Abstract
Objective: Alaska Native (AN) people have among the world’s highest rate of colorectal cancer (CRC). We assessed perceptions of AN people and their health care providers of a new take-home multitarget stool DNA test (MT-sDNA; Cologuard) relative to colonoscopy. Methods: Cross-sectional surveys of AN people aged 40 to 75 years (mailed) and providers (online). Results: Participants included 1616 AN patients (19% response rate) and 87 providers (26% response rate; 57% AN people). Over half (58%) of patients preferred colonoscopy for CRC screening, while 36% preferred MT-sDNA. Unscreened patients were significantly more likely to state a preference for MT-sDNA than previously screened patients (42% vs 31%, P < .05) as were younger patients (<60 years old) compared with older patients (40% vs 30%, P < .05). Most providers thought that MT-sDNA would improve screening rates (69%), would recommend if available (79%), and be implementable (79%). Perceived barriers differed substantially between patients and providers in both type and magnitude. Leading colonoscopy barriers reported by patients were travel (44%) and bowel preparation (40%), while providers thought that fear of pain (92%) and invasiveness of the test (87%) were the primary barriers. For MT-sDNA, patients’ belief that colonoscopy was better (56%) and not knowing how to do the test (40%) were primary barriers, while providers thought stool collection (67%) and having a stool sample in their home (63%) were leading barriers. Conclusions: This study found that MT-sDNA has potential acceptability among AN people and their health care providers. Both groups reported a willingness to use MT-sDNA and did not perceive major barriers to its use. This preference was especially true of unscreened and younger patients. The majority of providers indicated they would use MT-sDNA if available and that it would improve CRC screening rates. In this population, where colonoscopy access is limited, MT-sDNA has the potential to improve CRC screening adherence.
Keywords: Alaska Native, Cologuard, multitarget stool DNA testing, colonoscopy, colorectal cancer, screening, adherence, prevention
Introduction
Alaska Native (AN) people experience 2 times higher incidence and mortality from colorectal cancer (CRC) than US whites.1,2 CRC can be prevented through removal of precancerous polyps or treated more easily if detected early using screening tests.3-5 Although significantly improved, the AN CRC screening prevalence of 59% is still far from the national Healthy People 2020 goal of 70.5%.6,7
Because of high rates of colorectal neoplasia, colonoscopy has become the preferred CRC screening method in the AN population.8 Colonoscopy, however, is resource intensive, requires specially trained providers, and carries a risk of complications due to adverse reactions to anesthesia or bowel perforations.9 More than half of the AN population resides in widely distributed and remote roadless regions.10 In these regions there are 7 Tribal health facilities that provide colonoscopies, of which only 1 is connected by road to the communities that they serve. Therefore, colonoscopy generally requires travel in small aircraft for the patient and their medical escort, with concomitant costs and time away from work and dependent care. Additionally, colonoscopy appears to have limited effect on incidence or mortality of proximal CRC, which is of concern as over 41% of CRCs in AN people occur in the proximal colon.11-19 Compared with colonoscopy, take-home stool tests are less expensive, more easily distributed, eliminate the need for bowel preparation, and do not require costly travel or time away from work or caretaking responsibilities. CRC screening using guaiac-based fecal occult blood tests is not recommended for AN people because of false-positive results associated with a high prevalence of Helicobacter pylori infection and red meat consumption.8,20 Another take-home test, the fecal immunochemical test, which identifies intact human hemoglobin in stool, is available in the Alaska Tribal Health System.8 However, its use has been limited due to tribal leadership concerns that it does not detect precancerous polyps in this increased-risk population.
We previously evaluated the performance of a new take-home stool test, the multi-target stool DNA test (MT-sDNA; Cologuard, Exact Sciences, Madison, WI) in the AN population. Results were very similar to those in a large multicenter screening study; respective detection rates for CRC were 100% and 92% and for large polyps at greatest risk for progression (≥2cm) were 62% and 67% with respective specificities of 93% and 90%.21,22 MT-sDNA sensitivity for CRC is similar to that reported by colonoscopy, and was significantly higher than the fecal immunochemical test in the AN population.13-15
Patient willingness and ability to complete tests as well as provider recommendation are critical factors in improving CRC screening.23-28 Initial MT-sDNA studies have shown its use increases screening adherence, including among never-screened patients, and its use may actually increase the yield and quality of follow-up colonoscopies.29-31 In this study we assess the acceptability of MT-sDNA testing compared with colonoscopy among AN people and their healthcare providers. This study serves as a critical step in determining the feasibility and application of MT-sDNA; patient and provider barriers to MT-sDNA and colonoscopy; and provides insights for increasing CRC screening among other rural/remote populations.
Methods
This study was conducted in three rural/remote Alaska Tribal health organization regions from July-September 2017. The Alaska Area Institutional Review Board and relevant tribal research and ethics committees of each participating region approved the study. AN people aged 40 to 75 years (n = 8979) were invited to participate in a mailed survey, which in 2 regions included an invitational cover letter signed by the Tribal health organization’s Medical Director. Tribal health organization health care providers (n = 87), including mid-level providers, physicians, and community health aides/practitioners completed the survey online. Patient questions were adapted from the National Cancer Institute’s Health Information National Trends Survey32 and provider questions were adapted from the CRC Screening Practices: Survey of Primary Care Providers.33-35 Each survey contained 12 items and took about 5 to 10 minutes to complete. Likelihood ratio chi-square tests and Fisher’s exact test were used for categorical data using SAS version 9.4 (SAS Corporation, Cary, NC). All analyses were 2-tailed; P < .05 was considered statistically significant. Participants with missing data were excluded from the individual variable analysis and no corrections for multiple comparisons were made due to the small number of planned comparisons.
Results
Patient Characteristics
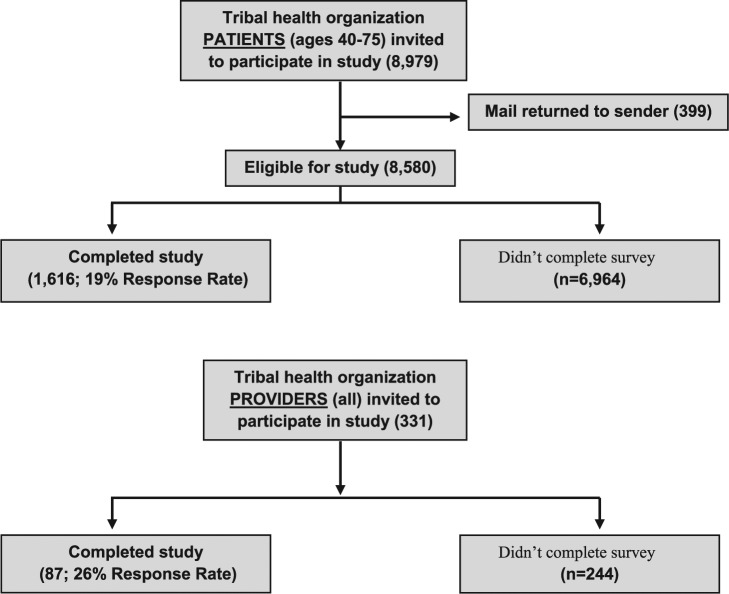
Of the 8580 patients with valid addresses, 1616 patient surveys were completed (19% response rate, range 18%-31%; Figure 1). A total of 21% of respondents were aged 40 to 49 years, 36% were aged 50 to 59 years, 34% were aged 60 to 69 years, and 7% were aged 70 to 75 years. Response rates were similar among men (51%) and women (49%). One-fifth (20%) of screening-eligible patients (men and women aged 40-75 years) had never heard of CRC screening tests and had never been screened. Of those who had heard about screening tests, 98% had heard of colonoscopy. About 22% had heard of take-home stool tests (fecal occult blood test or fecal immunochemical test), while only 16% had heard of MT-sDNA. When patients were asked about CRC screening, most agreed that being screened would make them feel they are doing something positive for their health (87%) or reassured (79%). A small minority reported not needing CRC screening because they feel fine (14%), no family history (9%), or afraid of having cancer (8%). Of note, 18% reported not seeking screening because of not knowing where to go. Men and women did not significantly differ in their general attitudes toward screening except for more men reporting not knowing where to go (21% vs 14%, P < .05) and not needing screening because no family history (11% vs 9%, P < .05).
Figure 1.
Study flow diagram.
Prior CRC Screening History and Reasons for Nonscreening
Over half of patients (58%) reported having been screened for CRC, mostly by colonoscopy (96%). Of the never screened, the top 5 reasons included no reason/never thought about it (46%); no symptoms (28%); no doctor suggestion (25%); didn’t need it/didn’t know needed it (21%); and too expensive/no insurance (13%). Less than 10% said they were unscreened because they “hadn’t gotten around to it,” 8% because they didn’t have a doctor, and 7% because of embarrassment or discomfort with the procedure. Participants could choose more than one answer, so results do not total 100%.
Patient Barriers to Colonoscopy and MT-sDNA
All participants (both screened and unscreened) were then asked about specific barriers (ie, conditions that make it difficult to be screened) to either colonoscopy or MT-sDNA, regardless if they were able to overcome those particular barriers to complete their screening. The top barriers reported by those who had ever undergone colonoscopy included colonoscopic preparation laxatives (51%), travel (47%), fear of injury (36%), discomfort with a tube in their rectum (34%), and fear of pain (32%). In contrast, the top barriers reported by those who had never undergone colonoscopy included fear of pain (65%), discomfort with a tube in their rectum (63%), travel (60%), colonoscopic preparation laxatives (57%), and fear of injury (49%). The top colonoscopy barriers overall included travel (44%), colonoscopic preparation laxatives (40%), fear of pain (35%), discomfort with a tube in their rectum (34%), and fear of injury (30%). Overall, 29% said that undergoing anesthesia or having to find a postprocedure escort would be barriers. Less than one-quarter reported taking time off work (23%), the need for dependent care (22%), embarrassment (18%), or the procedure taking too much time (16%) as colonoscopy barriers. Men and women did not differ substantially, although more women than men reported pain (40% vs 31%, P < .05) and undergoing anesthesia (33% vs 25%, P < .05) as barriers.
A smaller proportion of respondents reported barriers to getting screened using MT-sDNA (13%-53% answered yes to each of the listed barriers) compared with colonoscopy (38%-73%). The top 5 MT-sDNA barriers included belief that colonoscopy is better at preventing cancer (56%), having to learn how to do the test (40%), discomfort with stool collection (32%), needing a private place to perform the test (29%), and having to do MT-sDNA every 3 years (27%). Fewer patients reported having a stool sample in their home (26%), needing a toilet with a seat cover (25%), embarrassment (18%), or time (14%) as barriers. Of note, for each barrier queried, around 15% to 30% responded that they did not know how that barrier would affect their choice to be screened by MT-sDNA, reflecting an overall unfamiliarity with MT-sDNA. Men and women did not differ significantly in their reported MT-sDNA barriers.
Patient Comparison of Colonoscopy and MT-sDNA
There was no significant difference between colonoscopy and MT-sDNA in patient-reported embarrassment, discomfort, or time to do the test. After learning about MT-sDNA and colonoscopy, over half (58%) of respondents said they would prefer colonoscopy for CRC screening, while 36% said they would prefer MT-sDNA. A total of 12% said neither test, and 14% said either test or they were unsure. Unscreened patients were significantly more likely to state a preference for MT-sDNA than previously screened patients (42% vs 31%, P < .05). Likewise, younger patients (<60 years old) were significantly more likely to prefer MT-sDNA than older patients (40% vs 30%, P < .05).
Provider Characteristics
Of the 331 invited providers, 87 (26% response rate; range 16%-61%) completed the survey (Figure 1). Three-quarters (76%) were younger than 50 years, with 57% identifying as Alaska Native/American Indian. A total of 75% were women, similar to the proportion (86%) in the invited survey group.
Provider Willingness to Recommend MT-sDNA
Over two-thirds of providers (69%) thought that patients would be more likely to be screened for CRC if they could use MT-sDNA instead of colonoscopy, and 79% reported that if MT-sDNA became available at their organization they would recommend it to patients. There was no significant difference by provider sex, age (<50 vs 50+ years), or race (Alaska Native/American Indian vs White/other) in these 2 factors. Most providers reported that if MT-sDNA was available that it would be easy to distribute and implement in their practice (79%) as well as lead to CRC screening rate increases (79%). Less than a quarter (22%) reported that MT-sDNA would require more work because the test has to be performed every 3 years.
Provider Perceptions of Patient Barriers to Colonoscopy and MT-sDNA
The most common barriers that providers thought would affect their patients’ choice to get a colonoscopy were fear of pain or discomfort (92%), test invasiveness (87%), travel (77%), anesthesia (72%), taking laxatives (72%), and dependent care (66%). The barriers that providers thought would least affect their patients’ choice to have a colonoscopy were embarrassment (58%) and taking too much time (48%).
The top 5 provider-reported patient barriers to MT-sDNA were stool collection (67%), stool sample in the home (63%), belief that colonoscopy better at preventing cancer (56%), learning how to do the test (56%), and embarrassment (47%). Fewer providers reported needing a toilet with a seat cover (33%), a private place to do the test (32%), testing every 3 years (22%), or time (16%) as MT-sDNA barriers to patients.
Differences in Patient and Provider Assessment of Barriers
One notable finding was the difference between patients and providers in the magnitude and ranking of barriers to colonoscopy and MT-sDNA (Figure 2). Overall, patients were most likely to report travel and the bowel preparation as primary barriers to colonoscopy, while providers thought that fear of pain and test invasiveness were the primary barriers for their patients. For MT-sDNA, patients’ belief that colonoscopy was a better test and not knowing how to do the test were primary barriers, while providers thought that stool collection and having the sample in the patient’s house were the primary barriers for their patients. These differences were statically significant (P < .05). There was also a significant difference (P < .05) between patients and providers in concern over anesthesia (29% vs 72%), dependent care (22% vs 66%), and whether the colonoscopy would make them feel embarrassed (18% vs 57%) or take too much time (16% vs 48%).
Figure 2.
Differences in patient and provider assessment of barriers to multitarget stool DNA test and colonoscopy.
*Indicates statistically significant differences between patients and providers (P < .05).
Discussion
This study found that MT-sDNA has potential acceptability among AN people and their health care providers. Both groups reported a willingness to use MT-sDNA and did not perceive major barriers to its use. Even though it is not yet available in the Alaska Tribal Health System, over one-third (36%) of patients said they would prefer MT-sDNA for CRC screening instead of colonoscopy, similar to another study of minority populations (Black and Latino primary care patients), which found that 31% preferred MT-sDNA.36 The proportion of those who preferred MT-sDNA to colonoscopy was higher among unscreened and younger patients, which has important implications for improving screening uptake. Providers were likewise open to MT-sDNA, the majority of whom indicated they would use MT-sDNA if available and felt that it would improve CRC screening rates in this population. However, there was a lack of patient familiarity with completing MT-sDNA, provider uncertainty about its effectiveness, as well as a belief among both patients and providers that colonoscopy is better at preventing cancer. Because of the high false positive rates associated with guaiac-based fecal occult blood tests in the AN population there has been a reluctance among providers to use stool tests, which makes the interest in MT-sDNA evidenced by this study more surprising.
Many factors play a role in screening adherence and test preferences.24,37-49 Data from the National Health Interview Survey (NHIS) indicate that the most commonly reported reason (40%) for not having a CRC screening was “no reason or never thought about it,”50 which was similar to nonscreened AN respondents in the current study (46%). Other reasons for not having a CRC screening were about twice as high among AN respondents as the NHIS data.50
One of the most notable findings of this study was the difference in both the magnitude and ranking of barriers between patients and providers, especially for colonoscopy. Providers tended to overestimate how much pain and concern about the invasiveness of the test (colonoscopy) would affect their patient’s choice to be screened. Patients were much less likely to view potential issues as barriers, and the barriers that patients noted were mostly logistic, such as travel and bowel preparation issues. A sizeable minority of patients was unaware of the need for CRC screening and did not know where to complete their screening. These data indicate gaps in public health knowledge and messaging and suggest that health care providers should continue to strongly recommend and support CRC screening among the patients that they serve.
Limitations of this study include potential selection bias. Our provider response rate (26%) was low, but similar to other surveys of providers, especially surveys that do not offer incentives.51,52 Additionally, the provider groups included in the study were at multiple levels of practice, from community health aides to medical doctors. There may be differences in perceptions of MT-sDNA and colonoscopy by provider type that we were unable to observe. Similarly, although the patient response rate (19%) was not atypical for a mailed questionnaire study, especially in hard-to-reach populations53,54; patient respondents may differ from nonrespondents in their willingness to participate in a CRC screening survey. Patients may also have had low levels of literacy, which may have impeded their ability to complete the questionnaire. However, there is not an a priori reason that this would have biased our findings regarding preferences between colonoscopy and MT-sDNA. Patient respondents were similar in gender and age characteristics to the underlying Indian Health Service user population distributions in the participating Tribal health regions as well as similar in their screening status: A total of 58% patient respondents reported having been screened compared to 59% of AN people statewide who are up-to-date with CRC screening.6
Another potential limitation is that this was a hypothetical study of preferences. While over half of patients had been screened previously, primarily with colonoscopy, none had experience with MT-sDNA, which may have led them to over- or underestimate the relative benefits of MT-sDNA in comparison with colonoscopy. An intervention study in which patients are offered a choice of tests would help confirm these initial findings.
Multitarget stool DNA testing may represent a new strategy to expand CRC screening for AN people or other rural/remote populations and reduce both CRC incidence and mortality, especially where access to colonoscopy is limited. The results also highlight barriers to existing screening practices that can be used to identify areas for education for both patients and providers and strengthen CRC prevention and control.
Acknowledgments
We would like to acknowledge the contributions and support of the Alaska Native Tribal Health Consortium, the Bristol Bay Area Health Corporation, Chugachmiut, and the Yukon-Kuskokwim Health Corporation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Two authors (DAA and JBK) have disclosed relationships with Exact Sciences.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Richard M. Schulze Family Foundation.
ORCID iD: Diana G. Redwood  https://orcid.org/0000-0003-0360-5729
https://orcid.org/0000-0003-0360-5729
References
- 1. Perdue DG, Haverkamp D, Perkins C, Daley CM, Provost E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104(suppl 3):S404-S414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carmack AM, Schade TL, Sallison I, Provost EM, Kelly JJ. Cancer in Alaska Native People: 1969-2013: The 45-Year Report. Anchorage, AK: Alaska Native Tumor Registry, Alaska Native Epidemiology Center, Alaska Native Tribal Health Consortium; 2015. [Google Scholar]
- 3. Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57:90-104. [DOI] [PubMed] [Google Scholar]
- 4. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2564-2575. [DOI] [PubMed] [Google Scholar]
- 6. Indian Health Service. Alaska Area Aggregate GPRA (Government Performance and Results Act of 1993) Clinical Performance Report, CRS (Clinical Reporting System), Version 18.0. Anchorage, AK: Alaska Area Native Health Service; 2017. [Google Scholar]
- 7. Meester RG, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121:2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alaska Native Medical Center. Colorectal Cancer Screening Guidelines. Anchorage, AK: Alaska Native Tribal Health Consortium; 2013. [Google Scholar]
- 9. American Cancer Society. Colorectal Cancer Facts & Figures 2017-2019. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 10. US Census Bureau. Summary File 1, Tables PCT1, PCT2, PCT3. Race reporting for the American Indian and Alaska Native population by selected tribes: 2010. http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml. Published 2010. Accessed October 9, 2019.
- 11. Atkin WS, Edwards R, Kralj-Hans I, et al. ; UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [DOI] [PubMed] [Google Scholar]
- 12. Schoen RE, Pinsky PF, Weissfeld JL, et al. ; PLCO Project Team. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8. [DOI] [PubMed] [Google Scholar]
- 14. Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89-95. [DOI] [PubMed] [Google Scholar]
- 15. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422-428. [DOI] [PubMed] [Google Scholar]
- 17. Ahlquist DA, Wieand HS, Moertel CG, et al. Accuracy of fecal occult blood screening for colorectal neoplasia. A prospective study using Hemoccult and HemoQuant tests. JAMA. 1993;269:1262-1267. [PubMed] [Google Scholar]
- 18. Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128-1137. [DOI] [PubMed] [Google Scholar]
- 19. Kelly JJ, Alberts SR, Sacco F, Lanier AP. Colorectal cancer in Alaska Native people, 2005-2009. Gastrointest Cancer Res. 2012;5:149-154. [PMC free article] [PubMed] [Google Scholar]
- 20. Redwood D, Provost E, Asay E, et al. Comparison of fecal occult blood tests for colorectal cancer screening in an Alaska Native population with high prevalence of Helicobacter pylori infection, 2008-2012. Prev Chronic Dis. 2014;11:E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Redwood DG, Asay ED, Blake ID, et al. Stool DNA Testing for screening detection of colorectal neoplasia in Alaska Native people. Mayo Clin Proc. 2016;91:61-70. [DOI] [PubMed] [Google Scholar]
- 22. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. [DOI] [PubMed] [Google Scholar]
- 23. Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339-359. [DOI] [PubMed] [Google Scholar]
- 24. Wilkins T, Gillies RA, Harbuck S, Garren J, Looney SW, Schade RR. Racial disparities and barriers to colorectal cancer screening in rural areas. J Am Board Fam Med. 2012;25:308-317. [DOI] [PubMed] [Google Scholar]
- 25. Coughlin SS, Thompson T. Physician recommendation for colorectal cancer screening by race, ethnicity, and health insurance status among men and women in the United States, 2000. Health Promot Pract. 2005;6:369-378. [DOI] [PubMed] [Google Scholar]
- 26. Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939-944. [DOI] [PubMed] [Google Scholar]
- 27. Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41:23-29. [DOI] [PubMed] [Google Scholar]
- 28. Jones RM, Woolf SH, Cunningham TD, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010;38:499-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prince M, Lester L, Chiniwala R, Berger B. Multitarget stool DNA tests increases colorectal cancer screening among previously noncompliant Medicare patients. World J Gastroenterol. 2017;23:464-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berger BM, Schroy PC, 3rd, Rosenberg JL, et al. Colorectal cancer screening using stool DNA analysis in clinical practice: early clinical experience with respect to patient acceptance and colonoscopic follow-up of abnormal tests. Clin Colorectal Cancer. 2006;5:338-343. [DOI] [PubMed] [Google Scholar]
- 31. Johnson DH, Kisiel JB, Burger KN, et al. Multitarget stool DNA test: clinical performance and impact on yield and quality of colonoscopy for colorectal cancer screening. Gastrointest Endosc. 2017;85:657-665.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Institutes of Health, National Cancer Institute Division of Cancer Control and Population Science. Health Information National Trends Survey. hints.cancer.gov. Accessed October 18, 2016.
- 33. Hannon PA, Martin DP, Harris JR, Bowen DJ. Colorectal cancer screening practices of primary care physicians in Washington State. Cancer Control. 2008;15:174-181. [DOI] [PubMed] [Google Scholar]
- 34. Klabunde CN, Frame PS, Meadow A, Jones E, Nadel M, Vernon SW. A national survey of primary care physicians’ colorectal cancer screening recommendations and practices. Prev Med. 2003;36:352-362. [DOI] [PubMed] [Google Scholar]
- 35. Chapman K, Nicholls K, Sullivan MM, et al. Colorectal cancer screening practices in Alabama: a survey of primary care physicians. J Cancer Educ. 2012;27:687-694. [DOI] [PubMed] [Google Scholar]
- 36. Chablani SV, Cohen N, White D, Itzkowitz SH, DuHamel K, Jandorf L. Colorectal cancer screening preferences among Black and Latino primary care patients. J Immigr Minor Health. 2017;19:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ward SH, Parameswaran L, Bass SB, Paranjape A, Gordon TF, Ruzek SB. Resident physicians’ perceptions of barriers and facilitators to colorectal cancer screening for African Americans. J Natl Med Assoc. 2010;102:303-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolf RL, Zybert P, Brouse CH, et al. Knowledge, beliefs, and barriers relevant to colorectal cancer screening in an urban population: a pilot study. Fam Community Health. 2001;24:34-47. [DOI] [PubMed] [Google Scholar]
- 39. Rawl SM, Menon U, Champion VL, et al. Do benefits and barriers differ by stage of adoption for colorectal cancer screening? Health Educ Res. 2005;20:137-148. [DOI] [PubMed] [Google Scholar]
- 40. Quick BW, Hester CM, Young KL, Greiner KA. Self-reported barriers to colorectal cancer screening in a racially diverse, low-income study population. J Community Health. 2013;38:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walsh JM, Kaplan CP, Nguyen B, Gildengorin G, McPhee SJ, Pérez-Stable EJ. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino White Americans. J Gen Intern Med. 2004;19:156-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3:A123. [PMC free article] [PubMed] [Google Scholar]
- 43. Schroy PC, 3rd, Emmons KM, Peters E, et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43:573-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carcaise-Edinboro P, Bradley CJ. Influence of patient-provider communication on colorectal cancer screening. Med Care. 2008;46:738-745. [DOI] [PubMed] [Google Scholar]
- 46. Brown ML, Potosky AL, Thompson GB, Kessler LG. The knowledge and use of screening tests for colorectal and prostate cancer: data from the 1987 National Health Interview Survey. Prev Med. 1990;19:562-574. [DOI] [PubMed] [Google Scholar]
- 47. Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult US population. Cancer. 2004;100:2093-2103. [DOI] [PubMed] [Google Scholar]
- 48. Ling BS, Klein WM, Dang Q. Relationship of communication and information measures to colorectal cancer screening utilization: results from HINTS. J Health Commun. 2006;11(suppl 1):181-190. [DOI] [PubMed] [Google Scholar]
- 49. Stacy R, Torrence WA, Mitchell CR. Perceptions of knowledge, beliefs, and barriers to colorectal cancer screening. J Cancer Educ. 2008;23:238-240. [DOI] [PubMed] [Google Scholar]
- 50. Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: a systematic review. Eval Health Prof. 2007;30:303-321. [DOI] [PubMed] [Google Scholar]
- 52. Flanigan TS, McFarlane E, Cook S. Conducting survey research among physicians and other medical professionals: a review of current literature. Paper presented at: 63rd Annual Conference of the American Association for Public Opinion Research; 2008; New Orleans, LA. [Google Scholar]
- 53. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Edwards SL, Slattery ML, Edwards AM, et al. Factors associated with response to a follow-up postal questionnaire in a cohort of American Indians. Prev Med. 2009;48:596-599. [DOI] [PMC free article] [PubMed] [Google Scholar]