased from Sigma-Aldrich. Other cell culture reagents such as Dulbecco's modified eagle medium (DMEM) was purchased from Corning, while fetal bovine serum (FBS), bovine serum albumin (BSA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypsin-EDTA solution 10 X and antibiotic solution 100 X were purchased from HiMedia. All other reagents, such as concentrated sulfuric acid (H2SO4), concentrated nitric acid (HNO3), isopropanol etc, of analytical grade were purchased from Fisher Scientific and/or local suppliers (Chandigarh, India). Sample washing and solution preparation was done by Millipore deionized (DI) water (18 MV cm resistivity).
2.1. Synthesis of carboxy functionalized MWCNT (MWCNT-COOH)
Following a reported procedure for defect functionalization, the MWCNTs were chemically functionalized with a mixture of 3:1 concentrated sulfuric and nitric acid by sonicating it in an ultrasonic bath for 3 h at 30 °C. The acid solution was then added in a drop wise manner to cold Millipore water followed by filtration through a 0.2 μm polytetrafluoroethylene filter. The filtrate was then washed thoroughly with Millipore water till its pH was neutral. Finally, the filtrate was dried in a vacuum oven at 80 °C to give MWCNT-COOH [15].
2.2. Synthesis of amino functionalized MWCNT (MWCNT-NH2)
Further treatment of the MWCNT-COOH was performed with ethylenediamine (EDA) in presence of o-(benzotriazol-1-yl)-1,1,3,3-tetramethyluroniumhexafluorophosphate (HBTU) as catalyst. In brief, a sample of MWCNT-COOH was dispersed in EDA by sonicating it in an ultrasonic bath till a homogeneous mixture was achieved. The coupling agent, HBTU, was then added to the mixture and sonication was performed for 4 h at 30 °C. The sample was subsequently added to methanol and filtered using a 0.2 μm polytetrafluoroethylene filter paper. This was followed by extensive washing in methanol and drying in a vacuum oven at 80 °C for 4 h to give MWCNT-NH2 [15].
2.3. Materials characterization
The characterization of the MWCNT structure was analyzed using field-emission scanning electron microscope (FE-SEM, SU 8010, Hitachi). Energy dispersive x-ray spectrum (EDX) analysis was performed to quantify the elements of the sample which was also verified by elemental analysis on a CHNS–O elemental analyser (Flash 2000, Thermo Scientific). FTIR spectra were carried out on a Spectrum 400 FT-IR/FIR spectrometer (Perkin Elmer). Agglomeration studies were performed using visual observation after dispersing 1 mg of the various MWCNTs in 10 ml of MiliQ water and in 10 ml of DMEM (10 % FBS supplemented) to replicate the culture medium conditions and leaving them undisturbed for 7 days. Subsequently, the aqueous MWCNT samples after bath sonication were subjected to zeta potential analysis for estimation of charge to size ratio of the dispersed/dissolved nanoparticles.
2.4. Biocompatibility and cytotoxicity studies
The biocompatibility of pristine MWCNT, MWCNT-COOH and MWCNT-NH2 was studied using HEK 293 cell line. The HEK 293 cell line was obtained from cell repository, National Centre for Cell Science, Pune, Maharashtra, India. The cells were cultivated in T-75 culture flasks in DMEM medium, supplemented with 10 % FBS, 2 mM L-glutamine and antibiotics till 80–90 % confluency was achieved. Following which the cells were trypsinized (0.5 % trypsin and 0.2 % EDTA in 0.85 % normal saline) and used as per the experiments need in 96 or 24 well plates. The MWCNTs stock solutions were obtained by sonicating for a period of 30 min in DMEM media to achieve a concentration of 2 mg/ml. For the trypan blue assay, the cells were plated in 24 well plates at a seeding density of 5 × 104 cells/well and were incubated overnight. The cells were exposed to pristine and functionalized MWCNTs at concentrations varying from 25 μg/ml to 300 μg/ml for a period of 24 h along with appropriate controls lacking the MWCNTs. Following this the cells were trypsinized and counted using 0.4 % trypan blue. Relative values (%) of total number of viable cells for each time point were calculated on the basis of the total number of HEK 293 cells in control wells without MWCNTs, used as 100 %.
The cytotoxic analysis of MWCNTs on cells was further assessed by using (3-(4,5 dimethyl-2-thiazolyl)-2-5-diphenyl-2H-tetrazolium bromide) (MTT) assay. Briefly, 1 × 104 cells were seeded in 100 μl in 96 well plate and incubated overnight. This was followed by addition of MWCNTs at different concentrations for 24 h. 10 μl MTT was added to each well at a concentration of 5 mg/ml and further incubated for 4 h at 37 °C. The media was then removed and 100 μl of acidified isopropanol was added to each well and mixed thoroughly. The plate was then incubated at 37 °C for 30 min and afterwards absorbance was read with a microplate reader at 570 nm.
2.5. Measurement of ROS generation
Intracellular ROS activity was measured using DCFH-DA assay as the amount of fluorescence emitted per mg of total protein concentration as described by Wang and Joseph with slight modifications [16]. Briefly, 1 × 104 cells were plated in a 96 well plate and incubated overnight, this was followed by MWCNT exposure of pristine and functionalized MWCNTs (25 and 50 μg/ml) for 24 h. The cells were then washed twice with PBS and incubated for a period of 30 min in dark at 37 °C with DCHF-DA (DMSO, final concentration 20 μM). The cells were then again washed with PBS and the fluorescence intensity was monitored using a spectrofluorometer with excitation at 488 nm and emission at 520 nm. The amount of fluorescence was normalized to the protein content to estimate the relative ROS production. Bovine serum albumin was used as a standard to calculate the protein concentration.
2.6. In vivo toxicity analysis
2.6.1. Preparation of nanoparticle dispersions
The dilutions of the three MWCNT solutions namely, pristine MWCNT, MWCNT-COOH and MWCNT-NH2 were prepared in holtfreters medium to make final concentrations of 10 μg/ml, 25 μg/ml and 50 μg/ml. The suspensions were prepared and sonicated for 5 min each at energy of 25 KJ. These dispersions were used for dosing the zebra fish embryos.
2.6.2. In vivo experiments
No ethics approval is needed for such studies as during our experiments the embryos were collected as soon as they were laid and the experiment was completed within 120 h, a time point well before independent feeding starts.
2.6.2.1. Fish husbandry and embryo collection
Wild-type zebra fish (AB strain) were procured and raised according to standard breeding protocols (28 ± 0.5 °C with 14:10 day/night photoperiod). Reverse osmosis (pH 6.5–7.5) filtered water was supplied to the recirculation system with conductivity of 450–1000 s cm−1. Artemia and a dry flake diet were fed to zebra fish twice daily. Continuous observation of the development status of zebra fish embryos and larvae was done using an inverted microscope (Nikon, Japan). Adult zebra fish were spawned in tanks overnight with the sex ratio of 1:1 and embryos were collected within 1 h post fertilization.
2.6.2.2. Exposure
The collected embryos were then washed with holtfreters medium thrice and after 4 h post fecundation (hpf) these were identified under an inverted microscope. The embryos were then placed in 96 well flat bottom plates. One embryo was added to each well after discarding the dead embryos. Next, 100 μl of holtfreters medium containing the required concentration of respective MWCNTs were added to each well. This was followed by incubation of the 96 well plates at 28 °C. Finally, the zebra fish embryos were examined every 24 h till 120 hpf.
2.6.2.3. Hatching, malformation and mortality
The toxicity endpoints that are mortality and malformations were studied in embryo daily till 120 hpf. After every 24 h till 120 hpf, embryos were observed for mortality by looking at their heart beat, movement etc. Hatching was recorded at 72 hpf. Physical abnormalities such as head, yolk sac, tail and pericardia were observed and recorded at 96 hpf, respectively. Each experiment was performed independently in triplicate.
2.7. Statistical analysis
All the experiments were performed in triplicates and the data shown was calculated from the mean of three separate experimental readings with standard deviation as the error bars of the representative graphs. Statistical analysis was performed using two-way ANOVA analysis in Microsoft Excel software where, p < 0.05 was considered significant.
3. Results
3.1. MWCNT characterization
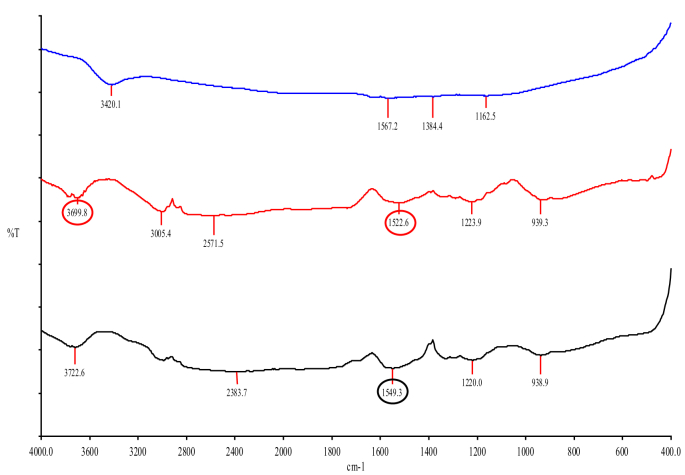
After the functionalization of MWCNTs, their structure and chemical composition were investigated. Fig. 1 shows the FTIR spectra of pristine MWCNTs (depicted as a blue line spectrum) and functionalized MWCNTs (depicted as red and black line spectrums for MWCNT-COOH and MWCNT-NH2, respectively). It was observed that the blue line spectrum of pristine MWCNTs is devoid of any prominent bands, however bands corresponding to the oxidation of MWCNTs appeared after treatment with the acid mixture in the red spectrum representing MWCNT-COOH. A broad band at 3699 cm−1 in the spectrum of MWCNT-COOH (Red line spectrum) was observed which is characteristic of an intermolecular OH stretching vibration. Another prominent peak at 1522 cm−1, which is indicative of a CO bond stretching frequency of the carboxylic group is visible in the MWCNT-COOH spectrum. These observations are clearly indicative of the addition of carboxyl group onto the CNT. The black line spectra of MWCNT-NH2 showed the disappearance of the band at 1522 cm−1 and the appearance of an amine specific band at 1549 cm−1, indicating the presence of an amide functional group.
Fig. 1.
The FTIR analysis of pristine MWCNT (Blue), MWCNT-COOH (Red) and MWCNT-NH2 (Black). A peak at 3699 cm−1 (red circle) in MWCNT-COOH (Red line spectrum) is characteristic of an intermolecular OH stretching vibration. Second peak at 1522 cm−1 is indicative of a CO bond stretching frequency of the carboxylic group on MWCNT-COOH (Red line spectrum). The black line spectra of MWCNT-NH2 showed disappearance of band at 1522 cm−1 and appearance of amine specific band at 1549 cm−1 indicating towards the presence of the amide functional group.
3.2. Scanning electron microscope (SEM)
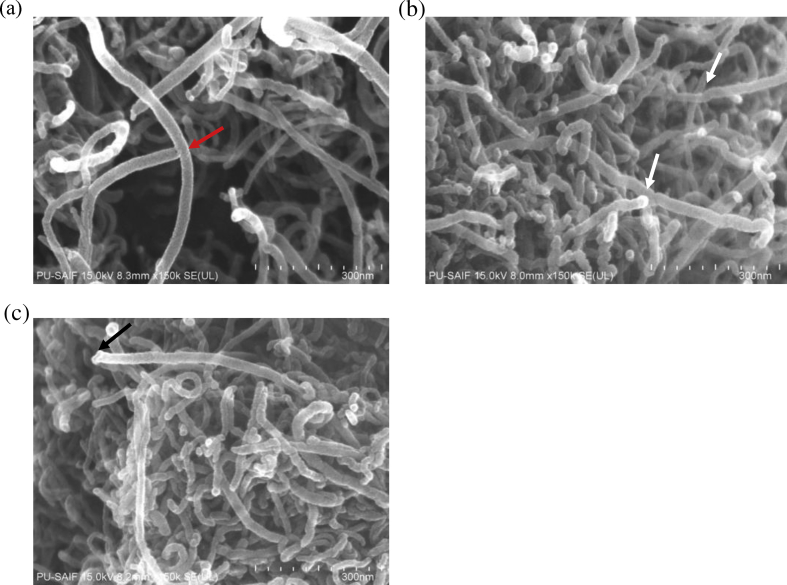
To reiterate the functionalization of MWCNTs, FE-SEM analysis was conducted as shown in Fig. 2. The Fig. 2a showed smooth side walls of the pristine MWCNTs (red arrow) whereas the side walls of functionalized MWCNTs, whether MWCNT-COOH Fig. 2b or MWCNT-NH2 Fig. 2c appeared to have rugged appearances with open cap structures (black arrow). This is indicative of defect generation along their walls wherein the new functional groups were added (generally carboxylic, amino and hydroxyl groups) leading to the appearance of disordered sites on the MWCNTs surface.
Fig. 2.
Representative FE-SEM images of (a) Pristine MWCNTs with the red arrow representing smooth wall structure (b) MWCNT-COOH with the white arrows representing rough walls after treatment with acid and the black arrow showing the uncapped end of the functionalized MWCNT (c) MWCNT-NH2 with the black arrow showing the uncapped end of the functionalized MWCNT.
3.3. Energy dispersion spectroscopy (EDS)
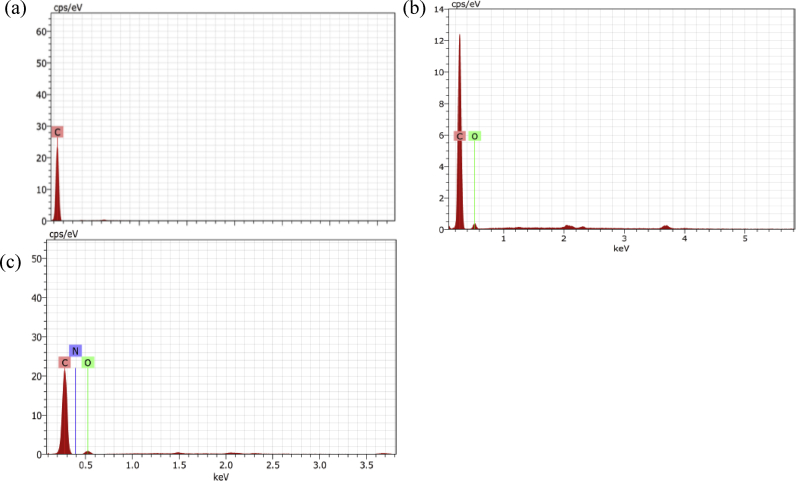
Furthermore, the analysis of the samples by energy dispersion spectroscopy provided a deeper insight into the functionalization efficiency of the nanotubes. The EDS spectrum revealed solely the presence of carbon in the pristine MWCNTs (Fig. 3a) whereas there was a presence of oxygen, upto about 7.53% in MWCNT-COOH (Fig. 3b); and MWCNT-NH2 showed an addition of nitrogen, amounting to 3.31% as depicted in Fig. 3c.
Fig. 3.
EDS analysis to demonstrate the presence of functional groups reassuring the addition of carboxy (b) and amino groups (c) to MWCNTs. The EDS graphs show peaks pertaining to peaks for (a) pristine MWCNT, (b) MWCNT-COOH and (c) MWCNT-NH2.
3.4. Elemental analysis
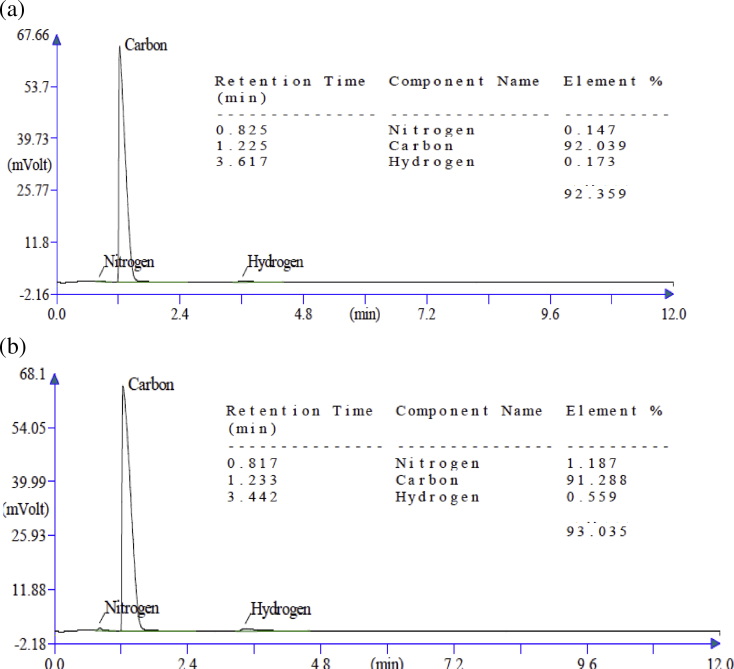
In order to further reiterate the functionalization of the MWCNTs, the elemental analysis was carried out. As shown by the elemental analysis graph of the MWCNTs (Fig. 4a), it seems there is an addition of about 7.64% oxygen (based on subtracting the cumulative percentage of the detected elements from 100; 100–92.359 = 7.64% oxygen) in MWCNT-COOH. This is consistent with the result observed in the EDS experiment (Fig. 3b). However, during amino functionalization as shown in Fig. 4b the nitrogen addition to the MWCNT was found to be about 1.18%. As expected, MWCNT-NH2 also show about 1% reduction in oxygen concentration in comparison to MWCNT-COOH.
Fig. 4.
Elemental analysis of functionalized MWCNTs (a) MWCNT-COOH and (b) MWCNT-NH2 and the table depicting the corresponding elemental composition detected.
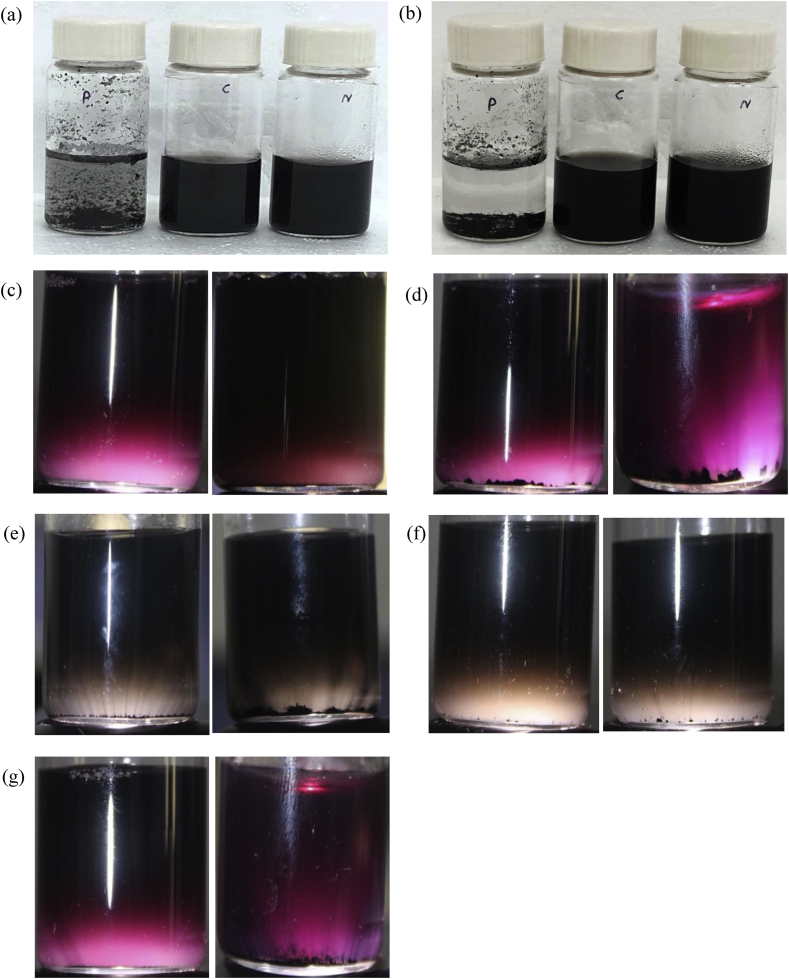
3.5. Agglomeration study
For a simple, semi quantitative and economical method for detecting the success of functionalization, the interaction of MWCNTs with aqueous media was studied. In the first study, 1 mg of MWCNTs were mixed in 10 ml of distilled water with the help of sonication in a water bath for 15 min. The second study explored the dispersion of MWCNTs in cell culture media used for cell culturing experiments (DMEM supplemented with 10 % FBS), with the remaining experimental conditions being the same. Finally, to visually characterize the functionalized MWCNTs’ solubility we illuminated the samples from below to check for aggregates. These pictures have been represented in pairs, capturing the moments at 0 day and after 7 days period. The observations for distilled water solubilization studies clearly depict a better solubilization of the functionalized MWCNTs when compared with pristine ones at both the time intervals as shown in Fig. 5a and b. However, upon closer inspection it was found that between MWCNT-COOH and MWCNT-NH2, MWCNT-COOH showed a more stable dispersion in distilled water after 7 days since the least amount of aggregates could be observed at the bottom of the vial as shown in Fig. 5c.
Fig. 5.
Agglomeration test of MWCNTs dissolved in MiliQ water and Cell culture medium followed by its dispersion using a bath sonicator. Here (a) MWCNT in distilled water at 0 day (b) MWCNT in distilled water at 7 days. (c) Comparison of day 0 and day 7 samples of MWCNT-COOH in distilled water. (d) Comparison of 0 day and after 7 days sample of MWCNT-NH2 in distilled water (e) Comparison of day 0 and day 7 samples of pristine MWCNT in cell culture media (f) Comparison of day 0 and day 7 samples of MWCNT-COOH in cell culture media (g) Comparison of day 0 and day 7 samples of MWCNT-NH2 in cell culture media.
Similarly, in the second study a similar trend was observed with MWCNT-COOH and MWCNT-NH2 being readily soluble after sonication and remained suspended in the media after 7 days. However, it was interesting to note that the initial observations (at time = 0 days) showed a relatively stable pristine MWCNTs dispersion in DMEM as compared to its dispersion in distilled water. On closer examination of the three samples dispersed in cell culture media it was observed that MWCNT-COOH had the least amount of aggregates sedimented at the bottom of the vial at both the tested time intervals 0 day and 7 days as shown in Fig. 5e, f and g.
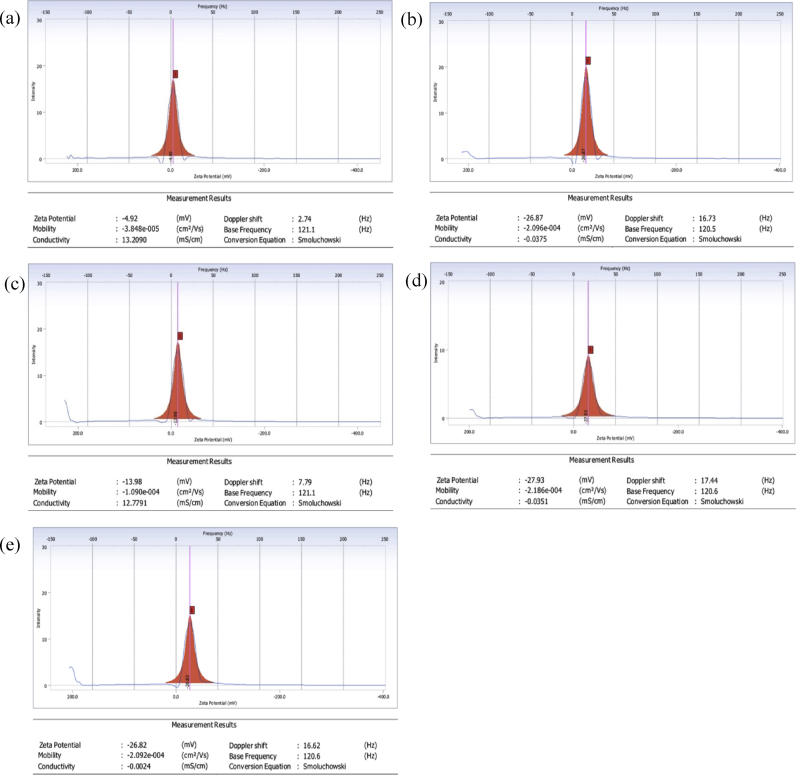
3.6. Zeta potential analysis
To get insights about the results of the agglomeration experiment a zeta potential analysis was performed to get the charge to size ratio of the functionalized MWCNTs in distilled water and in cell culture media (DMEM media supplemented with 10% FBS). The aqueous samples were subjected to the zeta potential analyzer after water bath sonication for a period of 15 min. However, the reading of pristine MWCNT could not be taken since its low solubility prevented the formation of a stable dispersion. As depicted in Fig. 6, the MWCNT-COOH in distilled water had an average reading of -27.93 mV whereas, the MWCNT-NH2 had a comparable average reading of -26.82 mV. In the case of MWCNTs in cell culture media, we found that pristine MWCNT had an average reading of -4.92 mV while the functionalized counter parts were at -26.87 mV and -13.97 mV for MWCNT-COOH and MWCNT-NH2 respectively.
Fig. 6.
The zeta potential graphs of the MWCNTs dissolved in culture media (a) Pristine MWCNT, (b) MWCNT-COOH and (c) MWCNT-NH2 and in distilled water using a bath sonicator (d) MWCNT-COOH and (e) MWCNT-NH2 has been illustrated here.
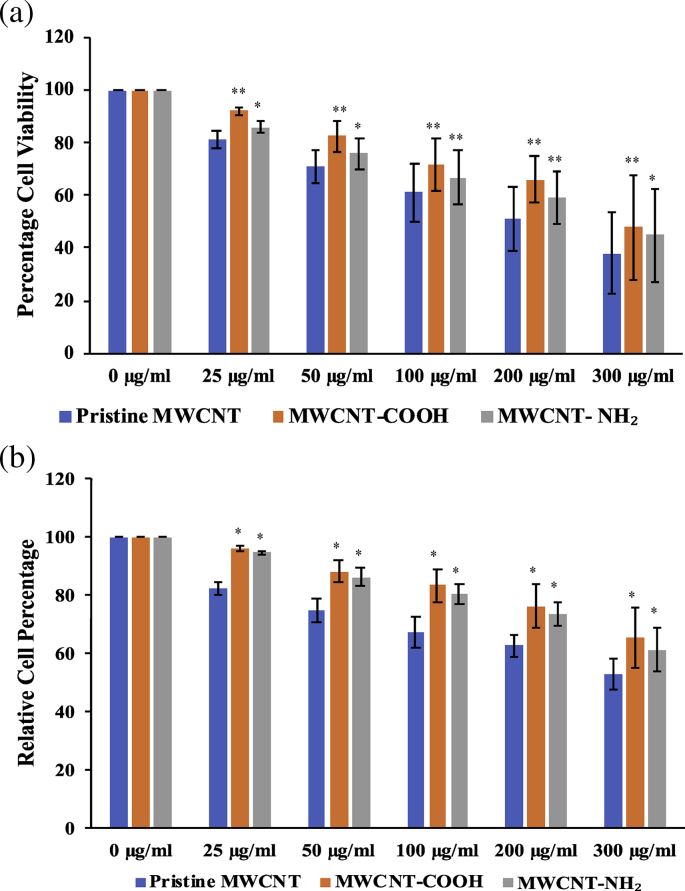
3.7. Cytotoxicity analysis
Following the functionalization and characterization studies, the cytotoxic analysis of the MWCNTs was undertaken using MTT assay. As shown in Fig. 7a, the cells growing at different concentrations of pristine MWCNT, MWCNT-COOH and MWCNT-NH2 from 25 μg/ml to 300 μg/ml responded in a dose dependent manner, showing increase in toxicity with increasing concentration of the nanotubes. However, the cytotoxicity of pristine MWCNTs was found to be exceedingly far more than that of the other two at each concentration tested (Fig. 7a).
Fig. 7.
Cytotoxicity assays of the functionalized MWCNTs. (a) MTT assay analysis of functionalized MWCNTs (b) Trypan blue dye exclusion assay analysis of functionalized MWCNTs. Analysis of variance (p < 0.05). Where, p < 0.05 (*) and p < 0.01 (**).
The pristine MWCNTs were found to be more toxic than any of the functionalized ones. As shown in Fig. 7a, even at a concentration of 25 μg/ml of pristine MWCNTs, the cell viability was reduced upto 81.57%. Between the functionalized MWCNTs, MWCNT-COOH showed a marginally better result than MWCNT-NH2, showing cell viabilities of 91.96 and 86.06 % respectively at a concentration of 25 μg/ml. Likewise, the trend remained the same at the highest concentration of the MWCNTs tested (300 μg/ml). As shown in Fig. 7a, at 300 μg/ml the pristine MWCNTs showed a cell viability of only 37.92 %, while the functionalized MWCNTs i.e. MWCNT-COOH and MWCNT-NH2 still retained the cell viability as high as 47.78 % and 45.02 %.
Trypan blue (TB) dye exclusion assay likewise reiterated the observations of MTT assay. As shown in Fig. 7b, it was observed that highest concentration of CNTs (300 μg/ml) exerted cytotoxic effect on the cells. Accordingly, the cell viability was found to be lowest at 300 μg/ml, showing only 53.01 %, 65.43 % and 61.37 % of cell viability as compared to the lowest CNT concentration tested (25 μg/ml) where the cell viabilities was as high as 82.48 %, 94.81 % and 92.26 % for pristine MWCNT, MWCNT-COOH and MWCNT-NH2 respectively. It was observed that significant toxicity starts to appear at a concentration of 50 μg/ml for all CNTs, with pristine MWCNTs showing an alarming 74.73 % of relative cell count; whereas MWCNT-COOH and MWCNT-NH2 fared significantly better, showing presence of about 88.32% and 86.30 % of the viable cells, respectively. Like in case of MTT assay it was observed that pristine MWCNTs are much more profound in eliciting toxic effects than the functionalized ones, reducing the viable cell count to about 50 % of the control at the highest concentration tested, 300 μg/ml. The comparison between the MWCNT-COOH (orange) and MWCNT-NH2 (grey) functionalized CNTs suggested that MWCT-COOH showed a relatively better tolerance to HEK 293 cells when compared to MWCNT-NH2.
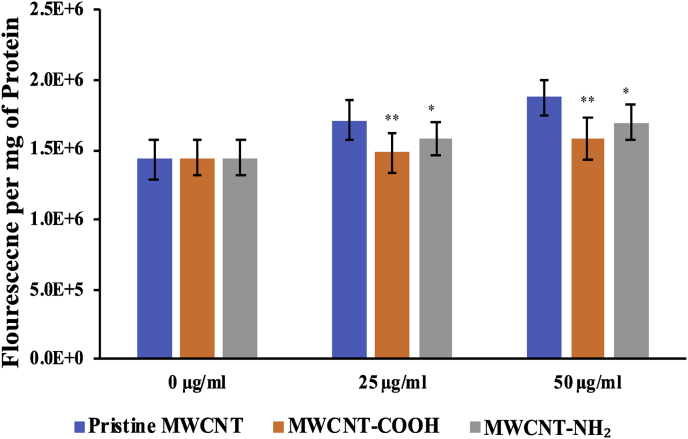
3.8. Measurement of ROS generation
Although the exact mechanism of toxicity of CNTs is widely debated upon, many reports hypothesize an intrinsic ROS generation pathway having an involvement in the characteristic toxicity observed in pristine MWCNTs, which eventually leads to oxidative stress in the cell [17, 18]. Probing further if functionalization has any effect on ROS generation we studied the effect of functionalized MWCNTs on the prooxidant status of HEK 293 cells. The cell line was treated with two different concentrations (25 and 50 μg/ml) of pristine MWCNTs, MWCNT-COOH and MWCNT-NH2 and the ROS pattern was analyzed. As shown in Shown in Fig. 8, the ROS levels remained ∼12 and 22 % higher for pristine MWCNTs versus the functionalized ones at 25 and 50 μg/ml concentrations, respectively. Both the carboxy and amino functionalization of MWCNT prevented the generation of prooxidant level and hence accounted for considerably low levels of ROS production.
Fig. 8.
Functionalization protects the cells against oxidative stress. DCFDA assay analysis for measuring the ROS following exposure of HEK 293 cells to functionalized MWCNTs (MWCNT-COOH & MWCNT-NH2) in comparison to pristine MWCNTs. The bars represent reactive oxygen species (ROS) generation. Y-axis represents percentage increase in ROS with respect to MWCNT concentration (x-axis). Analysis of variance (p < 0.05). Where, p < 0.05 (*) and p < 0.01 (**).
3.9. In vivo toxicity analysis
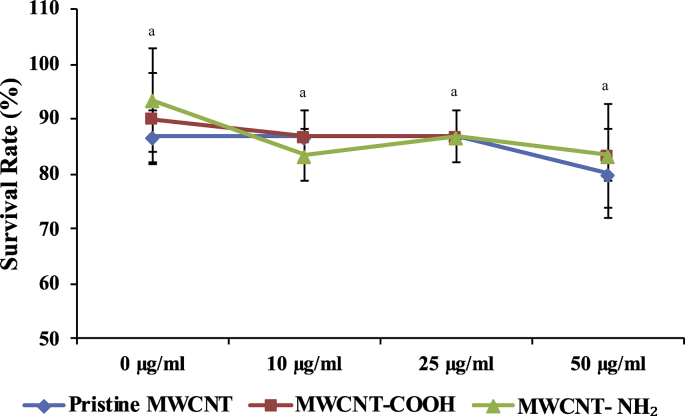
To get a better perspective of MWCNT toxicity under in vivo system we explored their effect on zebra fish embryos. Upon treating zebra fish embryos with different concentrations of pristine MWCNT, MWCNT-COOH and MWCNT-NH2 as shown in Fig. 9, it was observed that the survival rate of fish showed a non-significant decrease at concentrations as high as 50 μg/ml as compared to the control lacking the MWCNTs altogether (0 μg/ml). The lowest survival rate at highest concentration, i.e. 50 μg/ml, in case of both the pristine and functionalized MWCNTs was 80 percent only, which showed that the MWCNTs do not exhibit any toxicity in the zebra fish embryos.
Fig. 9.
Survivability rate of zebra fish embryos 120 h post fecundation (hpf) after treatment with pristine MWCNT, MWCNT-COOH and MWCNT-NH2 at 0 μg/ml, 10 μg/ml, 25 μg/ml and 50 μg/ml respectively. Analysis of variance (p < 0.05). Since p > 0.05, where ‘a’ represents non-significant effect.
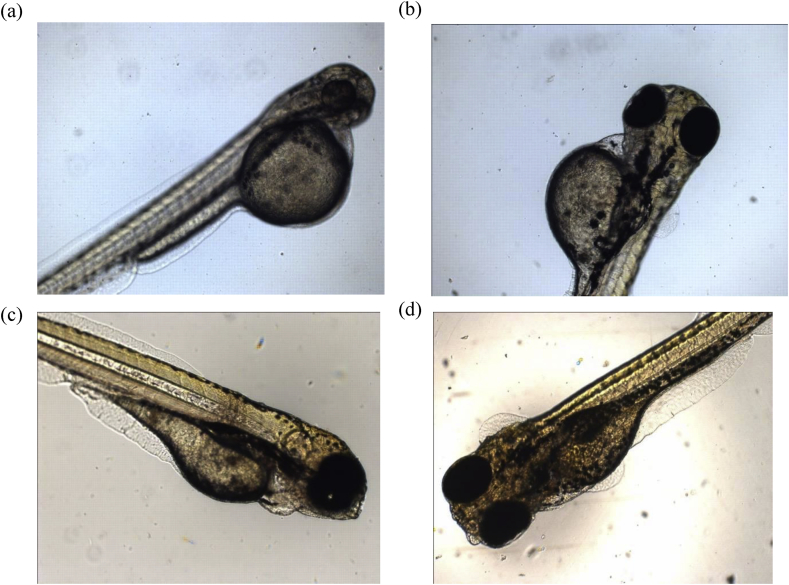
No visible malformations were observed in the treated or the control embryos under the light microscope as shown in Fig. 10. Similarly, no change in hatching rate was observed in zebra fish embryos which were treated with either of the MWCNTs, thereby assuring their nontoxic nature.
Fig. 10.
Zebra fish embryos 120 hpf with control (a) and MWCNT treated (each at a concentration of 50 μg/ml): pristine MWCNT (b), MWCNT-COOH (c) and MWCNT-NH2 (d).
4. Discussion
In the present study, we successfully achieved generation of the carboxy and amino functionalization of MWCNTs (employing biophysical methodologies) and further exploited these functionalized MWCNTs to show amelioration of cellular cytotoxicity by preventing prooxidant state in comparison to pristine MWCNTs. Functionalization of MWCNT in general, be it carbonyl, carboxyl, hydroxyl, amino, etc. has been shown to generate a profound effect on the toxicity character of the CNTs [19, 20]. Reports both in favor and against functionalization confront the precise role of MWCNTs towards cellular toxicity [15, 21, 22, 23]. To sort out such a conundrum our observations showed that functionalization reduces the cytotoxicity potential of pristine MWCNTs as reflected by MTT and trypan blue assays. This reduction in toxicity by functionalized MWCNTs was supported by decreased ROS generation in HEK 293 cells. The reason seems to be an important role being played by reduced agglomeration of functionalized MWCNTs in rendering these nano-particles non-toxic. Multiple studies in this regard did demonstrate functionalization of MWCNTs at varying degree and/or with different functional groups improved the dispersion of MWCNTs in medium and reducing their agglomeration leading to reduced cytotoxicity [19, 20]. Our observations indeed validate such an effect wherein, both MWCNT-COOH and MWCNT-NH2, while showing greater dispersion in the culture medium than pristine also showed reduced cytotoxicity with respect to the pristine ones. The zeta potential analysis further supported our claim since in water, the zeta potential of both the MWCNT-COOH and MWCNT-NH2 was comparable with an average value of -27.93 mV and -26.82 mV, respectively in comparison to that of pristine MWCNT as it could not be calculated due to non-formation of a stable dispersion with distilled water. Although when dispersed in culture media (DMEM supplemented with 10% FBS), all the MWCNTs though did show formation of suspensions giving a value of -4.92 mV, -26.87 mV and -13.98 mV for pristine, MWCNT-COOH and MWCNT-NH2 respectively but a low negative value of pristine MWCNTs depicts a low charge to size ratio and thus justifies the low solubility of pristine MWCNTs as compared to the functionalized one. Further, between the functionalized MWCNTs, MWCNT-COOH was found to have the highest negative score depicting a high charge to mass ratio thus more solubility. This effect is indeed accepted as majority of COOH have been involved in amide bond en route generation of MWCNT-NH2. It is important to discuss an observation by Chen et al [24] who suggested against such observations [24], as nerve growth factor (NGF) that was bound to both aminized MWCNTs and carboxy MWCNTs and tested on PC12 cells. The results showed enhanced adherence, reduced toxicity and significant cell differentiation in cells grown in aminized MWCNT-NGF in comparison to carboxy MWCNT-NGF. It was reasoned that ease of amino functionalized MWCNTs in forming stable constructs with active biomolecules may lower cytotoxicity. However, since in our experiment no bioactive component was added to the functionalized MWCNTs, the free functional groups were able to interact with the cellular components, thereby making MWCNT-NH2 more toxic than MWCNT-COOH on the basis of their exposed functional groups. Also, in our study, MWCNT oxidation was first carried out since it is known to increase the efficiency of subsequent amination reaction because MWCNT surface becomes activated only after oxidation with strong acids [24]. Thus, even with the presence of both functional groups on MWCNT-NH2 its ability to be more cytotoxic points towards the role of free amino groups on MWCNT-NH2 as contributors towards enhanced number of interactions with biological macromolecules, like cellular proteins, DNA, etc that might influence the cellular responses in a detrimental fashion [25]. Observation of Bianco et al [26] indeed corroborate to such possibility of biological interactions by MWCNT-NH2. Despite such observations, MWCNT-NH2 still manages to perform better than pristine MWCNTs on accounts of better solubility in the culture medium and less of cytotoxic.
Further, upon comparing the effectiveness of the different functional groups on the lowering of cytotoxicity the observations point out that carboxy functionalization was found to be more effective in rendering MWCNT nontoxic (MTT assay only) in comparison to the amino functionalization. It is therefore, pertinent to mention that overall effect of functionalization, whether carboxy or amino remained significantly effective in lowering cellular toxicity caused by pristine MWCNTs.
No doubt, functionalization of MWCNTs as shown in our study, did reduce the generation of ROS in the cells but a complete amelioration still could not be achieved. The reasons may be multiple however based on the available information the onus seems to be primarily on the strategies being employed for preparation of MWCNT. Different metals, including heavy metals, viz. Co, Fe, Ni, Mo, etc., are commonly used in the synthesis process of these CNTs and those get entrapped at the edges of the newly synthesized CNTs. These are difficult to remove and still persists in the CNT after harsh treatment of CNTs with inorganic acids [27, 28, 29]. Thus, it becomes difficult to remove these entrapped metals in case of pristine MWCNTs. While for the functionalized MWCNTs the defects did help to partially leach out comparable metal ions residues and hence there is minimal ROS generation following functionalization. Even for this minimum ROS generation by functionalized MWCNTs, the reaction of remnant heavy metal used in the basic preparation of MWCNTs still cannot be ruled out. Pulskamp et al 2007, did demonstrate generation of ROS following pristine and acid treated MWCNTs which was found to be greatly reduced following removal of entrapped metal nano-particles incorporated during generation of MWCNTs [30]. The biological applications of these functionalized MWCNTs thus necessitate the removal of entrapped metal nano particles during the functionalization process. Employing a greener chemistry approach for the generation of pristine MWCNTs would thus be a next mandatory step for their use in biological applications.
A surprising observation in the present study was the in vivo effects of these MWCNTs wherein embryo development in zebra fish remained refractory to increasing concentration of both pristine or functionalized MWCNTs. Such observations are though in accordance to the previous studies, which showed that pristine and functionalized MWCNTs do not cause any significant mortality or malformations in zebra fish embryos but certainly are in contrast to the in vitro studies using cell lines in which only pristine MWCNTs showed a significant level of toxicity [31]. The presence of chorion primary defense against the entry of pristine nanoparticles in zebra fish embryos seems to be the potential reason for such a refraction. Cheng & Cheng did demonstrate that when MWCNTs were directly injected inside the embryos, considerable toxicity was observed [32]. While under in vitro condition, due to agglomeration, these pristine nanoparticles tend to settle down on agglomeration, which brings them in close proximity to the cells attached at the bottom of the petriplates. However, in the case of zebra fish, settling of nanoparticles will inhibit their interactions with zebra fish embryos as after 72 h of fertilization, the zebra fish embryos hatch out of the chorion and start swimming in the medium, thereby limiting the effective concentration of exposure. Moreover, for in vivo effect the role played by immune defenses from zebra fish embryos/whole organism can also not be ruled out in comparison to in vitro system [33]. The observations of the present study thus pointed towards effectiveness of functionalization of MWCNTs towards weakening the toxicity of pristine MWCNTs under in vitro versus in vivo system. Thus, overall, besides targeting the methodology for generation of MWCNTs, as discussed earlier, the present observations like many other reports also sound a caution regarding the extrapolation of in vitro data to in vivo while toxicological analysis of such compounds are to be studied [34].
5. Conclusion
Based on the observations it is concluded that carboxy and amino functionalization render the MWCNTs both water soluble and biocompatible. In comparison to the pristine MWCNTs the functionalized MWCNTs significantly ameliorated the cellular toxicity and prooxidant potential in HEK 293 cells while zebra fish, as a model system, remained refractory to MWCNTs toxicity. Further, the observations also provide strong evidences towards the role of ROS generation in observed cellular toxicity besides the role being played by varying solubility index of different MWCNTs. Finally, it was also observed that the MWCNT-COOH fared better than MWCNT-NH2 in reducing MWCNTs toxicity to the cells.
Declarations
Author contribution statement
Sanjeev Puri: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ayush Chowdhry: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jasreen Kaur: Performed the experiments; Analyzed and interpreted the data.
Madhu Khatri, Veena Puri & Rakesh Tuli: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Research funding for this research was provided by Council of Scientific and Industrial Research, New Delhi [CSIR Sanction No.: 09/135(0694)/2012] and PURSE grant, UIET, PU, Chandigarh, India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors thankfully acknowledge Sophisticated Analytical Instrumentation Facility, Panjab University (PU), Chandigarh; Department of Central Sophisticated Instrument Cell, Post Graduate Institute of Medical Education and Research, Chandigarh; University Institute of Engineering and Technology (UIET), PU, Chandigarh; and Centre for Stem Cell and Tissue Engineering, PU, Chandigarh for supporting this research.
References
- 1.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991 Nov 7;354:56. [Google Scholar]
- 2.Sajid M.I., Jamshaid U., Jamshaid T., Zafar N., Fessi H., Elaissari A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016;501(1–2):278–299. doi: 10.1016/j.ijpharm.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 3.Mody N., Tekade R.K., Mehra N.K., Chopdey P., Jain N.K. Dendrimer, liposomes, carbon nanotubes and PLGA nanoparticles: one platform assessment of drug delivery potential. AAPS PharmSciTech. 2014;15(2):388–399. doi: 10.1208/s12249-014-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karousis N., Tagmatarchis N., Tasis D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010;110(9):5366–5397. doi: 10.1021/cr100018g. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L., Forman H.J., Ge Y., Lunec J. Multi-walled carbon nanotubes: a cytotoxicity study in relation to functionalization, dose and dispersion. Toxicol. In Vitro. 2017;42:292–298. doi: 10.1016/j.tiv.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J., Huang W., Wu L., Hu Y., Ye M. Study on amino-functionalized multiwalled carbon nanotubes. Mater. Sci. Eng. A. 2007;464(1–2):151–156. [Google Scholar]
- 7.Nayak T.R., Jian L., Phua L.C., Ho H.K., Ren Y., Pastorin G. Thin films of functionalized multiwalled carbon nanotubes as suitable scaffold materials for stem cells proliferation and bone formation. ACS Nano. 2010;4(12):7717–7725. doi: 10.1021/nn102738c. [DOI] [PubMed] [Google Scholar]
- 8.Kam N.W.S., Jan E., Kotov N.A. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2008;9(1):273–278. doi: 10.1021/nl802859a. [DOI] [PubMed] [Google Scholar]
- 9.Joddar B., Garcia E., Casas A., Stewart C.M. Development of functionalized multi-walled carbon-nanotube-based alginate hydrogels for enabling biomimetic technologies. Sci. Rep. 2016;6:32456. doi: 10.1038/srep32456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonelli F.M., Santos A.K., Gomes K.N., Lorencon E., Guatimosim S., Ladeira L.O. Carbon nanotube interaction with extracellular matrix proteins producing scaffolds for tissue engineering. Int. J. Nanomed. 2012;7:4511. doi: 10.2147/IJN.S33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumortier H., Lacotte S., Pastorin G., Marega R., Wu W., Bonifazi D. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6(7):1522–1528. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 12.Ji Z., Zhang D., Li L., Shen X., Deng X., Dong L. The hepatotoxicity of multi-walled carbon nanotubes in mice. Nanotechnology. 2009;20(44):445101. doi: 10.1088/0957-4484/20/44/445101. [DOI] [PubMed] [Google Scholar]
- 13.Bellucci S., Bergamaschi A., Bottini M., Magrini A., Mustelin T. IOP Publishing; 2007. Biomedical Platforms Based on Composite Nanomaterials and Cellular Toxicity; p. 95. [Google Scholar]
- 14.Magrez A., Kasas S., Salicio V., Pasquier N., Seo J.W., Celio M. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6(6):1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 15.Vuković G., Marinković A., Obradović M., Radmilović V., Čolić M., Aleksić R. Synthesis, characterization and cytotoxicity of surface amino-functionalized water-dispersible multi-walled carbon nanotubes. Appl. Surf. Sci. 2009;255(18):8067–8075. [Google Scholar]
- 16.Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 2005;39(10):1290. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y.-Y., Zhang J., Zheng Y.-F., Yang J., Zhu X.-Q. Cytotoxic and genotoxic effects of multi-wall carbon nanotubes on human umbilical vein endothelial cells in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011;721(2):184–191. doi: 10.1016/j.mrgentox.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Thurnherr T., Brandenberger C., Fischer K., Diener L., Manser P., Maeder-Althaus X. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol. Lett. 2011;200(3):176–186. doi: 10.1016/j.toxlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Alshehri R., Ilyas A.M., Hasan A., Arnaout A., Ahmed F., Memic A. Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity: miniperspective. J. Med. Chem. 2016;59(18):8149–8167. doi: 10.1021/acs.jmedchem.5b01770. [DOI] [PubMed] [Google Scholar]
- 20.Coccini T., Roda E., Sarigiannis D., Mustarelli P., Quartarone E., Profumo A. Effects of water-soluble functionalized multi-walled carbon nanotubes examined by different cytotoxicity methods in human astrocyte D384 and lung A549 cells. Toxicology. 2010;269(1):41–53. doi: 10.1016/j.tox.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Bottini M., Bruckner S., Nika K., Bottini N., Bellucci S., Magrini A. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 2006;160(2):121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton R.F., Jr., Xiang C., Li M., Ka I., Yang F., Ma D. Purification and sidewall functionalization of multiwalled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal. Toxicol. 2013;25(4):199–210. doi: 10.3109/08958378.2013.775197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foldvari M., Bagonluri M. Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomed. Nanotechnol. Biol. Med. 2008;4(3):183–200. doi: 10.1016/j.nano.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Chen W., Xiong Q., Ren Q., Guo Y., Li G. Can amino-functionalized carbon nanotubes carry functional nerve growth factor? Neural Regen. Res. 2014;9(3):285. doi: 10.4103/1673-5374.128225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Z., Yang Z., Hu Y., Li J., Fan X. Multiple functionalization of multi-walled carbon nanotubes with carboxyl and amino groups. Appl. Surf. Sci. 2013;276:476–481. [Google Scholar]
- 26.Bianco A., Kostarelos K., Partidos C.D., Prato M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005;(5):571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 27.Shigemoto-Mogami Y., Fujimori K., Ikarashi Y., Hirose A., Sekino Y., Sato K. Residual metals in carbon nanotubes suppress the proliferation of neural stem cells. Fundamental Toxicol. Sci. 2014;1(3):87–94. [Google Scholar]
- 28.Pumera M. Carbon nanotubes contain residual metal catalyst nanoparticles even after washing with nitric acid at elevated temperature because these metal nanoparticles are sheathed by several graphene sheets. Langmuir. 2007;23(11):6453–6458. doi: 10.1021/la070088v. [DOI] [PubMed] [Google Scholar]
- 29.Shvedova A., Castranova V., Kisin E., Schwegler-Berry D., Murray A., Gandelsman V. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J. Toxicol. Environ. Health Part A. 2003;66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 30.Pulskamp K., Diabaté S., Krug H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007;168(1):58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Liu X.T., Mu X.Y., Wu X.L., Meng L.X., Guan W.B., Ma Y.Q. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014;27(9):676–683. doi: 10.3967/bes2014.103. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J., Cheng S.H. Influence of carbon nanotube length on toxicity to zebrafish embryos. Int. J. Nanomed. 2012;7:3731–3739. doi: 10.2147/IJN.S30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur J., Khatri M., Puri S. Toxicological evaluation of metal oxide nanoparticles and mixed exposures at low doses using zebra fish and THP1 cell line. Environ. Toxicol. 2019;34(4):375–387. doi: 10.1002/tox.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voigt N., Henrich-Noack P., Kockentiedt S., Hintz W., Tomas J., Sabel B.A. Toxicity of polymeric nanoparticles in vivo and in vitro. J. Nano Res. 2014;16(6):2379. doi: 10.1007/s11051-014-2379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]