Abstract
Background:
Postoperative pancreatic collection (POPC) is a frequent complication after pancreatectomy. Although percutaneous drainage (PD) has been the treatment of choice for POPC with encapsulation, endoscopic ultrasound-guided transmural drainage (EUS-TD) was recently reported effective for this condition. The main aim of this retrospective study was to compare EUS-TD and PD in terms of effectiveness and safety as the first procedure in patients with noncapsulated POPC.
Methods:
Consecutive patients who underwent pancreatectomy and developed noncapsulated POPC requiring EUS-TD or PD between April 2003 and May 2018 were enrolled. Noncapsulated POPC was defined as pancreatic collection appearing within 28 days postoperatively and lacking a thick encapsulating inflammatory wall on contrast-enhanced computed tomography. The effectiveness of drainage was compared between the two groups before and after propensity-score matching of patient characteristics. Outcomes of interest included re-intervention rate, number of re-interventions, immediate complication, remote complication, and time to clinical resolution after the procedure.
Results:
A sum of 81 patients were included: 14 underwent EUS-TD, and 67 underwent PD. There were significant differences between groups in POPC size and type of surgery. Propensity-score matching selected 13 patients who underwent EUS-TD and 28 who underwent PD. Re-intervention rate (p = 0.045), and number of re-interventions (p = 0.026) were significantly lower in the matched EUS-TD group than in the matched PD group. There were no significant between-group differences in immediate complication and remote complication. The time to clinical resolution after the procedure was significantly shorter in the matched EUS-TD than in the matched PD group (14 versus 26 days; p < 0.0001).
Conclusion:
EUS-TD is more effective than PD for drainage of noncapsulated POPC. EUS-TD should be considered as the first treatment of choice for noncapsulated POPC visible on EUS.
Keywords: endoscopic ultrasound-guided drainage, percutaneous drainage, postoperative pancreatic collection
Introduction
Postoperative pancreatic collection (POPC) is a frequent complication of pancreatic surgery, encountered in 20–30% of patients who undergo pancreaticoduodenectomy, distal pancreatectomy, or enucleation of a lesion within the body of the pancreas.1–8 POPC can lead to severe pain, gastric-outlet obstruction, intra-abdominal infection, and sepsis. Conservative management of POPC may include long-term jejunal feeding, total parenteral nutrition, with or without treatment with octreotide with or without antibiotics. POPC may also require drainage, such as percutaneous drainage (PD), endoscopic retrograde cholangiopancreatography with placement of a pancreatic stent, endoscopic ultrasound (EUS)-guided transmural drainage (EUS-TD), or surgical cyst–gastrostomy.9 Conventionally, PD is the first choice for treatment of POPC, with open surgery being an alternative.10 Although PD provides effective drainage of POPC, the presence of an external catheter may lead to a significant deterioration in quality of life (QOL).11 EUS-TD was recently reported safe and effective for POPC,8–10 suggesting that EUS-TD may be an alternative method of choice for POPC drainage.8–10 Although most previous studies have assessed the effectiveness of EUS-TD for POPC with encapsulation,8,12,13 few studies have evaluated the effectiveness of EUS-TD for noncapsulated POPC. Moreover, few studies, and no prospective randomized controlled trials, have compared the effectiveness of PD and EUS-TD for POPC, and assessment of studies comparing EUS-TD with PD suggests a patient selection bias.
This study compared EUS-TD with PD to determine the effectiveness of EUS-TD for treatment of noncapsulated POPC. To minimize any possible selection bias, this retrospective study used propensity-score analysis to match patients who underwent PD and EUS-TD for POPC. In addition, a subgroup analysis compared the effectiveness of EUS-TD and PD for POPC after distal pancreatectomy.
Materials and methods
This retrospective cohort study was approved by the Institutional Review Board of the Wakayama Medical University Hospital (registry number: 2401). Oral consent was obtained from all patients or proxies. Written consent from patients is not necessary for observational studies according to Japanese law.
Study design
The main aim of this retrospective study was to compare EUS-TD and PD in terms of effectiveness and safety as the first procedure in patients with noncapsulated POPC. To minimize any possible selection bias, propensity-score analysis was performed to match patients who underwent PD and EUS-TD for POPC. In addition, a subgroup analysis compared the effectiveness of EUS-TD and PD for POPC after distal pancreatectomy.
Patients
The medical records of Wakayama Medical University Hospital were examined to identify all patients who underwent pancreatic resection between April 2003 and May 2018 and afterwards developed symptomatic noncapsulated POPC requiring EUS-TD or PD. In this hospital, patients with infected POPC undergo drainage treatment regardless of whether POPC is encapsulated. Among the patients, only those who met the inclusion criteria described below were enrolled in the study. Noncapsulated POPC was defined as pancreatic collection that appeared within 28 days postoperatively and lacked a thick inflammatory wall on contrast-enhanced computed tomography (CECT). Capsulated POPC was defined as pancreatic collection with a thick encapsulating inflammatory wall on CECT. Patients were included if they (a) had been diagnosed with noncapsulated POPC by CECT, (b) had undergone EUS-TD or PD for the first 28 days postoperatively, and (c) had clinically relevant POPC, manifested by one or more of the following: abdominal pain, nausea, or vomiting, fever, or leukocytosis. Patients were excluded if they (a) had POPC cured by conservative drainage during surgery, (b) were not followed up for 1 month or longer, or (c) had undergone both EUS-TD and PD on the same day.
Percutaneous drainage technique
All ultrasound-guided PD procedures were performed by one of five expert doctors. A 16-gauge needle was percutaneously inserted into the POPC that was aspirated under ultrasound and fluoroscopy. A 0.018-inch guidewire was inserted into the POPC, followed by placement of an indwelling drainage catheter (7 Fr percutaneous transhepatic cholangiodrainage set; Create Medic, Yokohama, Japan). If necessary, additional drainage catheters were inserted into other locations. The collected fluid was emptied as completely as possible, followed by postdrainage imaging. Catheter exchange or removal was based on clinical improvement as well as drainage catheter output, catheter malfunction or dislodgement, and evidence of persistent fluid on repeated imaging. Catheters were removed from patients who showed clinical improvement of the POPC and no drainage from the external fistula tube of the PD, followed by discharge and follow up.
EUS-guided transmural drainage
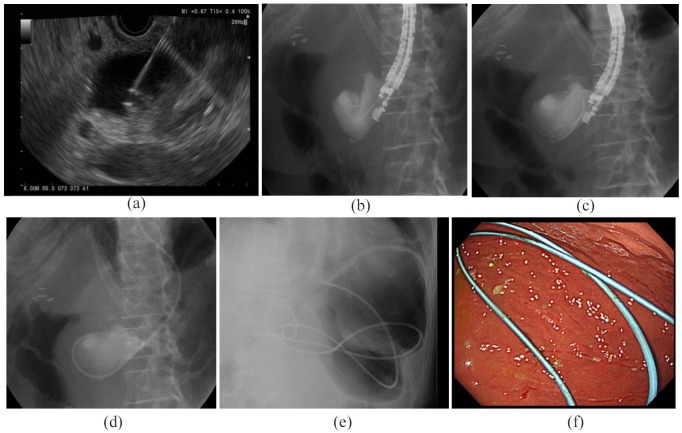
All EUS-TD procedures were performed by one of four endosonographers, all of whom had at least 5 years of experience in performing EUS and had performed more than 100 pancreatic EUS-guided fine-needle aspiration procedures prior to this study. All procedures were performed while patients were in the left lateral position and sedated either with midazolam, dexmedetomidine hydrochloride plus pentazocine, or propofol plus pentazocine. EUS imaging under Doppler-flow guidance was used to assess the local vasculature and determine the POPC puncture site. The primary puncture into the POPC was made with a 19-gauge needle [SonoTip Pro Control Tip; Medi-Globe, Achenmühle, Germany; Figure 1(a)]. Successful POPC access was demonstrated by injection of contrast medium to confirm the location [Figure 1(b)]. A 0.025-inch guidewire (VisiGlide2; Olympus Corporation, Tokyo, Japan) was inserted through the needle and coiled into the POPC [Figure 1(c)]. The needle was withdrawn, and the guidewire was left in the POPC. The cyst–gastrostomy tract was initially dilated using a 4 mm biliary dilating balloon (Ren; Kaneka Medical, Osaka, Japan) or tapered dilator (ES dilator; Zeon Medical, Tokyo, Japan). Using the 0.025 inch guidewire, a 7 Fr endoscopic nasobiliary drainage (ENBD) tube (Flexima ENBD catheter; Boston Scientific, Natick, MA, USA) was inserted into the POPC as an external drainage tube [Figure 1(d)].
Figure 1.
Endoscopic ultrasound-guided transmural drainage.
(a) Endoscopic ultrasound image showing puncture of a POPC with a 19-gauge needle, (b–d) Fluoroscopy images showing (b) puncture of POPC and injection of contrast agent into the POPC cavity, (c) insertion of a guidewire through the fine needle into the POPC, and (d) insertion of an ENBD tube into the POPC cavity. Making an ENBD tube an internal drainage tube: (e) a fluoroscopic image showing placement of the tips of an ENBD tube; one in the POPC and the other in the stomach; (f) an endoscopic image showing placement of the tip of an ENBD tube in the stomach.
ENBD, endoscopic nasobiliary drainage; POPC, postoperative pancreatic collection.
Catheter exchange or addition was based on clinical improvement, drainage catheter output, catheter malfunction or dislodgement, and evidence of persistent fluid on repeated imaging.
Internalization the ENBD tube
When patients showed no abdominal pain and inflammation, and there was no drainage from the external fistula tube of the POPC, the ENBD tube was cut and deployed into the stomach using endoscopy guidance. The tube was subsequently used as an internal fistula catheter for drainage of the POPC [Figure 1(e, f)]. Immediately after creation of the internal fistula, these patients were discharged. The internalized ENBD tube was removed within 1 year and the patient was followed up for at least 3 months after removal.
Outcome measures
The outcome of the analysis was time to clinical resolution after the first procedure,12–14 with clinical resolution defined as patients who were stable and ambulatory, with no external drainage or antibiotic treatment, and on normal oral food intake.14
The other outcomes were placement duration of the external fistula tube, duration of the internalized ENBD tube, re-intervention rate, number of re-interventions, clinical resolution rate, technical success rate and complications. In addition, complications were divided into immediate complications and remote complications according to when they occurred. Immediate complications were defined as those that occurred within 3 days after the first procedure in each group. Remote complications were defined as those that occurred on day 4 or later after the first procedure in each group or as those that occurred during placement of the external fistula tube in the matched PD group. Other treatment outcomes included C-reactive protein (CRP) concentrations 3, 7, and 14 days after first drainage and maximum POPC diameters 7 and 14 days after first drainage, as measured on CT. Technical success of EUS-TD and PD was defined as the successful placement of a transmural stent or percutaneous catheter, respectively, into the POPC. Complications were defined as any newly developed complication after the procedure. Time to clinical resolution was also assessed in the subgroup of patients who underwent distal pancreatectomy.
Statistical analysis
Categorical variables were compared using the χ2 test, Fisher’s exact test, and the Wilcoxon rank-sum test. Because the clinical characteristics of patients in the EUS-TD and PD groups differed markedly, propensity-score-matching analysis was performed to adjust for the effect of patient selection bias. The propensity scores of patients in the EUS-TD group were calculated with a multivariate logistic regression model using possible confounders, such as age, operation type, size of POPC, CRP concentration, and time after operation to the first re-intervention. The propensity score of each patient in the EUS-TD group was calculated based on the estimated probability of the logistic model. Based on these propensity scores, a nearest neighbor matching technique with a caliper coefficient of 0.2 was used to create a one-to-two match to patients in the PD group. After matching, time to clinical resolution in each matched group was estimated using the Kaplan–Meier method and then compared using the log-rank test. Patients who could not be discharged were censored at the date of last follow up or death. In addition, differences in categorical variables between the matched groups were analyzed with the χ2 test, Fisher’s exact test, and the Wilcoxon rank-sum test. p values ⩽ 0.05 were considered statistically significant. Statistical analyses were performed using Statistical Analysis JMP(Version Pro 13.2.1)(SAS Institute Inc; Tokyo: Japan) and EZR(Easy R)(Version 1.37) (Jichi Medical University; Saitama: Japan).
Results
Study population
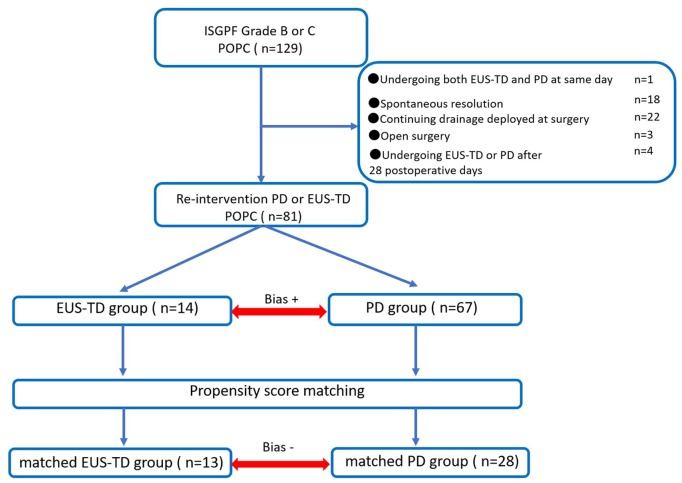
Between April 2003 and May 2018, 1073 pancreatectomies were performed at our institution, with POPC developing in 129 patients (12%). Forty-eight patients were excluded because POPC spontaneously resolved with conservative treatment alone (n = 18), drainage deployed at surgery was continued for POPC (n = 22), reoperation was performed (n = 3), patients underwent EUS-TD or PD > 28 days after surgery (n = 4), or patients underwent both EUS-TD and PD on the same day (n = 1). Eighty-one patients were therefore considered eligible for this study (Figure 2).
Figure 2.
Flow chart showing the inclusion and exclusion of patients and schematic representation of the propensity-score-matching analysis.
To balance the characteristics of the patient groups, the EUS-TD and PD groups were subjected to 1:2 propensity-score matching. Propensity scores were calculated for 82 patients based on a logistic analysis of clinical characteristics. The 14 patients in the EUS-TD group were matched 1:2 to 28 of the 68 patients in the PD group.
EUS-TD, endoscopic ultrasound-guided transmural drainage; PD, percutaneous drainage; POPC, postoperative pancreatic collection.
Patient characteristics
Clinical baseline characteristics of the included patients are summarized in Table 1.
Table 1.
Patient characteristics.
| All patients |
Propensity-matched patients |
|||||
|---|---|---|---|---|---|---|
|
EUS-TD group
(n = 14) |
PD group
(n = 67) |
p value * |
Matched EUS-TD group
(n = 13) |
Matched PD group
(n = 28) |
p value * | |
| Sex (male/female) | 10/4 | 23/44 | 0.34 | 10/3 | 16/12 | 0.21 |
| Age (years) | 64.5 ± 16.4 | 68.6 ± 8.0 | 0.22 | 64.2 ± 12.0 | 67.0 ± 8.3 | 0.55 |
| Surgery type (pancreaticojejunostomy/distal pancreatectomy) | 3/11 | 54/13 | <0.001 * | 3/10 | 15/13 | 0.10 |
| Diameter of POPC (mm) | 75.0 ± 18.9 | 59.4 ± 21.8 | 0.006 * | 75.3 ± 19.6 | 72.6 ± 23.6 | 0.55 |
| Number of postoperative days until procedure | 13.8 ± 7.5 | 10.9 ± 4.4 | 0.24 | 14.5 ± 7.3 | 11.0 ± 4.9 | 0.18 |
| C-reactive protein concentration (mg/l) | 10.6 ± 6.5 | 11.0 ± 7.1 | 0.88 | 11.1 ± 6.5 | 10.9 ± 6.5 | 0.72 |
| Pathology | ||||||
| Pancreatic adenocarcinoma | 8 | 18 | 0.03 | 7 | 10 | 0.28 |
| Neuroendocrine tumor | 0 | 3 | 1.00 | 0 | 3 | 0.54 |
| Intraductal papillary mucinous neoplasm | 1 | 9 | 1.00 | 1 | 3 | 0.76 |
| Papillary carcinoma | 1 | 13 | 0.28 | 1 | 2 | 0.95 |
| Biliary adenocarcinoma | 1 | 18 | 0.17 | 1 | 7 | 0.19 |
| Others | 3 | 6 | 0.18 | 3 | 3 | 0.30 |
Data are shown as n or as mean ± SD. p values were determined by chi-squared and Wilcoxon rank-sum tests, as appropriate.
p values < 0.05 (bold) were considered statistically significant.
EUS-TD, endoscopic ultrasound-guided transmural drainage; PD, percutaneous drainage; POPC, postoperative pancreatic collection.
Among these characteristics, type of operation and POPC size differed significantly in the EUS-TD and PD groups, whereas age, CRP concentration, and number of postoperative days until the procedure did not.
To minimize the effect of selection bias between the two groups, propensity-score-matching analysis was performed, based on selected factors (age, operation type, size of POPC, CRP concentration, and time after operation to the first re-intervention). Propensity matching selected 13 patients in the EUS-TD group and 28 in the PD group.
The C statistic calculated by the receiver operating characteristic curve of our model was 0.87, showing that this model had good ability to distinguish between the EUS-TD and PD groups (Figure 2). After matching, there were no significant differences in clinical characteristics (such as age, operation type, size of POPC, CRP concentration, and time after operation to the first re-intervention) between the two groups (Table 1).
Outcomes
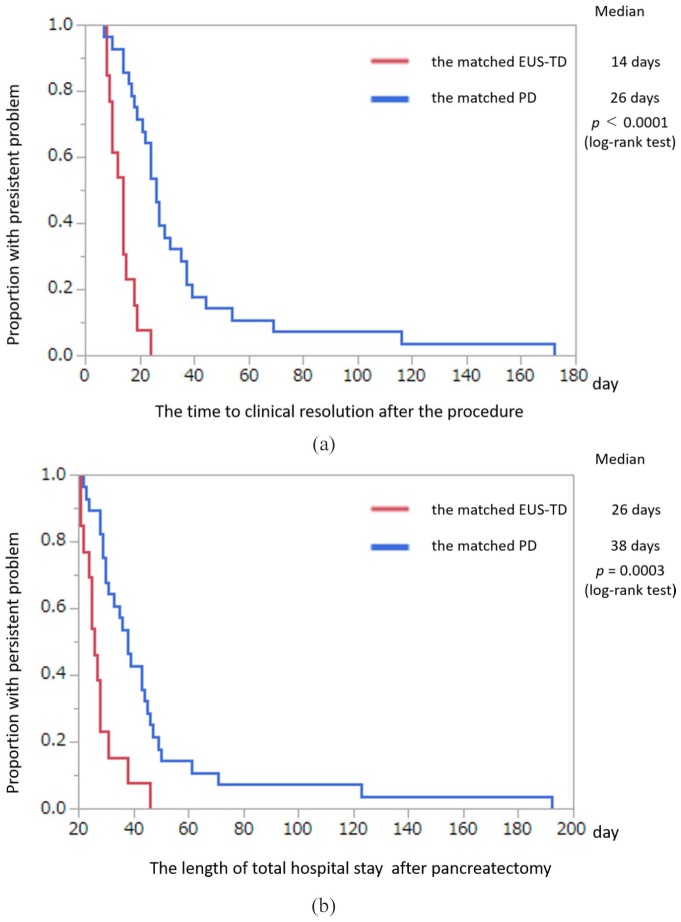
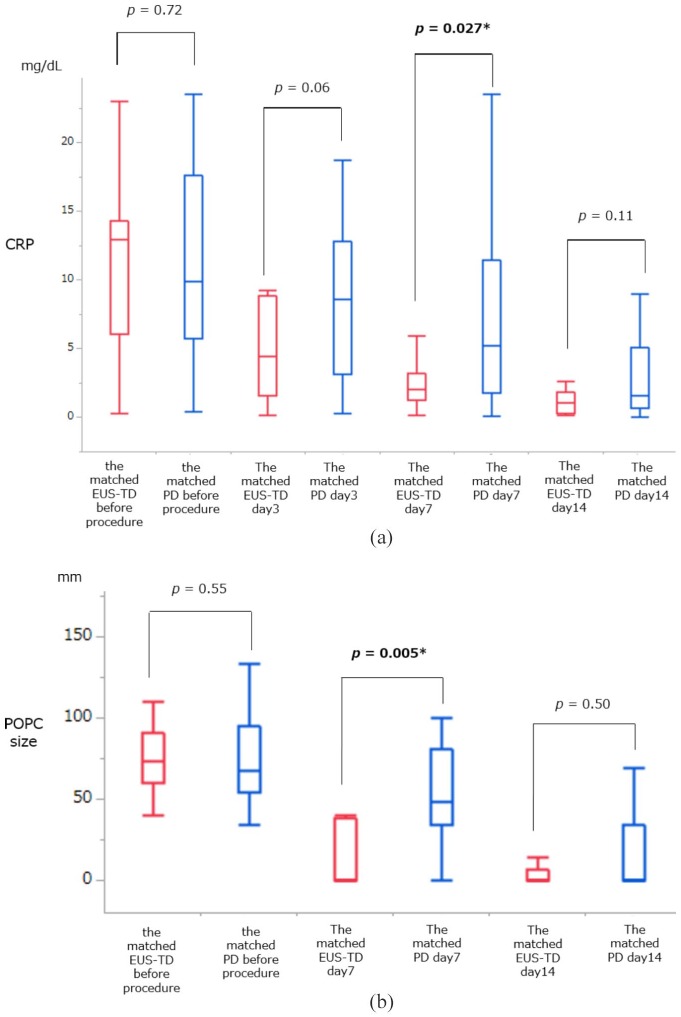
Kaplan–Meier analysis showed that the median time to clinical resolution after the procedure was significantly shorter in the matched EUS-TD group than in the matched PD group [14 versus 26 days, p < 0.0001, Figure 3(a)]. Kaplan–Meier analysis also showed that the median length of total hospital stay after pancreatectomy was significantly shorter in the matched EUS-TD than in the matched PD group [26 versus 38 days, p = 0.0003, Figure 3(b)]. Other secondary endpoints are shown in Table 2. The number of re-interventions per patient and the re-intervention rate were significantly lower in the matched EUS-TD than in the matched PD group (0.2 ± 0.4 versus 0.8 ± 1.1, p = 0.026; 15.3% versus 50.0%, p = 0.045). By contrast, there were no significant between-group differences in technical success, clinical success, immediate complication, and remote complication. In addition, there was no immediate complication in either group. However, there were three cases of remote complications, namely wound infection (n = 2), and intra-abdominal bleeding (n = 1) in the matched PD group. There was one complication in the matched EUS-TD group: one patient had a recurrence of POPC during internalization of the ENBD tube. The placement duration of the external fistula tube in the matched EUS-TD group was significantly shorter than that in the matched PD group (6 versus 17 days; p = 0.0008). The median number days of internalization of the ENBD in the matched EUS-TD group was 66.5 days. Although POPC size and serum CRP concentration before drainage were comparable in the two groups, POPC size (p = 0.005) and serum CRP concentration (p = 0.027) were significantly lower in the matched EUS-TD group after 7 days. However, serum CRP concentrations after 3 and 14 days and POPC size after 14 days did not differ significantly in the two propensity-matched groups [Figure 4(a, b)].
Figure 3.
Kaplan–Meier analysis of variables in relation to the propensity-matched EUS-TD and PD groups (intention-to-treat analysis).
(a) Kaplan–Meier analysis of time to clinical resolution in the propensity-matched EUS-TD and PD groups (intention-to-treat analysis). Median time to resolution was significantly shorter in the matched EUS-TD than in the matched PD group (p = 0.0003, log-rank test); (b) Kaplan–Meier analysis of total hospital stay after pancreatectomy in the propensity-matched EUS-TD and PD groups (intention-to-treat analysis). Median time to discharge was significantly shorter in the matched EUS-TD than in the matched PD group (p = 0.0003, log-rank test).
EUS-TD, endoscopic ultrasound-guided transmural drainage; PD, percutaneous drainage.
Table 2.
Results in the propensity-matched EUS-TD and PD groups.
| Matched EUS-TD group n = 13 |
Matched PD group n = 28 |
p value* | |
|---|---|---|---|
| Technical success | 13 (100%) | 28 (100%) | – |
| Re-intervention | 2 (15.3%) | 14 (50%) | 0.045 * |
| Number of re-interventions | 0.2 ± 0.4 | 0.8 ± 1.1 | 0.026 * |
| Clinical success | 13 (100%) | 28 (100%) | – |
| CRP concentration after 3 days (mg/l) | 5.0 ± 3.6 | 8.5 ± 5.4 | 0.06 |
| CRP concentration after 7 days (mg/l) | 2.3 ± 1.6 | 6.6 ± 5.8 | 0.027 * |
| CRP concentration after 14 days (mg/l) | 1.1 ± 0.8 | 3.5 ± 4.2 | 0.11 |
| POPC diameter after 7 days (mm) | 19.8 ± 28.5 | 54.1 ± 39.2 | 0.005 * |
| POPC diameter after 14 days (mm) | 14.6 ± 40.7 | 16.8 ± 30.2 | 0.50 |
| Duration of placing external drainage tube (day) | 8 (6–13) | 17 (4–47) | 0.0008 * |
| Period of placing the internalization tube of ENBD tube (day) | 66.5 (43–308) | – | |
| ISGPF grade (B/C) | 13/0 | 25/3 | 0.22 |
| Complication | |||
| Immediate complication | 0 (0%) | 0 (0%) | – |
| Remote complication | |||
| Complication during placing external fistula tube¶ | 0 (0%) | 3 (10.7%)¶ | 0.54 |
| Complication after internalization of ENBD tube§ | 1 (7.7%)§ | – | – |
Data are shown as mean ± SD or median (range) or as n (%) p values were calculated by chi-squared, Wilcoxon rank-sum tests, as appropriate.
p values < 0.05 (bold) were considered statistically significant.
Wound infection (n = 2), intra-abdominal bleeding (n = 1)
Recurrence of POPC (n = 1).
CRP, C-reactive protein; ENBD, endoscopic nasobiliary drainage; EUS-TD, endoscopic ultrasound-guided transmural drainage; ISGPF, International Study Group on Pancreatic Fistula; PD, percutaneous drainage; POPC, postoperative pancreatic collection.
Figure 4.
One-way analyses of variance of serum CRP concentrations and POPC diameter in the matched EUS-TD and PD groups before drainage.
(a) One-way analysis of variance of serum CRP concentrations in the matched EUS-TD and PD groups before drainage and 3, 7, and 14 days after the procedure. Serum CRP concentrations before and 3 and 14 days after drainage were comparable in the two groups. However, CRP concentrations after 7 days were significantly lower in the matched EUS-TD than in the matched PD group (p = 0.005 by Wilcoxon rank-sum test); (b) one-way analysis of variance of POPC diameter of the matched EUS-TD and PD groups before drainage and 7 and 14 days after the procedure. POPC diameter before and 14 days after drainage were comparable in the two groups. However, POPC diameter after 7 days was significantly smaller in the matched EUS-TD than in the matched PD group (p = 0.005 by Wilcoxon rank-sum test).
*p values < 0.05 (bold) were considered statistically significant.
CRP, C-reactive protein; EUS-TD, endoscopic ultrasound-guided transmural drainage; PD, percutaneous drainage; POPC, postoperative pancreatic collection.
Patients who underwent distal pancreatectomy
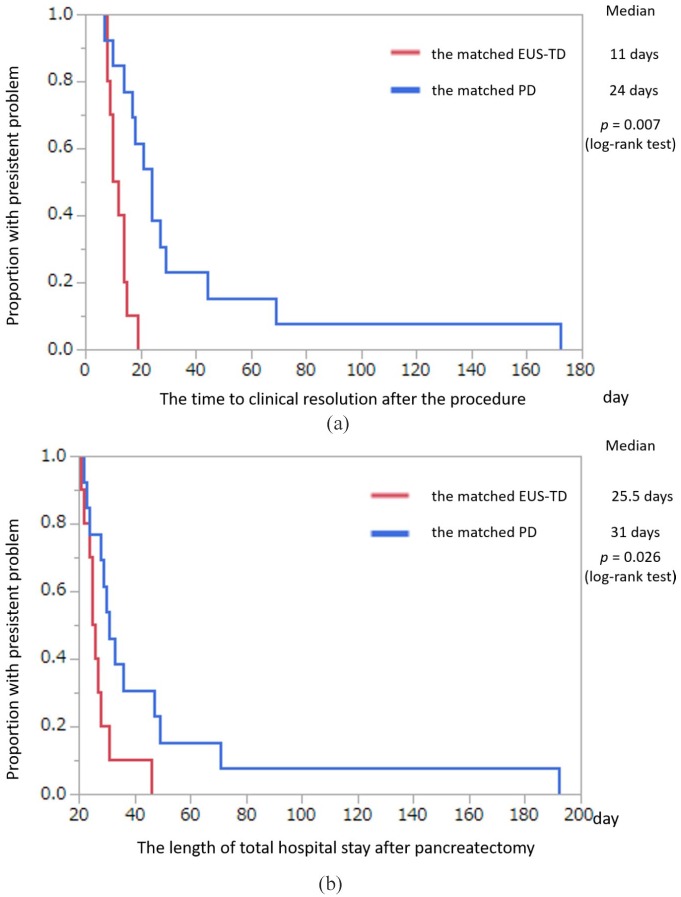
The clinical characteristics of the included subgroup of patients who underwent distal pancreatectomy are summarized in Table 3. Kaplan–Meier analysis of the subgroup of patients who underwent distal pancreatectomy showed that the median time to clinical resolution after the procedure [11 versus 24 days, p = 0.007, Figure 5(a)] and the median postoperative hospital stay after surgery [25.5 versus 31 days, p = 0.0026, Figure 5(b)] were both significantly shorter in the matched EUS-TD than in the matched PD group.
Table 3.
Characteristics of propensity-matched patients who underwent distal pancreatectomy.
| Matched EUS-TD group (n = 10) |
Matched PD group (n = 13) |
p value* | |
|---|---|---|---|
| Sex (male/female) | 7/3 | 9/4 | 0.96 |
| Age (years) | 62.6 ± 12.8 | 64.3 ± 8.5 | 1.00 |
| POPC diameter (mm) | 74.8 ± 20.0 | 56.0 ± 16.3 | 0.017 * |
| CRP concentration (mg/dl) | 10.8 ± 6.8 | 9.3 ± 6.9 | 0.56 |
| Number of postoperative days until procedure | 15.8 ± 6.8 | 10.6 ± 5.5 | 0.10 |
| Pathology | |||
| Pancreatic adenocarcinoma | 6 | 7 | 0.76 |
| Neuroendocrine tumor | 0 | 3 | 0.10 |
| Intraductal papillary mucinous neoplasm | 1 | 1 | 0.85 |
| Others | 3 | 2 | 0.40 |
Data are reported as n or as mean ± SD. p values were calculated using chi-squared or Wilcoxon rank-sum tests, as appropriate.
p values < 0.05 (bold) were considered statistically significant.
CRP, C-reactive protein; EUS-TD, endoscopic ultrasound-guided transmural drainage; PD, percutaneous drainage; POPC, postoperative pancreatic collection.
Figure 5.
Kaplan–Meier analysis of time to clinical resolution and total hospital stay in the propensity-matched EUS-TD and PD groups of patients who had undergone distal pancreatectomy (intention-to-treat analysis).
(a) Kaplan–Meier analysis of time to clinical resolution in the propensity-matched EUS-TD and PD groups of patients who had undergone distal pancreatectomy (intention-to-treat analysis). Median time to resolution was significantly shorter in the matched EUS-TD than in the matched PD group (p = 0.007, log-rank test); (b) Kaplan–Meier analysis of total hospital stay in the propensity-matched EUS-TD and PD groups of patients who had undergone distal pancreatectomy (intention-to-treat analysis). Median time to discharge was significantly shorter in the matched EUS-TD than in the matched PD group (p = 0.026, log-rank test).
EUS-TD, endoscopic ultrasound-guided transmural drainage; PD, percutaneous drainage.
Discussion
This study showed that, in propensity-matched patients requiring drainage for POPC, the time to clinical resolution after the procedure was shorter in those who underwent EUS-TD than in those who underwent PD. Previous studies reported that EUS-TD was safe and effective for POPC.8–12 In addition, EUS-TD is an effective treatment for POPC that is difficult to resolve with PD, according to Rena and colleagues.15 However, the few studies to date comparing EUS-TD with PD have assessed their effectiveness in drainage of encapsulated POPC. These studies reported that the time to clinical resolution was significantly shorter with EUS-TD than with PD in patients with encapsulated POPC.12–14,16 To our knowledge, the present study is the first to report that EUS-TD was more effective than PD for early resolution of noncapsulated POPC.
The previous retrospective studies comparing EUS-TD with PD may be limited by selection bias, as POPC location can depend on the type of surgery and POPC characteristics may depend on the timing of drainage. This study found that some patient characteristics, including maximum POPC diameter and type of surgery, differed significantly in the EUS-TD and PD groups. To control for these differences, and to eliminate selection bias, the EUS-TD and PD groups were subjected to 1:2 propensity-score matching, thereby minimizing the effects of any inherent bias and reducing the effects of confounding factors identified in observational studies.17–20 Treatment effects in randomized trials and propensity-score analysis were found to be similar in similar populations.20
After matching, serum CRP concentrations and POPC sizes 7 days after the procedure were found to be significantly lower in the EUS-TD than in the PD group. Moreover, the number of re-interventions per patient was significantly lower in the matched EUS-TD group than in the matched PD group. These findings indicated that EUS-TD is a more reliable and effective drainage treatment than PD for POPC. In addition, the drainage tube inserted into the POPC could be internalized in all patients in the EUS-TD group by cutting the tube and dropping it into the stomach. This internalization procedure was partly responsible for the reductions in time to clinical resolution and total hospital stay.
Drainage of pancreatic cysts using EUS-TD is designed to be performed after formation of a thick POPC capsule. EUS-TD for POPC is usually performed more than 4 weeks after surgery, because it is not difficult to internalize the drainage tube into the POPC.8,12,13,16,21 Téllez and colleagues reported that EUS-TD does not necessarily require an external fistula tube to drain encapsulated POPC.16 However, POPC may become life threatening in some patients, suggesting that drainage treatment should be performed before the POPC becomes encapsulated. If the internal fistula tube is deployed into the POPC before encapsulation by EUS-TD, it may cause leakage of gastrointestinal fluid into the abdominal cavity. Therefore, an external drainage tube should be used to treat noncapsulated POPC.
This study demonstrated that EUS-TD was as easily performed as PD for noncapsulated POPC within 4 weeks after surgery. Although another method of EUS-TD, in which an external drainage tube (6 Fr pigtail endoscopic ENBD tube) was deployed into the noncapsulated POPC within 4 weeks after surgery has been described, time to clinical resolution did not differ between these EUS-TD and PD groups.10 In that study, 63.6% of patients who underwent EUS-TD needed a second procedure, in which the ENBD tube was replaced with double pigtail stents to internalize the drainage tube. In the present study, however, using EUS-TD for drainage of noncapsulated POPC, a 7 Fr ENBD tube was inserted into the POPC, followed by internal drainage through cutting of the ENBD tube and leaving it in the stomach. Re-intervention was only required in 15.3% patients in the matched EUS-TD group. There were fewer patients who needed re-intervention in the present study than in the previous study, in which the ENBD tube was removed from patients with POPC. We suggest that it is the internalization of the ENBD tube that prevents the recurrence of POPC.10 This internalization contributed to the shorter time to clinical resolution of noncapsulated POPC.
Subgroup analysis of propensity-matched patients who had undergone distal pancreatectomy showed no between-group differences in patient age, serum CRP concentration, and number of postoperative days until the procedure. The POPC maximum diameter was significantly greater in the matched EUS-TD group than in the matched PD group. However, the time to clinical resolution after the procedure was significantly shorter in the matched EUS-TD group than in the matched PD group. This result indicated that EUS-TD is a more effective drainage treatment than PD in patients who underwent distal pancreatectomy.
This study had several limitations. First, the number of patients in the EUS-TD group was small, particularly the number who underwent pancreaticoduodenectomy. EUS-TD is not necessarily indicated for all patients who undergo pancreaticoduodenectomy because of anatomic variations in the position of the stomach.13 EUS-TD requires that the POPC be in contact with the stomach. Second, despite propensity-score matching, it may not be possible to completely eliminate selection bias between the EUS-TD and PD groups. Prospective, randomized, controlled trials are necessary to compare the effectiveness of EUS-TD with PD for drainage of POPC.
In conclusion, EUS-TD is more effective than PD for noncapsulated POPC after pancreatectomy. EUS-TD is also as technically feasible and safe as PD. EUS-TD should be considered for first-line treatment of patients with noncapsulated POPC.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the present study was supported by grants from the Japan Society for Promotion of Science.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Masayuki Kitano  https://orcid.org/0000-0001-6885-9223
https://orcid.org/0000-0001-6885-9223
Contributor Information
Takashi Tamura, Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
Masayuki Kitano, Second Department of Internal Medicine, Wakayama Medical University, 811-1 Kimiidera, Wakayama City, Wakayama 641-8509, Japan.
Manabu Kawai, Second Department of Surgery, Wakayama Medical University, Wakayama, Japan.
Kensuke Tanioka, Clinical Study Support Center, Wakayama Medical University, Wakayama, Japan.
Masahiro Itonaga, Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
Yuki Kawaji, Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
Junya Nuta, Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
Keiichi Hatamaru, Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
Yasunobu Yamashita, Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
Yuji Kitahata, Second Department of Surgery, Wakayama Medical University, Wakayama, Japan.
Motoki Miyazawa, Second Department of Surgery, Wakayama Medical University, Wakayama, Japan.
Seiko Hirono, Second Department of Surgery, Wakayama Medical University, Wakayama, Japan.
Ken-ichi Okada, Second Department of Surgery, Wakayama Medical University, Wakayama, Japan.
Hiroki Yamaue, Second Department of Surgery, Wakayama Medical University, Wakayama, Japan.
References
- 1. Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg 1999; 229: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010; 211: 503–539. [DOI] [PubMed] [Google Scholar]
- 3. Goh BK, Tan YM, Chung YF, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg 2008; 143: 956–965. [DOI] [PubMed] [Google Scholar]
- 4. Kleeff J, Diener MK, Z’graggen K, et al. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg 2007; 245: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrone CR, Warshaw AL, Rattner DW, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg 2008; 12: 1691–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008; 248: 438–446. [DOI] [PubMed] [Google Scholar]
- 7. Kawai M, Kondo S, Yamaue H, et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011; 18: 601–608. [DOI] [PubMed] [Google Scholar]
- 8. Varadarajulu S, Trevino JM, Christein JD, et al. EUS for the management of peripancreatic fluid collections after distal pancreatectomy. Gastrointest Endosc 2009; 70: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 9. Kwon YM, Gerdes H, Schattner MA, et al. Management of peripancreatic fluid collections following partial pancreatectomy: a comparison of percutaneous versus EUS-guided drainage. Surg Endosc 2013; 27: 2422–2427. [DOI] [PubMed] [Google Scholar]
- 10. Futagawa Y, Imazu H, Mori N, et al. The effectiveness and feasibility of endoscopic ultrasound-guided transgastric drainage of postoperative fluid collections early after pancreatic surgery. Surg Laparosc Endosc Percutan Tech 2017; 27: 267–272. [DOI] [PubMed] [Google Scholar]
- 11. Malecka-Panas E, Juszynski A, Chrzastek J, et al. Pancreatic fluid collections: diagnostic and therapeutic implications of percutaneous drainage guided by ultrasound. Hepatogastroenterology 1998; 45: 873–878. [PubMed] [Google Scholar]
- 12. Azeem N, Baron TH, Topazian MD, et al. Outcomes of endoscopic and percutaneous drainage of pancreatic fluid collections arising after pancreatic tail resection. J Am Coll Surg 2012; 215: 177–185. [DOI] [PubMed] [Google Scholar]
- 13. Onodera M, Kawakami H, Kawatani M, et al. Endoscopic ultrasound-guided transmural drainage for pancreatic fistula or pancreatic duct dilation after pancreatic surgery. Surg Endosc 2012; 26: 1710–1717. [DOI] [PubMed] [Google Scholar]
- 14. Jürgensen C, Distler M, Arlt A, et al. EUS-guided drainage in the management of postoperative pancreatic leaks and fistulas (with video). Gastrointest Endosc 2019; 89: 311–319. [DOI] [PubMed] [Google Scholar]
- 15. Rana SS, Sharma R, Gupta R. Endoscopic treatment of refractory external pancreatic fistulae with disconnected pancreatic duct syndrome. Pancreatology 2019; 19: 608–613. [DOI] [PubMed] [Google Scholar]
- 16. Téllez-Ávila F, Carmona-Aguilera GJ, Valdovinos-Andraca F, et al. Postoperative abdominal collections drainage: percutaneous versus guided by endoscopic ultrasound. Dig Endosc 2015; 27: 762–766. [DOI] [PubMed] [Google Scholar]
- 17. Gum PA, Thamilarasan M, Watanabe J, et al. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: a propensity analysis. JAMA 2001; 286: 1187–1194. [DOI] [PubMed] [Google Scholar]
- 18. Luo Z, Gardiner JC, Bradley CJ, et al. Applying propensity score methods in medical research: pitfalls and prospects. Med Care Res Rev 2010; 67: 528–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuss O, Legler T, Börgermann J, et al. Treatments effects from randomized trials and propensity score analyses were similar in similar populations in an example from cardiac surgery. J Clin Epidemiol 2011; 64: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 21. Tilara A, Gerdes H, Allen P, et al. Endoscopic ultrasound-guided transmural drainage of postoperative pancreatic collections. J Am Coll Surg 2014; 218: 33–40. [DOI] [PubMed] [Google Scholar]