Abstract
With age, joints become subject to chronic inflammatory processes that lead to degeneration of articular cartilage. Although multifactorial, cytokines have been shown to play a role in the pathogenesis of these chronic disease states. Stromal cell-derived factor 1 (SDF-1) is a chemokine that has been shown to be active in homeostatic mechanisms and developmental processes throughout the body, such as endochondral bone formation. SDF-1 plays a role in the transition from cartilage to bone. Although it has been shown to be a factor in normal development, it has also been shown to involve in the pathogenesis of rheumatoid arthritis (RA) and osteoarthritis (OA). In RA, SDF-1 has been shown to stimulate the recruitment of proinflammatory cells, as well as osteoclasts to the synovium, aiding in the facilitation of synovial degradation. Similarly, in OA, SDF-1 has been shown to regulate key proteins involved in the degradation of the cartilage of the joint. Because of its role in degenerative joint disease, SDF-1 has been investigated as a potential therapeutic target. Animal studies have been employing SDF-1 inhibitors, such as AMD3100 and T140, to study their effects on attenuating degenerative joint disease. These studies have shown promising results in slowing the progression of cartilage degradation and could potentially be used as therapeutic target for humans OA and RA.
Keywords: osteoarthritis, rheumatoid arthritis, stromal cell-derived factor-1 (SDF1)
Introduction
Cytokines are small molecules first identified for their chemoattractant properties and central role in promoting cell mobilization, but also are now recognized for their many roles in recruitment, development, homeostasis, and tissue engineering. Consequently, dysregulated expression of cytokines can have various implications in regards to pathological conditions.1 Among cytokines, stromal cell-derived factor 1 (SDF-1) is an important chemokine that has been shown to be active in numerous homeostatic mechanisms and developmental processes of mature and immature cell types.2 SDF-1 binds to the receptor, CXCR4, which activates a G-protein, initiating a cascade of signaling molecules, which prompts various biological processes.1
SDF-1 signaling has broad implications in multiple tissue types. The highest levels of SDF-1 have been detected in the liver, heart, pancreas, and spleen.1 SDF-1 has been shown to have dose-dependent effects on cardiomyocytes by acting as an attractant for stem cells for tissue repair for the ischemic heart, but can paradoxically interact with inflammatory cytokines, contributing to cellular injury.3 CXCR4 is found to be constitutively expressed in hepatocytes, and contributes to liver homeostasis and injury responses.4 SDF-1 also has major implications in the progression of cancer. It has the ability to affect tumor angiogenesis, cell proliferation, and chemotherapy resistance and its binding to CXCR4 has been widely implicated in targeting secondary sites for tumor growth.1,5
Recent studies have shown the role of SDF-1 on musculoskeletal development and pathogenesis. SDF-1 plays a critical role in bone marrow mesenchymal stem cell differentiation, migration, recruitment, and engraftment as well as their survival, and proliferation.6 With age, the SDF-1–CXCR4 axis becomes dysregulated, possibly playing a role in age-related cartilage pathology and osteoarthritis (OA). SDF-1 is well known for regulating inflammatory and immune responses, suggesting its role in RA. In this paper, we aimed to review the role of SDF-1 in cartilage pathobiology specifically rheumatoid arthritis (RA) and OA.
SDF-1 and endochondral bone development
SDF-1 has been shown to play multiple roles during embryogenesis. These roles include lymphopoiesis, hematopoietic stem cell homing, heart ventricular septum formation, cerebellar and hippocampal neuron migration, structuring of the intestinal vasculature, and limb skeletal muscle cell migration.7 The primary role of SDF-1 during these processes is regulating cell migration, even during gastrulation in early embryonic development. Migrating cells, mesoderm and definitive endoderm, contain CXCR4, whereas embryonic ectoderm expresses the SDF-1 ligand. This pattern of complementary CXCR4 and SDF-1 has not only been shown to be present in the early stages of embryogenesis, but also in many other developing tissue types, including vascular endothelium/mesoderm, thyroid endodermal epithelial/mesenchyme, and nasal ectodermal epithelium/mesenchyme.8 A growing area of interest is the implications of SDF-1 in the early development of the musculoskeletal system.
During early musculoskeletal development, endochondral ossification occurs when the cartilage matrix is replaced by bone. Hypertrophic chondrocytes undergo apoptosis followed by the resorption of the mineralized matrix by matrix metalloproteinase (MMP)-9 and MMP-13. Osteoblastic cells then migrate from the underlying bone marrow to form the new bony layer.9 There are two major transition points: the entry of the cartilaginous hypertrophic stage from the proliferation stage, and the departure from the hypertrophic stage to bone, which occurs at the growth plate. Recent studies have shown the complementary pattern of the SDF-1–CXCR4 axis on chondrocyte hypertrophy at the growth plate to stimulate this transition process.10 The expression of SDF-1 and CXCR4 at the chondro-osseous junction provides evidence for these molecules plays important role in transition from cartilage to bone.
The distribution pattern of SDF-1 and CXCR4 at the growth plate is important in order to understand the differentiation process of chondrocytes to bone during endochondral bone formation. Hypertrophic cartilage has been shown to be positive for CXCR4, whereas its neighboring bone marrow expresses SDF-1.10 Wei and colleagues demonstrated that the transfection of CXCR4 into pre-hypertrophic chondrocyte increased the expression of MMP-13 and type X collagen, which are markers for hypertrophic cartilage, in response to SDF-1. This suggests that SDF-1 in bone marrow has the diffusing capabilities to infiltrate the cartilage and aid in the progression of endochondral bone growth. In addition, when tibia growth plate was incubated with SDF-1, it helped in elongation of the hypertrophic zone at the distal end of the growth plate toward the proximal end, which points to the possibility that the SDF-1–CXCR4 axis is involved in the closure of the growth plate.10 Murata and colleagues11 was able to show similar results. Their study showed that embryonic humeri of SDF-1–/– mice were shorter than those of wild-type mice. In fact, this study was able to look at the actin cytoskeleton of the cells and found that in SDF-1–/– mice, the actin cytoskeleton was interrupted. With the addition of SDF-1 these impairments were reversed, suggesting that SDF-1 can regulate actin polymerization and stimulate bone growth through managing chondrocyte hypertrophy. Because of the role of SDF-1 in development, it has implications in the pathogenesis of degenerative joint disease, such as RA and OA.
RA pathogenesis
RA is a chronic, progressive inflammatory autoimmune disease that affects the lining of joints. Although the exact cause remains partly unknown, studies show a strong genetic basis developed from the HLA-DRB1*04 cluster as well as environmental factors, including smoking and infection, which can influence the progression and severity of disease.12 Early in the disease process, damage of the synovial lining is initially caused by the innate immune response through presentation of specific antigens and the secretion of several proinflammatory cytokines. These cytokines not only mediate several destructive processes, but also orchestrate the migration of other cell types, such as macrophages and T-lymphocytes to the synovium.12
A number of cytokines play key roles in the destructive processes such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-2, and interferon (IFN)-γ. These cytokines infiltrate the synovial membrane and produce an inflammatory response and drive cell migration. Additionally, these cytokines can activate synoviocytes, stimulating the release of MMPs into the synovial fluid, causing cartilage degradation. Macrophage-driven osteoclastogenesis requires the interaction between receptor activator of nuclear factor κΒ (RANK) and its ligand (RANKL), which is regulated by cytokines such as TNF-α, IL-1, IL-6, and IL-17.12
In RA, there are massive increases in synovial cellularity, hyperplasia of synovial cells, known as fibroblast-like synoviocytes (FLSs), and activation/proliferation/differentiation of infiltrating immune cells leading to the formation of the pannus, which is found at the interface of the cartilage and bone.12,13 FLSs mediate joint destruction in RA and are able to invade collagenous structures by secreting factors that promote inflammation, neovascularization, and cartilage degradation. Though there are many moving parts of this autoimmune attack on the synovial lining, there is a push for new and more effective therapies and so other factors involved in pathogenesis are being studied. One of those molecules is SDF-1, which has been shown to play a role in the progression of this disease process.
SDF-1 and RA pathogenesis
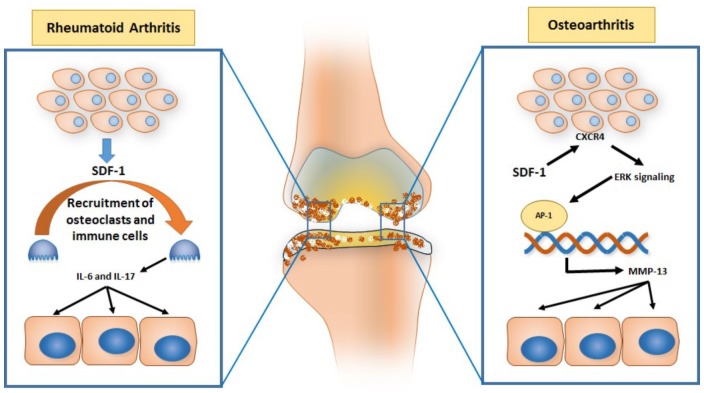
SDF-1 has been shown to be a key factor in regulating many of the molecules involved in the pathogenesis of RA (Figure 1). As mentioned above, important steps in the disease process of RA are cell migration into the synovium and osteoclastogenesis. Migration of T-cells, B-cells, and macrophages into the inflamed synovium is promoted by various chemokines, especially SDF-1. In addition, because osteoclasts are highly positive for CXCR4, SDF-1 can stimulate the recruitment of circulating osteoclast precursor cells directly and promote their activity within the synovium by increasing their precursor numbers, multinucleated cell fusion, cell size, tartrate-resistant acid phosphatase (TRAP) activity, and prevent osteoclast apoptosis.14 SDF-1 also interacts with other cell types and cytokines. Kim and colleagues have shown the interaction between SDF-1 and FLSs and a link with T-cell-derived IL-17.15 Moreover, SDF-1 has been shown to upregulate certain cytokines important in the pathogenesis of RA, such as IL-6.16 Specifically, the IL-6 signaling pathway shows the recruitment of inflammatory mononuclear cells, which contribute to joint destruction by secreting proteolytic enzymes and reactive oxygen intermediates as well as promoting T-cell recruitment by regulating chemokine secretion during inflammation.
Figure 1.
Schematic diagram showing CXCL1–12/SDF-1 signaling in osteoarthritis and rheumatoid arthritis pathophysiology.
IL, interleukin; MMP, matrix metalloproteinase; SDF-1, stromal cell-derived factor 1.
In osteoclastogenesis, RANKL is a major mediator of osteoclast differentiation and activation. As the RANK receptor is stimulated by RANKL, osteoclasts become activated, and resorption of bone is initiated. Kim and colleagues cultured RA synovial fibroblasts with CD4+ cells and found a synergistic effect on the production of SDF-1, which, in turn, increased the production of RANKL. They were able to identify the intracellular signaling pathways mediating the effects of SDF-1-induced RANKL expression through various inhibitors of intracellular signal molecules. Furthermore, they showed that SDF-1 induces phosphorylation of Akt, p38 MAPK, JNK, and IkBa in CD4+ T-cells, further upregulating RANKL expression.14 In addition, Grassi and colleagues demonstrated that osteoclasts grown in the presence of SDF-1 displayed a two-fold increase in their ability to resorb bone-like mineral matrix, and secreted increased amounts of MMP-9, one of the major proteolytic enzymes involved in the bone mineral matrix.17
SDF-1 has been shown to have multiple effects on the proinflammatory cytokines responsible for the damage done to the synovium. IL-17 is a cytokine derived from helper T (Th)17 cells that stimulates the production of proinflammatory cytokines from monocytes and macrophages and RA synovial fibroblasts. In addition, it contributes to bone erosion and tissue destruction in RA by inducing chondrocytes and synovial fibroblasts to produce prostaglandin E2. Kim and colleagues demonstrated that IL-17, induced the production of SDF-1. This process occurs by first secreting SDF-1 by FLSs and then recruiting T-cells to the inflamed synovium. The T-cells produce IL-17 and stimulate more production of SDF-1 from the FLSs. This overproduction of SDF-1 results in augmentation and acceleration of the inflammatory response and bone destruction in RA.15 In chronic inflammatory conditions, IL-6, a predominant mediator of the acute-phase response, is elevated. Chen and colleagues showed that SDF-1a–CXCR4 stimulates the activation of the PI3K, Akt, and activator protein (AP)-1 pathways leading to upregulated IL-6 expression. This supports the hypothesis that SDF-1 promotes the inflammatory response.16 SDF-1 has been shown to play a major role in RA, but it also has implications in age-related degenerative joint disease, such as OA.
Therapeutic use of SDF-1 in RA animal studies
By identifying the role of SDF-1 in the pathogenesis of RA, current and past research has sought to identify potential therapeutics to aid in the attenuation of RA-associated joint inflammation (Table 1). Several promising agents have been manufactured to target specific cytokines, such as IL-1, IL-6, INF-γ, and TNF-α and have produced promising results in clinical therapy of patients with RA. Furthermore, several agents have been identified in antagonizing the SDF-1–CXCR4 axis. These include the peptide T140, AMD3100, plant-based celastrol, and microRNA-137.18–21 The T140 analog, 4F-bonzoyl-TN14003, also implicated as an HIV-entry inhibitor and anticancer metastatic agent, shows potential as a promising agent in inhibiting SDF-1-mediated chemotaxis. One study used human Jurkat cells, an immortalized line of human T-cell lymphocytes, and mice splenocytes that both contain CXCR4, to demonstrate the inhibitory effects of 4F-bonzoyl-TN14003 on cell migration. In another study, mice were injected with bovine type II collagen to create collagen-induced arthritis (CIA), an autoimmunity-driven inflammatory disease of joints, and used as a study model. 4F-bonzoyl-TN14003 was osmotically pumped into the joint spaces of CIA joints. Compared with the control, 4F-bonzoyl-TN14003 inhibited SDF-1-induced cell migration. Symp-toms of RA were lower in the 4F-bonzoyl-TN14003-treated mice, which included an evalua-tion of the CIA score. When compared with indomethacin and methotrexate, both known therapeutic agents against RA symptoms, 4F-bonzoyl-TN14003 mirrored their inhibitory activity against RA symptoms.18
Table 1.
Use of SDF-1–CXCR4 inhibitors in rheumatoid arthritis.
| Author | Animal | Species | Inhibitor | Results |
|---|---|---|---|---|
| Tamamura and colleagues19 | Mice | BALB/c mice | 4F-benzoyl-TN14003 | Inhibits cell migration, lowers CIA score, body weight loss, ankle swelling, and limbs, weight gain |
| Matthys and colleagues20 | Mice | IFN-γR KO mice of DBA/a strain | AMD3100 | Delayed onset of clinical disease, decreased clinical scores, reduction of leukocytes, hyperplasia of the synovium, and pannus formation |
| Du and colleagues21 | Rats | Wistar rats | miRNA miR-137 | Inhibited RA-FLS proliferation, migration, and invasion, decreased expression of TNF-α, IL-6, IL-8, and IL-1B, decreased total level of SDF-1 mRNA and protein |
| Li and colleagues22 | Human | RA fibroblast-like synoviocytes | Celastrol | Suppresses hypoxia-induced FLS migration and invasion, inhibits hypoxia-induced CXCR4 expression, binds to the CXCR4 promoter and inhibits binding of HIF-1a preventing transcription |
CIA, collagen-induced arthritis; FLS, fibroblast-like synoviocytes; IFN, interferon; IL, interleukin; RA, rheumatoid arthritis; SDF-1, stromal cell-derived factor 1; TNF, tumor necrosis factor.
Matthys and colleagues used AMD3100, which is also a CXCR4 antagonist.19 They used the CIA model in IFN-γR-deficient mice that can develop joint lesions identical to wild-type mice in addition to increased SDF-1 mRNA in inflamed joints versus asymptomatic joints. They showed that in mice treated with phosphate-buffered saline (PBS), arthritis started to appear on day 19 after immunization. AMD3100-treated mice began showing arthritis-like symptoms 2 weeks later. In addition to delaying the onset of disease, with AMD3100 initiated 7 days after immunization, clinical disease scores were lower and the overall incidence of RA was decreased. Histologically, AMD3100-treated mice revealed a reduction in the infiltration of leukocytes, as well as reduced hyperplasia of the synovium, and pannus formation.19 Du and colleagues demonstrated an inhibitory role of microRNA-137 on SDF-1 expression in FLSs. MicroRNAs are small, noncoding RNAs that regulate gene expression. The aim of that study was to examine the proliferation, migration, invasion, and expression of inflammatory cytokines within the inflamed synovium when exposed to miRNA, specifically, miR-137. miR-137 targets the 3′-untranslated region of the C-X-C motif, CXCL12 (SDF-1). Using male Wistar rats, they found that miR-137 expression was upregulated in RA-FLSs, suggesting it may be involved in the pathogenesis of RA. However, when overexpressed, miR-137 significantly inhibited RA-FLS proliferation, migration, and invasion compared with the control. The rats also exhibited decreased expression levels of TNF-α, IL-6, and IL-1B as well as decreased SDF-1 mRNA and protein compared with the control.20
Li and colleagues studied the effects of the plant-derived pentacyclic-triterpene extract, celastrol, as it has also been shown to produce antiarthritic effects. By studying the effects of celastrol under hypoxic physiological conditions of the joint space, they were not only able to show that celastrol suppressed the migration and invasion of hypoxia-induced FLSs, but also its mechanism through CXCR4–SDF1 signaling. Hypoxia-inducible factor-1a (HIF-1a) is a protein important for the transcriptional activity of cells to allow them to survive in a hypoxic microenvironment. Li and colleagues observed that celastrol inhibits the binding of HIF-1a to the promotor on the CXCR4 gene, halting its transcription, therefore, suppressing SDF-1–CXCR4-induced migration and invasion of proinflammatory cells into the joint space.21 These studies all show the promising effects of different agents and their proposed mechanisms of actions at combating the influx of inflammatory cytokines and cells that damage the joints and cause RA.
OA pathogenesis
OA is the most prevalent joint disease and is the leading musculoskeletal cause of impaired mobility in the elderly. Clinically, this disease results in chronic pain, joint instability, stiffness, deformities, and joint space narrowing. It mainly affects the articular cartilage of large joints such as knees, hips, and spines, but can affect the smaller joints in the hands causing synovial inflammation, alteration of periarticular bone, and osteophyte formation.22,23 Articular cartilage is composed of type II collagen, proteoglycans, and other extracellular matrix components. It forms the architecture of the collagen matrix composed of interwoven cartilage fibrils containing a dense meshwork with tensile and resilient strength, allowing the joint to maintain its stability and biomechanical function. As the articular chondrocytes mature, they are able to synthesize matrix components and degrading enzymes that allows for minimal flux of the overall cartilage support system.22
Progression to osteoarthritis begins through damage to the articular cartilage, chondrocytes, which typically have little regenerative capacity, by abnormal mechanical loading or injury.22 The changes in the structure and composition of the articular cartilage further stimulate the articular chondrocytes to secrete more catabolic and degenerative factors. Further breakdown stimulates the articular chondrocytes to undergo apoptosis, leading to reduced joint space and friction between the bones, causing pain and limited mobility.22
There is growing evidence for the role of osteoblasts in the progression of the pathogenesis of OA. Osteoblasts are key cells in the regulation of bone metabolism and homeostasis by synthesizing bone matrix that becomes mineralized over time. One of their main functions is to stimulate the differentiation of osteoclast precursor cells to mature osteoclast through the RANK–RANKL axis. Evidence suggests that osteoblast dysregulation plays a key role in OA through abnormal expression of the RANK–RANKL axis and osteoprotegerin (OPG), which is responsible for abnormal bone remodeling and decreased mineralization.23 Maruotti and colleagues noted that two groups of osteoblasts have been identified in OA. Low OA osteoblasts have decreased OPG and increased RANKL, whereas high OA osteoblasts have elevated OPG and lower levels of RANKL, suggesting that low OA osteoblasts are more involved in bone resorption. However, high OA osteoblasts exhibit higher levels of proinflammatory cytokines, such as PGR-2 and IL-6. This abnormal osteoblast metabolism may be responsible for the abnormal mineralization of subchondral bone in OA.23 Osteoblast dysregulation can further be explained by the consequences of SDF-1 acting on the RANK–RANKL pathway as well as its ability to increase cartilage destroying enzymes.
SDF-1 and OA pathogenesis
As mentioned above, chondrocytes are capable of expressing various proteolytic enzymes such as aggrecanases and MMPs. Under normal conditions, these enzymes maintain homeostasis via regulating the very low matrix turnover responsible for cartilage remodeling.24 However, in OA, due to the disruption of the homeostasis environment, aggrecanases and MMPs become upregulated. Li and colleagues were able to simulate the chondrocyte microenvironment by creating a hypoxic environment for the cells to more accurately describe this mechanism. Under hypoxic conditions, CXCR4 was shown to be upregulated in OA chondrocytes. By binding to CXCR4, SDF-1 upregulated the expression of MMP-13 mRNA expression and enzyme activity. MMP-13 is paramount to the pathogenesis of OA, as it degrades type II collagen.25 Chiu found that one of the possible explanations for the increase in degradation enzymes is, in fact, the chemokine SDF-1. SDF-1 is found in the synovium, and its receptor, CXCR4, is located on the surface of chondrocytes. By binding to CXCR4, SDF-1 activates the ERK signaling pathway and its downstream transcription factors. This results in the activation of AP-1 on the MMP-13 promoter and the upregulation of MMP-13, allowing it to further contribute to cartilage destruction in OA.24 This supports the idea that SDF-1 plays a role in regulating the specific transcription factors that are important for the regulation of the MMP family of proteins and the overall pathogenesis of OA (Figure 1).
SDF-1 has also been found to have implications as OA progresses over time with its interaction with osteoblasts. We previously discussed the dysregulation of osteoblasts in the pathogenesis of OA with the abnormal expression of the RANK–RANKL pathway.23 By isolating cells from mouse bone marrow, Goto and colleagues studied the effects of the SDF-1–CXCR4 axis in the presence of RANKL and macrophage-colony stimulating factor. In their study, they found that by removing the CXCR4+CD45– cells in the presence of RANKL, osteoclastogenesis was strongly suppressed.26 Lisignoli and colleagues27 found evidence that SDF-1 significantly induced proliferation of osteoblasts in patients with OA as well as the upregulation of collagen type I. Although this may seem like a protective mechanism to counteract the damaged bone resulting from the articular cartilage degeneration, it leads to enhanced bone density, increased subchondral bone metabolism, and osteophyte formation.25 As the mechanism for the role of SDF-1 becomes clearer, it has potential to be used as a target for therapeutic drugs in hope for attenuating the disease process of OA.
Therapeutic use of SDF-1 in OA animal studies
As more research is done on the SDF-1–CXCR4 axis and its role in OA and other degenerative joint disease, it has become an area of research for its potential as a target for therapeutics. As several studies have shown increases in either CXCR4 and SDF-1 in the pathogenesis of OA, these same studies have addressed the use of CXCR4 inhibitors to block the effect of SDF-1 in the synovium to prevent OA pathogenesis (Table 2).28,29 Of those inhibitors, AMD3100 and T140 are popular choices. Both AMD3100 and T140 have been approved for human use in HIV and cancer therapy, with T140 being implicated in the treatment of chronic inflammatory diseases.29,30 Dong and colleagues transected the anterior cruciate ligament (ACLT) in C57BL/6J mice and infused AMD3100, a CXCR4 inhibitor to study its effects on the subchondral bone in post-traumatic OA. Using micro computed tomography, it was found that treatment with AMD3100 in ACLT mice significantly slowed the progression of tibial subchondral bone loss compared with the mice treated with a vehicle.28 Wei and colleagues showed similar results through India ink staining of the medial tibial plateaus, which showed deep and wide fissures in PBS-treated guinea pigs and a significant improvement in the AMD3100 treated guinea pigs, where cartilage damage was much less.31
Table 2.
Use of SDF-1–CXCR4 inhibitors in in osteoarthritis.
| Author | Animal | Species | Inhibitor | Results |
|---|---|---|---|---|
| Wang and colleagues31 | Guinea pigs | Hartley | T140 | Decreased SDF-1 in serum, attenuated severity of OA cartilage damage in vivo, downregulated expression of MMPs, upregulated mRNA expression of type II collagen and aggrecan, reduced degradation of type II collagen |
| Dong and colleagues30 | Mice | C57Black6/J | AMD3100 | Attenuated tibial subchondral bone loss, decreased TRAP-positive multinucleated cells, attenuated the degeneration of articular cartilage, decreased serum SDF-1a, decreased osteoclastogenesis |
| Thomas and colleagues32 | Mice | C57Black6/J | AMD3100 | Preserved intact cartilage, decreased expression of MMPs, decreased extent of OA damage |
| Wei and colleagues33 | Guinea pigs | Hartley | AMD3100 | Attenuates severity of OA cartilage damage, decreased SDF-1, MMPs, IL-B1, GAGs |
GAG, glycosaminoglycans; IL, interleukin; MMP, matrix metalloproteinase; OA, osteoarthritis; SDF-1, stromal cell-derived factor 1; TNF, tumor necrosis factor; TRAP, tartrate-resistant acid phosphatase.
In addition, ACLT mice treated with AMD3100 showed a reduction in the number of CXCR4+ cells and TRAP+ multinucleated cells within the tibial subchondral bone, as well as attenuating the degeneration of articular cartilage through reduced expression of MMP-13.28 Thomas and colleagues demonstrated, through immunohistochemistry, that the expression of MMP-13 after destabilizing medial meniscectomy of the right knee in C57BL6/J mice treated with AMD3100 was significantly reduced compared with the destabilizing medial meniscectomy/PBS-treated group and the sham control.30 When treated with an alternative CXCR4 inhibitor, T140, a small peptide that is antagonist of CXCR4, the outcomes were similar. Wang and colleagues demonstrated that guinea pigs treated with T140 showed downregulation of the expression of several members of the MMP family, such as MMP-3, MMP-9 and MMP-13 but showed upregulation in the expression of type II collagen and aggrecan.29 Both type II collagen and aggrecan are thought to be protective factors that help maintain the elasticity and hardness of collagen.29 These studies even addressed the changes in osteoclast differentiation with the use of a CXCR4 inhibitor. It is understood that SDF-1 promotes osteoclast differentiation through the interaction between RANK–RANKL; however, when treated with AMD3100, it neutralizes the effect of SDF-1 in both in vivo and vitro studies.28 These studies suggest that post-traumatic osteoarthritis associated with articular cartilage degradation can be attenuated with AMD3100 and other CXCR4 antagonists.30
Many of these therapeutic options for inhibiting the SDF-1–CXCR4 axis in RA and OA have been shown to be efficacious against other pathological conditions. There is a growing body of work in which AMD3100 has been implicated in the treatment of HIV and many different cancers, including several types of leukemias and solid tumors and has demonstrated clinical efficacy through the mobilization of hematopoietic stem cells.32 Similarly, T140 analogs were first discovered through the antagonism of CXCR4 in patients with HIV to prevent entry of the virus into the cell. It has also been studied for efficacy against malignancy, such as breast cancer, melanoma and several other blood-related cancers.33
Many of the clinical trials performed with CXCR4 inhibitors have been in the field of hematologic malignancies and their ability to make the tumor cells more chemosensitive in addition to stem cell mobilization. Only a few clinical trials are underway in evaluating the efficacy against solid tumors. Many of these clinical trials have shown these drugs to be well tolerated, but are still evaluating for clinical efficacy.34 With success shown in animal studies against both RA and OA, these therapies have the potential to be studied on human patients, as they are already being studied in humans with other pathologies.
Conclusion
SDF-1 is a cytokine that has been shown to have many different functions, from the anabolic effects of endochondral bone formation to playing a role in the development of both RA and OA. In RA, SDF-1 contributes to the migration of osteoclast precursor cells within the synovium as well as increasing cell size, TRAP activity, and preventing osteoclast apoptosis. Similarly, in OA, SDF-1 contributes to the degeneration of cartilage through the upregulation of proteolytic enzymes, such as aggrecanases and MMP-13, in addition to contributing to the induction of osteoblast proliferation. In proving that SDF-1 contributes to these degenerative diseases, it has a potential to be used as a therapeutic target. Several SDF-1–CXCR4 antagonists, such as AMD3100 and T140 have been shown to be beneficial in slowing the progression of subchondral bone loss by reduction in the formation of proteolytic enzymes, such as MMPs. Further studies need to be done to solidify the therapeutic effects in large animal models, which then can hopefully progress from the bench to bedside in patients with RA and OA.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This publication is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research, and Development Program (VA Merit Award 1I01CX000930 01, W.D.H. and S.F.) and the National Institutes of Health (National Institute on Aging-AG036675 W.D.H., M.M.L., S.F., M.H. and C.S.). The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government. The above-mentioned funding did not lead to any conflict of interests regarding the publication of this manuscript.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Sadanand Fulzele  https://orcid.org/0000-0002-3510-6759
https://orcid.org/0000-0002-3510-6759
Contributor Information
Robert Bragg, Departments of Orthopedic Surgery, Augusta University, Augusta, GA, USA.
William Gilbert, Departments of Orthopedic Surgery, Augusta University, Augusta, GA, USA.
Ahmed M. Elmansi, Department of Pathology and Laboratory Medicine, Medical University of South Carolina, and the Ralph H. Johnson VAMC, Charleston, SC, USA
Carlos M. Isales, Department of Medicine, Augusta University, Augusta, GA, USA
Mark W. Hamrick, Department of Cell Biology and Anatomy, Augusta University, Augusta, GA, USA
William D. Hill, Department of Pathology and Laboratory Medicine, Medical University of South Carolina, and the Ralph H. Johnson VAMC, Charleston, SC, USA
Sadanand Fulzele, Department of Orthopedic Surgery, Augusta University, 1459 Laney Walker, Augusta, GA, 30904, USA.
References
- 1. Pozzobon T, Goldoni G, Viola A, et al. CXCR4 signaling in health and disease. Immunol Lett 2016; 177: 6–15. [DOI] [PubMed] [Google Scholar]
- 2. Wright LM, Maloney W, Yu X, et al. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 2005; 36: 840–853. [DOI] [PubMed] [Google Scholar]
- 3. Jarrah AA, Schwarskopf M, Wang ER, et al. SDF-1 induces TNF-mediated apoptosis in cardiac myocytes. Apoptosis 2018; 23: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liepelt A, Tacke F. Stromal cell-derived factor-1 (SDF-1) as a target in liver diseases. Am J Physiol Gastrointest Liver Physiol 2016; 311: G203–G209. [DOI] [PubMed] [Google Scholar]
- 5. Meng W, Xue S, Chen Y. The role of CXCL12 in tumor microenvironment. Gene 2018; 641: 105–110. [DOI] [PubMed] [Google Scholar]
- 6. Carbone LD, Buzkova P, Fink HA, et al. Association of plasma SDF-1 with bone mineral density, body composition, and hip fractures in older adults: the cardiovascular health study. Calcif Tissue Int 2017; 100: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Andres C, Torres M. Comparative expression pattern analysis of the highly conserved chemokines SDF1 and CXCL14 during amniote embryonic development. Dev Dyn 2010; 239: 2769–2777. [DOI] [PubMed] [Google Scholar]
- 8. McGrath KE, Koniski AD, Maltby KM, et al. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol 1999; 213: 443–456. [DOI] [PubMed] [Google Scholar]
- 9. Hinton RJ, Jing Y, Jing J, et al. Roles of chondrocytes in endochondral bone formation and fracture repair. J Dent Res 2017; 96: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei L, Kanbe K, Lee M, et al. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol 2010; 341: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murata K, Kitaori T, Oishi S, et al. Stromal cell-derived factor 1 regulates the actin organization of chondrocytes and chondrocyte hypertrophy. PLoS One 2012; 7: e37163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012; 51(Suppl. 5): v3–v11. [DOI] [PubMed] [Google Scholar]
- 13. Asif Amin M, Fox DA, Ruth JH. Synovial cellular and molecular markers in rheumatoid arthritis. Semin Immunopathol 2017; 39: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim HR, Kim KW, Kim BM, et al. Reciprocal activation of CD4+ T cells and synovial fibroblasts by stromal cell-derived factor 1 promotes RANKL expression and osteoclastogenesis in rheumatoid arthritis. Arthritis Rheumatol 2014; 66: 538–548. [DOI] [PubMed] [Google Scholar]
- 15. Kim KW, Cho ML, Kim HR, et al. Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes: role of interleukin-17 and CD40L-CD40 interaction. Arthritis Rheum 2007; 56: 1076–1086. [DOI] [PubMed] [Google Scholar]
- 16. Chen HT, Tsou HK, Hsu CJ, et al. Stromal cell-derived factor-1/CXCR4 promotes IL-6 production in human synovial fibroblasts. J Cell Biochem 2011; 112: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 17. Grassi F, Cristino S, Toneguzzi S, et al. CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J Cell Physiol 2004; 199: 244–251. [DOI] [PubMed] [Google Scholar]
- 18. Tamamura H, Fujisawa M, Hiramatsu K, et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett 2004; 569: 99–104. [DOI] [PubMed] [Google Scholar]
- 19. Matthys P, Hatse S, Vermeire K, et al. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-y receptor-deficient mice. J Immunol 2001; 167: 4686–4692. [DOI] [PubMed] [Google Scholar]
- 20. Du J, Zhang F, Guo J. miR137 decreases proliferation, migration and invasion in rheumatoid arthritis fibroblastlike synoviocytes. Mol Med Rep 2018; 17: 3312–3317. [DOI] [PubMed] [Google Scholar]
- 21. Li GQ, Liu D, Zhang Y, et al. Anti-invasive effects of celastrol in hypoxia-induced fibroblast-like synoviocyte through suppressing of HIF-1alpha/CXCR4 signaling pathway. Int Immunopharmacol 2013; 17: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 22. Xia B, Di C, Zhang J, et al. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int 2014; 95: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maruotti N, Corrado A, Cantatore FP. Osteoblast role in osteoarthritis pathogenesis. J Cell Physiol 2017; 232: 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiu YC, Yang RS, Hsieh KH, et al. Stromal cell-derived factor-1 induces matrix metalloprotease-13 expression in human chondrocytes. Mol Pharmacol 2007; 72: 695–703. [DOI] [PubMed] [Google Scholar]
- 25. Li P, Deng J, Wei X, et al. Blockade of hypoxia-induced CXCR4 with AMD3100 inhibits production of OA-associated catabolic mediators IL-1beta and MMP-13. Mol Med Rep 2016; 14: 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goto Y, Aoyama M, Sekiya T, et al. CXCR4+ CD45– cells are niche forming for osteoclastogenesis via the SDF-1, CXCL7, and CX3CL1 signaling pathways in bone marrow. Stem Cells 2016; 34: 2733–2743. [DOI] [PubMed] [Google Scholar]
- 27. Lisignoli G, Toneguzzi S, Piacentini A, et al. CXCL12 (SDF-1) and CXCL13 (BCA-1) chemokines significantly induce proliferation and collagen type I expression in osteoblasts from osteoarthritis patients. J Cell Physiol 2006; 206: 78–85. [DOI] [PubMed] [Google Scholar]
- 28. Dong Y, Liu H, Zhang X, et al. Inhibition of SDF-1alpha/CXCR4 signalling in subchondral bone attenuates post-traumatic osteoarthritis. Int J Mol Sci 2016; 17: E943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Wang K, Li Y, Han R, et al. T140 blocks the SDF-1/CXCR4 signaling pathway and prevents cartilage degeneration in an osteoarthritis disease model. PLoS ONE 2017; 12: e0176048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas NP, Li P, Fleming BC, et al. Attenuation of cartilage pathogenesis in post-traumatic osteoarthritis (PTOA) in mice by blocking the stromal derived factor 1 receptor (CXCR4) with the specific inhibitor, AMD3100. J Orthop Res 2015; 33: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei F, Moore DC, Wei L, et al. Attenuation of osteoarthritis via blockade of the SDF-1/CXCR4 signaling pathway. Arthritis Res Ther 2012; 14: R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu T, Li X, You S, et al. Effectiveness of AMD3100 in treatment of leukemia and solid tumors: from original discovery to use in current clinical practice. Exp Hematol Oncol 2015; 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia 2009; 23: 43–52. [DOI] [PubMed] [Google Scholar]
- 34. Wald O. CXCR4 based therapeutics for non-small cell lung cancer (NSCLC). J Clin Med 2018; 7: E303. [DOI] [PMC free article] [PubMed] [Google Scholar]