Abstract
Background:
Obesity is recognized as an independent risk factor for chronic kidney disease through multiple direct and indirect biological pathways. Bariatric surgery is a proven, effective method for sustained weight loss. However, there is a relative paucity of data on the impact of bariatric surgery on renal outcomes.
Objective:
The primary objective was to evaluate the change in urine albumin/creatinine ratio (ACR) in patients undergoing bariatric surgery, at 12 months after the procedure. Secondary objectives were to determine the changes in ACR at (6 and 24 months), estimated glomerular filtration rate (eGFR; 6, 12, and 24 months), and hemoglobin A1c (HbA1c); 12 and 24 months) after the procedure.
Design:
This observational retrospective cohort study included consecutive obese patients who underwent bariatric surgery.
Setting:
Provincial Bariatric Surgery Clinic at the Regina General Hospital, Saskatchewan.
Patients:
This study includes 471 consecutive obese adult patients who underwent bariatric surgery between 2008 and 2015.
Measurements:
We studied the impact of bariatric surgery on body mass index (BMI), renal outcomes (urine ACR and eGFR) and metabolic outcomes (fasting glucose, total cholesterol, low-density lipoprotein, triglycerides, and HbA1c) in 471 patients.
Methods:
Patients were followed for 2 years postsurgery in the bariatric clinic. Mixed linear models that accounted for the repeated nature of the data were used to access changes in outcomes over time.
Results:
Patients were predominantly female (81%) with a mean age (±SD) of 46 ± 10 years. Most patients (87%) had a BMI > 40 kg/m2 and 81% of the patients underwent Roux-en-Y gastric bypass. The mean BMI decreased from 47.7 ± 7.8 kg/m2 at baseline to 37.1 ± 7.9 kg/m2 at 6 months and 34.8 ± 8.8 kg/m2 at 12 months. In a subcohort of patients with microalbuminuria, ACR showed an improvement from a median [interquartile] value of 5.1 [3.7-7.5] mg/mmol at baseline to 2.3 [1.2-3.6] mg/mmol at 6 months (P = .007), to 1.4 [0.9-3.7] mg/mmol at 2-year follow-up (P < .001). Similarly, eGFR increased in patients with microalbuminuria from 109 ± 10 mL/min/1.73 m2 at baseline to 120 ± 36 mL/min/1.73 m2 at 2-year follow-up (P = .013). There were statistically significant reductions in triglycerides, fasting glucose, and HbA1c.
Limitations:
This was a retrospective chart review, with the lack of a control group. Patients with eGFR less than 60 mL/min/1.73 m2 were not considered for surgery, and we had to measure renal outcomes predominantly on the presence of proteinuria.
Conclusions:
Our results suggest bariatric surgery significantly decreased weight and consequently improved renal and metabolic outcomes (eGFR, ACR, fasting glucose, cholesterol, and triglycerides) in patients with elevated BMI.
Keywords: obesity, bariatric surgery, renal outcomes, albuminuria, glomerular filtration rate, urine albumin/creatinine ratio
Abrégé
Contexte:
L’obésité est reconnue comme un facteur de risque indépendant d’insuffisance rénale chronique (IRC) via de multiples voies biologiques directes et indirectes. La chirurgie bariatrique est une méthode efficace et éprouvée pour perdre du poids de façon durable. Or, il existe peu de données mesurant l’impact de cette intervention sur les issues rénales.
Objectifs:
L’objectif principal était de mesurer la variation du rapport albumine/créatinine (RAC) urinaire chez des patients subissant une chirurgie bariatrique, 12 mois après l’intervention. On souhaitait aussi mesurer le RAC (6 mois et 24 mois), le débit de filtration glomérulaire estimé (DFGe) (6, 12 et 24 mois) et le taux d’hémoglobine glyquée (HbA1c) (12 et 24 mois) à intervalles réguliers après l’intervention.
Type d’étude:
Étude de cohorte rétrospective observationnelle portant sur des patients obèses ayant subi une chirurgie bariatrique.
Cadre:
La clinique provinciale de chirurgie bariatrique du Regina General Hospital (Saskatchewan).
Sujets:
Un total de 471 patients consécutifs ayant subi une chirurgie bariatrique entre 2008 et 2015.
Mesures:
Nous avons étudié l’impact de la chirurgie bariatrique sur l’indice de masse corporelle (IMC), les issues rénales (RAC, DFGe) et les résultats métaboliques (glycémie à jeun, cholestérol total) de 471 patients.
Méthodologie:
Les patients ont été suivis dans une clinique bariatrique jusqu’à deux ans après l’intervention. Des modèles mixtes linéaires tenant compte de la nature répétitive des données ont été employés pour évaluer les variations dans les résultats au fil du temps.
Résultats:
La cohorte était majoritairement féminine (81%) et l’âge moyen (±SD) se situait à 46 ± 10 ans. La majorité des sujets (87%) présentait un IMC supérieur à 40 kg/m2 et 81% des patients avaient subi une dérivation gastrique de type Roux-en-Y. L’IMC moyen est passé de 47,7 ± 7,8 kg/m2 (initial) à 37,1 ± 7,9 kg/m2 après 6 mois, et à 34,8 ± 8,8 kg/m2 après 12 mois. Dans une sous-cohorte de patients atteints de microalbuminurie, le RAC est passé d’une valeur médiane (EIQ) initiale de 5,1 [3,7-7,5] mg/mmol à 2,3 [1,2-3,6] mg/mmol après 6 mois (P = 0,007), et à 1,4 [0,9-3,7] mg/mmol après deux ans de suivi (P < 0,001). Parallèlement, dans cette même sous-cohorte, le DFGe est passé de 109 ± 10 mL/min/1,73 m2 (initial) à 120 ± 36 mL/min/1,73 m2 après deux ans de suivi (P = 0,013). Des réductions statistiquement significatives ont également été observées pour les triglycérides, la glycémie à jeun et l’HbA1c.
Limites:
Il s’agit d’une analyze de dossiers rétrospective sans groupe contrôle. Les patients avec un DFGe inférieur à 60 mL/min/1,73 m2 n’ont pas été pris en compte pour l’intervention et nous avons dû mesurer les issues rénales principalement en fonction de la présence d’une protéinurie.
Conclusion:
Nos résultats suggèrent que la chirurgie bariatrique entraîne une perte significative de poids et, conséquemment, une amélioration des issues rénales et métaboliques (DFGe, RAC, glycémie à jeun, taux de cholestérol et de triglycérides) chez les patients présentant un IMC élevé.
What was known before
Bariatric surgery is an effective alternative to achieve significant and sustained weight loss. There was a relative lack of long-term data on renal outcomes.
What this adds
This study adds to the growing literature on renal outcomes after bariatric surgery. It adds to long-term (2 year) safety of the procedure and positive sustained impact on renal outcomes.
Background
Both obesity and chronic kidney disease (CKD) constitute high-prevalence medical conditions with a significant impact on health care systems. In 2014, 20% of adult Canadians were obese. At the same time, the global prevalence of CKD is estimated to be around 13%.1 There seems to be a strong pathophysiological association between these 2 entities.2 In addition to increasing the risk of hypertension and diabetes, excessive adipose tissue is responsible for the activation of the sympathetic nervous system and eventually renin-angiotensin system leading to hypertension.3 It also leads to insulin resistance through disruption of insulin signaling pathways from lipolysis and release of inflammatory markers.3 Obesity is also considered an independent risk factor for the development of CKD through multiple biological pathways including compression-induced increased renal sodium reabsorption leading to afferent arteriolar vasodilation and consequently, hyperperfusion, hyperfiltration, and worsening albuminuria/proteinuria.4-6 Furthermore, increased adipose tissue has been associated with the presence of histological changes in the kidney including focally thickened basement membrane, dilated capillary loops, thickened foot processes, glomerulomegaly, and eventually glomerulosclerosis.7-9 The combined direct effect of obesity and indirect impact of hypertension, diabetes, and metabolic syndrome on the glomerular barrier can be clinically evaluated by measuring albuminuria and altered glomerular filtration rates (GFRs).
Given the strong association between obesity and CKD, it is plausible that interventions leading to significant weight loss may benefit renal function. Previous studies have estimated that every kilogram of intentional weight loss may result in a 4% reduction of proteinuria, regardless of the beneficial effect of weight loss in blood pressure.10 Bariatric surgery is a proven, effective procedure for sustained weight loss and is increasingly being considered for patients with morbid or severe obesity. Options for bariatric surgery include Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric banding, and laparoscopic vertical sleeve gastrectomy (LSG).2,11,12 All of these procedures are associated with significant and sustained weight loss and potential remission of diabetes, hypertension, and dyslipidemia.13-15
While there is strong evidence of improved metabolic outcomes, there is paucity of long-term data on renal outcomes (proteinuria and estimated glomerular filtration rate [eGFR]) in Canadian patients. Previous studies looking at renal measures in obese subjects have been limited by heterogeneity in outcomes and variable duration of follow-up.16-20 In this single center, retrospective population-based cohort, we attempted to identify the long-term impact of bariatric surgery on renal function of obese patients treated in a single-center provincial bariatric program.
Methods
Study Design
Retrospective review of electronic medical records of all the patients who underwent bariatric surgery at the Regina General Hospital between 2008 and 2015 was performed. Demographic, clinical, and biochemical data were collected. Demographic data included age (years) and sex. Clinical data included height (cm) and weight (kg), BMI (kg/m2), and the type of bariatric surgery (RYGB or LSG). Biochemical data included albumin (g/L), creatinine (μmol/L), albumin/creatinine ratio (ACR; mg/mmol), glucose (mmol/L), hemoglobin A1c (HbA1c; %), total cholesterol (mmol/L), triglyceride (mmol/L), and low-density lipoprotein (LDL) cholesterol (mmol/L). Diabetes was defined according to the American Diabetic Association (fasting glucose >7.0 mmol/L and HbA1c >6.5%) and normoglycemia (fasting glucose <5.6 mmol/L and HbA1c <5.7%). Microalbuminuria was defined as ACR >2.0 and <20 mg/mmol in men and >2.8 and <28 mg/mmol in women, and macroalbuminuria was defined as ACR >20 mg/mmol in men and >28 mg/mmol in women. Glomerular filtration rate was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.21 The study was approved by the Research Ethics Board of the former Regina Qu’Appelle Health Region (REB-17-86).
Surgical Eligibility Criteria
The provincial bariatric surgical program at the Regina General Hospital, Saskatchewan, Canada, accepts referrals from family doctors across the region for assessment for weight reduction surgery. Criteria for surgery were based on the National Institute of Health guidelines and included the following: body mass index (BMI) ≥40 kg/m2 or 35 to 40 kg/m2 with 1 obesity-related comorbidity and a prior attempt to lose weight. Patients aged <18 or >60 years and had GFR <60 mL/min/1.73 m2 were not eligible for bariatric surgery. Patients were initially assessed by a multidisciplinary program (psychologist, exercise therapist, and dietitian) along with the bariatric surgeon. They were instructed to follow a diet reduction regime for a period of 6 months and subsequently re-evaluated by the multidisciplinary team.
Surgical Interventions and Follow-Up
Two procedures (RYGB and LSG) were predominantly performed for weight loss surgery at the Regina General Hospital. In RYGB, the stomach is divided into an upper stomach pouch (15-30 mL) and a lower gastric remnant. The stomach pouch is then anastomosed to the mid jejunum, and the jejuno-jenunal anastomosis is created to connect the biliopancreatic limb and the gastric remnant, thereby allowing the gastric, pancreatic, and biliary secretion to mix with the food in the jejuno-jejunal anastomosis.2 Laparoscopic vertical sleeve gastrectomy is a restrictive surgery that involves the removal of 70% to 80% of the lateral stomach.2 Both procedures are widely accepted and validated as appropriate surgical options for weight loss by the American Society of Bariatric and Metabolic surgery. Postoperatively, patients were seen at scheduled intervals for 2 years to monitor for any surgical, metabolic, or nutritional complications from their procedures.
Statistics
All statistical analyses were performed using SPSS version 22 (SPSS Inc, Chicago, IL). Continuous variables are summarized as mean ± SD or median (interquartile range [IQR]), as appropriate. Differences in outcomes over time were assessed using mixed linear models that accounted for the repeated nature of the data and within-subject correlation; both intercept and time were specified as fixed effects and an unstructured covariance was specified. Time (visit) was treated as a repeat measure and was categoric when specified in the model. A P-value <.05 was considered statistically significant.
Results
Patient Characteristics
Baseline characteristics of the 471 study participants are shown in Table 1. Most of the study participants were women (81%), and the mean age at surgery was 46 ± 10 years.
Table 1.
Baseline Patient and Surgery Characteristics.
| Variable | |
|---|---|
| Age at surgery (Mean ± SD), y | 46 ±10 |
| Sex, No. (%) | |
| Female | 380 (80.7) |
| Male | 91 (19.3) |
| Type of surgery, No. (%) | |
| Roux-en-Y gastric bypass | 380 (80.7) |
| Laparoscopic sleeve gastrectomy | 90 (19.3) |
| Diabetic, No. (%) | 116 (27.8)a |
| Before diabetes, No. (%) | 160 (38.3)a |
| Microalbuminuria, No. (%) | 60 (12.7) |
| Macroalbuminuria, No. (%) | 7 (1.5) |
| Height (Mean ± SD), cm | 166 ± 10 |
| Weight (Mean ± SD), kg | 133 ± 28 |
| Body mass index (Mean ± SD), kg/m2 | 47.7 ± 7.8 |
| Estimated glomerular filtration rate (mean ± SD), mL/min/1.73 m2 | 107.5 ± 10.5 |
| Albumin/creatinine ratio (median [interquartile range]), mg/mmol | 0.8 [0.5-1.8] |
| Glucose fasting (mean ± SD), mmol/L | 6.6 ± 2.5 |
| Hemoglobin A1c (mean ± SD), % | 6.2 ± 1.1 |
| Cholesterol (mean ± SD), mmol/L | 4.6 ± 1.0 |
| Low-density lipoprotein (mean ± SD), mmol/L | 2.7 ± 0.9 |
| Triglyceride (mean ± SD), mmol/L | 1.8 ± 1.1 |
n = 417 due to missing data.
Weight loss and changes in metabolic parameters
There were significant changes in weight and BMI following surgery (Figure 1). Three percent of patients had a BMI of 30 to 34.9 kg/m2, 11% had a BMI between 35 and 35.9 kg/m2, and 87% had a BMI >40 kg/m2. Eighty-one percent of the patients underwent RYGB and 19% underwent LSD. The BMI was significantly lower 6 months after bariatric surgery for up to a 2-year follow-up period (Figure 1). The mean BMI decreased from 47.7 ± 7.8 kg/m2 at baseline to 37.1 ± 7.9 kg/m2 at 6 months after the procedure. The BMI further declined from 37.1 ± 7.9 kg/m2 to 34.8 ± 8.8 kg/m2 at 1 year. However, there was no further significant decrease in BMI in the second year after the bariatric surgery as compared with the first year. At 2-year follow-up, BMI was 34.8 ± 7.9 kg/m2.
Figure 1.

Change in body mass index (A) and weight (B) over the study period.
Renal outcomes
Mean creatinine was 62 ± 16 μmol/L at baseline, 70 ± 15 μmol/L at 12 months, and 67 ± 18 μmol/L at 2-year follow-up. The mean eGFR was 107.5 ± 10.5 mL/min/1.73 m2 at baseline, 102.1 ± 22.4 mL/min/1.73 m2 at 1 year, and 106.2 ± 27.0 mL/min/1.73 m2 at 2-year follow-up. These results suggest that there was a decline in eGFR postsurgery that lasted up to a year and reverted back to baseline by 24 months (Table 2). The majority of patients (84%) had normal albumin excretion rates. In patients with microalbuminuria (n = 60, 12.7%), ACR and eGFR showed improvements (Figure 2). In patients with microalbuminuria, ACR showed an improvement from a median [IQR] value of 5.1 [3.7-7.5] mg/mmol at baseline to 2.3 [1.2-3.6] mg/mmol at 6 months (P = .007), to 1.4 (0.9-3.7) mg/mmol at 2-year follow-up (P < .001). Similarly, eGFR increased in patients with microalbuminuria changing from 109 ± 10 mL/min/1.73 m2 at baseline to 120 ± 36 mL/min/1.73 m2 at 2-year follow-up (P = .013).
Table 2.
Descriptive statistics of renal and metabolic measures over time for all patients.
| Baseline | 6 months | 12 months | 24 months | P-value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | ||
| (median [IQR]) | (median [IQR]) | (median [IQR]) | (median [IQR]) | ||||||
| Body mass index, kg/m2 | 471 | 47.7 ± 7.8 | 362 | 37.1 ± 7.9 | 322 | 34.8 ± 8.8 | 264 | 34.8 ± 7.9 | <.001 |
| Weight, kg | 471 | 133 ± 28 | 362 | 102 ± 21 | 281 | 97 ± 30 | 231 | 96 ± 25 | <.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 471 | 107.5 ± 10.5 | 172 | 103.0 ± 22.9 | 164 | 102.1 ± 22.4 | 182 | 106.2 ± 27.0 | .002 |
| Creatinine, μmol/L | 471 | 62 ± 16 | 172 | 69 ± 14 | 164 | 70 ± 15 | 182 | 67 ± 18 | <.001 |
| Albumin/creatinine ratio, mg/mmol | 416 | 0.8 [0.5-1.8] | 235 | 0.8 [0.5-1.4] | 213 | 0.6 [0.5-1.1] | 174 | 0.7 [0.5-1.2]) | .265 |
| Glucose fasting, mmol/L | 407 | 6.6 ± 2.5 | 154 | 5.6 ± 1.2 | 150 | 5.5 ± 1.3 | 141 | 5.6 ± 1.4 | <.001 |
| HbA1c, % | 169 | 6.2 ± 1.1 | 53 | 5.6 ± 0.6 | 31 | 5.6 ± 1.0 | 14 | 5.5 ± 0.8 | <.001 |
| Cholesterol, mmol/L | 407 | 4.6 ± 1.0 | 157 | 4.3 ± 0.9 | 150 | 4.4 ± 0.9 | 139 | 4.5 ± 0.9 | <.001 |
| Low-density lipoprotein, mmol/L | 407 | 2.7 ± 0.9 | 156 | 2.6 ± 0.8 | 150 | 2.6 ± 0.7 | 138 | 2.6 ± 0.8 | .062 |
| Triglyceride, mmol/L | 408 | 1.8 ± 1.1 | 156 | 1.2 ± 0.5 | 150 | 1.1 ± 0.5 | 138 | 1.1 ± 0.6 | <.001 |
significant change from baseline (P < .05).
Figure 2.
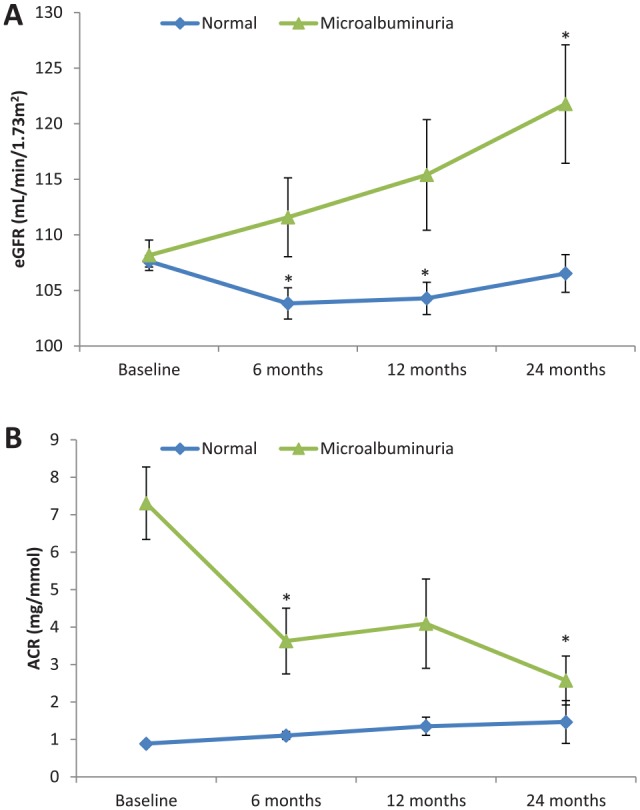
Two-year follow-up evaluation of estimated glomerular filtration rate (A) and albumin/creatinine ratio (B) in the cohort stratified according to the presence of baseline proteinuria.
Note. Estimated marginal means and standard error are presented at each time point using linear mixed model analysis. Asterisks (*) indicate significant change from baseline (P < .05) using Bonferroni adjustment to account for multiple comparisons.
Diabetes and lipid profile
A significant reduction in fasting glucose, and glycated hemoglobin levels was observed in our study at 6-month follow-up and maintained at 2-year follow-up (P < .001). There were significant improvements in serum total cholesterol and triglyceride levels at 6 months, which were maintained for 2 years (Table 2). Based on these lab values, the proportion of patients with diabetics reduced significantly from 27.8% (116/417) at baselines to 7.8% (14/179) at 6 months, 7.4% (12/162) at 1 year and 9.6% (16/146) at 2 years.
Discussion
In this single-center study, we examined the impact of bariatric surgery on renal and metabolic function using eGFR, albuminuria, serum lipid profile, glucose fasting, and HbA1c as biologic markers. We observed significant improvement in BMI from 47.7 ± 7.8 to 34.8 ± 8.8 kg/m2 by 1 year after the surgery. Our results are similar to the other published studies that show significant reduction in weight after the procedure.2,19 We also described a beneficial effect in glucose homeostasis and lipid profile comparable to those described by other groups.22 Furthermore, we observed a reduction in albumin excretion rates amongst those subjects with albuminuria at baseline. These results are consistent with a recent systematic review that observed an overall reduction in proteinuria after bariatric surgery.20
Over the years, bariatric surgery has built a safety record and has become an effective alternative to achieve a significant and sustained weight reduction. However, data regarding the impact and safety of bariatric procedures in patients with kidney impairment are lacking mainly because most centers will consider patients with significant renal impairment as noneligible for bariatric surgery. Preserved GFR with microalbuminuria and macroalbuminuria is the earliest clinical manifestation of renal involvement in obese patients.20,23 Although patients with abnormal albumin excretion rates contributed to only 16% of the study population, we observed a significant improvement in albuminuria after the surgery. The observed difference might have been different with a larger sample size.
Animal studies from the mid-1990s revealed several pathways for the development of proteinuria. These include elevated glomerular pressure by stretching of mesangial cells by increased hydraulic pressure,24,25 increased production of transforming growth factor beta, which impair the lysosomal enzyme activity in tubular cells26 and endothelial cell dysfunction.27,28 There are human data to suggest that reduction in albuminuria following bariatric surgery is correlated with an improvement in insulin sensitivity,29 favorable alterations in the levels of gut hormones that control insulin secretion,30 reduction in renal cytokines,31 and improvement in anti-inflammatory adipokine adiponectin. Irrespective of the pathogenesis of albuminuria in patients with obesity, improvement in albumin excretion rates following bariatric surgery suggests improved glomerular and/or systemic capillary function.
We observed that eGFR decreased for our entire cohort over the first year, which is in contrast to few other published studies showing an increase after the procedure.32,33 Obesity is hemodynamically associated with elevations in glomerular filtration (hyperfiltration).34 Brochner-Mortensen et al35 were the first group to demonstrate that GFR decreased after intestinal bypass surgery in obese subjects. Chagnac et al36 also showed that after the bariatric surgery, there was a decrease in GFR, renal plasma flow, and microalbuminuria in obese patients. Our study is comparable with other published studies that showed a reduction in measured glomerular filtration rate (mGFR) due to a resolution of glomerular hyperfiltration after the bariatric surgery.36-38 We did not perform mGFR as performed in other studies and instead used creatinine-based formula (CKD-EPI equation) to estimate the GFR. It is practically impossible to measure iothalamate GFR in routine clinical care. Measurement of cystatin C would have provided a more accurate eGFR than creatinine, but we were hampered by the lack of an available assay in our Institution.
The STAMPEDE trial39 and other randomized controlled trials40,41 further showed that bariatric surgery was superior to intensive medical therapy in improvement in lipid levels and quality of life along with better diabetic control. We similarly noticed statistically significant changes in total cholesterol and triglyceride levels, but not in LDL levels. As the key treatment goal of surgical intervention in obesity is prevention of long-term complications, it was reassuring to see results of Swedish Obesity Subject Study (SOS), show a reduction in microvascular and macrovascular complications after the bariatric surgery.42
The effects of weight loss on diabetes status and HbA1c in our study were identical to previously published studies.43,44 Although we did not capture medication use (oral hypoglycemic agents and insulin), a significant decline in fasting glucose and HbA1c levels was seen in our cohort. Improved blood glucose regulation has been reported after the bariatric surgery leading to a reduction in the amount of insulin, and, in many cases, often discontinuation of insulin or oral hypoglycemic agents.39,43 A meta-analysis by Panunzi et al45 reported that most obese patients (90%) entered remission of their type 2 diabetes after metabolic procedures in the short to medium term. It has been estimated that 24.2% of kidney disease cases in the United States among men and 34% among women could be prevented if overweight and obesity were eliminated.46 Overall, the results confirm the success of bariatric surgery in our Institution. The strengths of this study include improved metabolic outcomes and reduction in albuminuria after the bariatric surgery. In addition, this is the largest Canadian cohort with 24 months of follow-up. Navaneethan et al29 looked at 13 patients and followed them for 12 months. Similarly, the first published study from 1980 by Brochner-Mortensen et al looked at 8 patients after jejuno-ileal bypass surgery and followed them for 12 months.35 In 2008, Agarwal et al47 reported on 94 patients with microalbuminuria and followed them over 12 months. Serpa Neto et al48 reported on 140 patients and followed them over 8 months. Fenske et al49 looked at 34 patients and followed them over 12 months. Similarly, Stephenson et al50 and Ruiz-Tovar et al51 reported on 23 and 50 patients, respectively, and followed them over only 12 months. In contrast, in this single-center retrospective study, we present data on 471 patients followed over 24 months and is the largest Canadian cohort to our knowledge. Chang et al2 followed 985 patients, but only 91 of the 985 patients had GFR <60 mL/min. More recent studies (Imam et al looked at 714 patients and Swedish Obesity Study) reported on a bigger cohort and followed patients over a longer period of time.52,53
Limitations of Study
Our study was a retrospective chart review, and there was no “control group” that underwent routine care in comparison, making it challenging to ascertain the kidney-specific risks and benefits of bariatric surgery. Patients with GFR less than 60 mL/min/1.73 m2 were not offered surgery by the surgical team due to perceived increased perioperative risk of acute kidney injury. Our assessment of renal outcomes was predominantly on the presence of proteinuria. Patients with impaired albumin excretion rates contributed to only 16% of patients at the time of surgery, and there was significantly high clinic dropout rate creating an opportunity for bias. We did not collect information on short- or longer-term complications related to bariatric surgery. Finally, we used CKD-EPI formula for the calculation of GFR. Creatinine-based eGFR equations have not been validated in morbidly obese adults or in patients with change in body composition after bariatric surgery. We were limited in our ability to explore the impact of the filtration rate any further because we did not measure iothalamate GFR and cystatin-based assays are currently not performed in our Institution.
Acknowledgments
The authors wish to acknowledge Regina Qu’Appelle Health Region, Research and Performance Support and Dr Christian Rueda-Classen, MD PhD for assisting with this study.
Footnotes
Ethics Approval and Consent to Participate: The study was approved by the Research Ethics Board of the former Regina Qu’Appelle Health Region (REB 17-86).
Consent for Publication: Not applicable as there is no patient identifying information in this manuscript.
Availability of Data and Materials: The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions: B.P. edited the final manuscript and conceived, designed, and assisted with the multiple drafts. M.M. wrote the initial draft. G.K. assisted with the drafts. A.C. and W.B. performed the statistical analysis. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bhanu Prasad  https://orcid.org/0000-0002-1139-4821
https://orcid.org/0000-0002-1139-4821
References
- 1. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang AR, Grams ME, Navaneethan SD. Bariatric surgery and kidney-related outcomes. Kidney Int Rep. 2017;2(2):261-270. doi: 10.1016/j.ekir.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakkis JI, Weir MR. Obesity and kidney disease. Prog Cardiovasc Dis. 2018;61(2):157-167. [DOI] [PubMed] [Google Scholar]
- 4. Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33(1):14-22. [DOI] [PubMed] [Google Scholar]
- 5. Ting SM, Nair H, Ching I, Taheri S, Dasgupta I. Overweight, obesity and chronic kidney disease. Nephron Clin Pract. 2009;112(3):c121-c127; discussion c127. [DOI] [PubMed] [Google Scholar]
- 6. Garland JS. Elevated body mass index as a risk factor for chronic kidney disease: current perspectives. Diabetes Metab Syndr Obes. 2014;7:347-355. doi: 10.2147/DMSO.S46674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498-1509. [DOI] [PubMed] [Google Scholar]
- 8. Xu T, Sheng Z, Yao L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front Med. 2017;11(3):340-348. doi: 10.1007/s11684-017-0570-3. [DOI] [PubMed] [Google Scholar]
- 9. D’Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453-471. [DOI] [PubMed] [Google Scholar]
- 10. Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25(4):1173-1183. doi: 10.1093/ndt/gfp640. [DOI] [PubMed] [Google Scholar]
- 11. Meijer RI, vanWagensveld BA, Siegert CE, Eringa EC, Serne EH, Smulders YM. Bariatric surgery as a novel treatment for type 2 diabetes mellitus: a systematic review. Arch Surg. 2011;146(6):744-750. doi: 10.1001/archsurg.2011.134. [DOI] [PubMed] [Google Scholar]
- 12. Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Med Clin North Am. 2011;95(5):1009-1030. [DOI] [PubMed] [Google Scholar]
- 13. Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19(12):1605-1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 14. Padwal RS, Majumdar SR, Klarenbach S, et al. The Alberta population-based prospective evaluation of the quality of life outcomes and economic impact of bariatric surgery (APPLES) study: background, design and rationale. BMC Health Serv Res. 2010;10:284. doi: 10.1186/1472-6963-10-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3-14. doi: 10.1007/s11695-018-3525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reid TJ, Saeed S, McCoy S, Osewa AA, Persaud A, Ahmed L. The effect of bariatric surgery on renal function. Surg Obes Relat Dis. 2014;10(5):808-813. [DOI] [PubMed] [Google Scholar]
- 17. Li K, Zou J, Ye Z, et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One. 2016;11(10):e0163907. doi: 10.1371/journal.pone.0163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohan S, Tan J, Gorantla S, Ahmed L, Park CM. Early improvement in albuminuria in non-diabetic patients after Roux-en-Y bariatric surgery. Obes Surg. 2012;22(3):375-380. doi: 10.1007/s11695-011-0437-7. [DOI] [PubMed] [Google Scholar]
- 19. Clerte M, Wagner S, Carette C, et al. The measured glomerular filtration rate (mGFR) before and 6 months after bariatric surgery: a pilot study. Nephrol Ther. 2017;13(3):160-167. doi: 10.1016/j.nephro.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 20. Bilha SC, Nistor I, Nedelcu A, et al. The effects of bariatric surgery on renal outcomes: a systematic review and meta-analysis. Obes Surg. 2018;28(12):3815-3833. doi: 10.1007/s11695-018-3416-4. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warkentin LM, Majumdar SR, Johnson JA, et al. Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: two-year prospective cohort study. BMC Med. 2014;12:175. doi: 10.1186/s12916-014-0175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17(12 suppl 3). doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 24. Hirakata M, Kaname S, Chung UG, et al. Tyrosine kinase dependent expression of TGF-beta induced by stretch in mesangial cells. Kidney Int. 1997;51(4):1028-1036. doi: 10.1038/ki.1997.144. [DOI] [PubMed] [Google Scholar]
- 25. Riser BL, Cortes P, Heilig C, et al. Cyclic stretching force selectively up-regulates transforming growth factor-beta isoforms in cultured rat mesangial cells. Am J Pathol. 1996;148(6):1915-1923. [PMC free article] [PubMed] [Google Scholar]
- 26. Schenk O, Ling H, Sebekova K, Vamvakas S, Heidland A. High-glucose media enhance the responsiveness of tubular cells to growth promoters: effect on lysosomal cathepsins and protein degradation. Miner Electrolyte Metab. 1998;24(4):254-260. [DOI] [PubMed] [Google Scholar]
- 27. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601-2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arcaro G, Zamboni M, Rossi L, et al. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23(9):936-942. [DOI] [PubMed] [Google Scholar]
- 29. Navaneethan SD, Kelly KR, Sabbagh F, Schauer PR, Kirwan JP, Kashyap SR. Urinary albumin excretion, HMW adiponectin, and insulin sensitivity in type 2 diabetic patients undergoing bariatric surgery. Obes Surg. 2010;20(3):308-315. doi: 10.1007/s11695-009-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737. [DOI] [PubMed] [Google Scholar]
- 31. Bueter M, Dubb SS, Gill A, et al. Renal cytokines improve early after bariatric surgery. Br J Surg. 2010;97(12):1838-1844. doi: 10.1002/bjs.7264. [DOI] [PubMed] [Google Scholar]
- 32. Neff KJ, Baud G, Raverdy V, et al. Renal function and remission of hypertension after bariatric surgery: a 5-year prospective cohort study. Obes Surg. 2017;27(3):613-619. doi: 10.1007/s11695-016-2333-7. [DOI] [PubMed] [Google Scholar]
- 33. Mirajkar N, Bellary S, Ahmed M, Singhal R, Daskalakis M, Tahrani AA. The impact of bariatric surgery on estimated glomerular filtration rate in patients with type 2 diabetes: a retrospective cohort study. Surg Obes Relat Dis. 2016;12(10):1883-1889. doi: 10.1016/j.soard.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 34. Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324(3):127-137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 35. Brochner-Mortensen J, Rickers H, Balslev I. Renal function and body composition before and after intestinal bypass operation in obese patients. Scand J Clin Lab Invest. 1980;40(8):695-702. doi: 10.3109/00365518009095584. [DOI] [PubMed] [Google Scholar]
- 36. Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480-1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 37. Friedman AN, Moe S, Fadel WF, et al. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol. 2014;39(1):8-15. doi: 10.1159/000357231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49(6):1774-1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 39. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mingrone G, Panunzi S, DeGaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964-973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 41. Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the diabetes surgery study. JAMA. 2018;319(3):266-278. doi: 10.1001/jama.2017.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56-65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 43. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567-1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 45. Panunzi S, DeGaetano A, Carnicelli A, Mingrone G. Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? a meta-analysis. Ann Surg. 2015;261(3):459-467. doi: 10.1097/SLA.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 46. Saiki A, Nagayama D, Ohhira M, et al. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes. 2005;29(9):1115-1120. doi: 10.1038/sj.ijo.0803009. [DOI] [PubMed] [Google Scholar]
- 47. Agrawal V, Khan I, Rai B, et al. The effect of weight loss after bariatric surgery on albuminuria. Clin Nephrol. 2008;70(3):194-202. doi: 10.5414/cnp70194. [DOI] [PubMed] [Google Scholar]
- 48. Serpa Neto A, Bianco Rossi FM, Dal Moro Amarante R, Alves Buriti N, Cunha Barbosa Saheb G, Rossi M. Effect of weight loss after Roux-en-Y gastric bypass, on renal function and blood pressure in morbidly obese patients. J Nephrol. 2009;22(5):637-646. [PubMed] [Google Scholar]
- 49. Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9(4):559-568. doi: 10.1016/j.soard.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 50. Stephenson DT, Jandeleit-Dahm K, Balkau B, Cohen N. Improvement in albuminuria in patients with type 2 diabetes after laparoscopic adjustable gastric banding. Diab Vasc Dis Res. 2013;10(6):514-519. doi: 10.1177/1479164113498083. [DOI] [PubMed] [Google Scholar]
- 51. Ruiz-Tovar J, Giner L, Sarro-Sobrin F, Alsina ME, Marco MP, Craver L. Laparoscopic sleeve gastrectomy prevents the deterioration of renal function in morbidly obese patients over 40 years. Obes Surg. 2015;25(5):796-799. doi: 10.1007/s11695-014-1486-5. [DOI] [PubMed] [Google Scholar]
- 52. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741-752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 53. Imam TH, Fischer H, Jing B, Burchette R, Henry S, DeRose SF, Coleman KJ. Estimated GFR before and after bariatric surgery in CKD. Am J Kidney Dis. 2017;69(3):380-388. doi: 10.1053/j.ajkd.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


