Abstract
Background
Underfeeding in intensive care patients on enteral nutrition is commonplace and multifactorial. This can be exacerbated by interruptions caused by routine fasting for procedures and investigations. Our study aims to demonstrate that a volume based feeding protocol can overcome the barriers of underfeeding and safely increase energy and protein delivery in UK intensive care patients, potentially improving clinical outcomes.
Methods
In this single centre cohort study, data were collected from adult mechanically ventilated patients. We compared the standard care of rate based feeding, from an International Nutrition Survey (2014/15) to the new intervention of volume based feeding, in a mixed medical and surgical intensive care unit. The primary outcomes were the proportion of energy and protein daily targets delivered. Secondary outcomes compared the effects on gastrointestinal tolerance, glycaemic control, mortality, mechanical ventilation days, length of stay in intensive care unit and hospital.
Results
From a total of 82 patients (rate based feeding = 27, volume based feeding = 55), volume based feeding patients received significantly more prescribed energy (52% versus 81%; p < 0.001) and protein (40% versus 74%; p < 0.001). There was no significant difference in gastrointestinal symptoms such as gastric residual volumes (p = 0.62), glycaemic control (p = 0.94) or insulin usage (p = 0.75). Although there was an improvement in energy and protein delivery, there were no differences in mechanical ventilation days (p = 0.12), mortality (p = 0.06), length of stay in intensive care unit (p = 0.93) and hospital (p = 0.72) between the groups.
Conclusion
Compared to rate based feeding, volume based feeding significantly improved energy and protein provision with no adverse effects on glycaemic control or gastrointestinal tolerance, clinical outcomes were not affected.
Keywords: Underfeeding, enteral nutrition, volume based feeding, rate based feeding, intensive care, clinical outcome
Introduction
Nutrition support is an essential part of treatment in patients requiring intensive care. Timely provision of greater energy and protein intake is associated with lower mortality and a faster time to discharge alive.1,2 However, underfeeding in intensive care patients is commonplace and multifactorial.3 In response to stress, underfeeding can lead to malnutrition and poor clinical outcomes, including increased mechanical ventilation days, infectious complications, length of stay (LOS) in the intensive care unit (ICU) and in hospital, with an increase in associated healthcare costs.4–8
Enteral nutrition (EN) remains the preferred route of feeding in ICUs, providing both nutritional and non-nutritional benefits.9–12 However, there is currently insufficient evidence for the optimal EN delivery method in the literature for intensive care patients, with options including rate based feeding (RBF) or bolus feeding.13,14 Frequent interruptions to EN including routine fasting for procedures and investigations exacerbate underfeeding in ICU patients15,16 and recent studies have demonstrated that RBF is ineffective in addressing this issue.17–20 Despite this, RBF remains the most common method of EN delivery throughout ICUs in Europe. The recent International Nutrition Survey (INS, 2014/15) demonstrated that adequacy of energy and protein in enterally fed ICU patients in Europe was 58 and 54%, respectively (unpublished data; Darren Heyland, 2017, personal communication).
A volume based feeding (VBF) approach has been recommended to address the challenges of frequent interruptions and optimise the delivery of EN,12,14 designed to adjust the infusion rate to make up for daily interruptions in delivery, enabling a greater volume of EN to be delivered compared to a fixed hourly RBF.18 This recommendation for VBF is based on studies in North America.18–21 To date there are no studies evaluating VBF alone and its effect on EN delivery or clinical outcomes outside of North American healthcare institutes. Although the practice of intensive care medicine is universal in most countries, there can be significant differences in healthcare and populations in this already heterogeneous patient group; these previous VBF studies may not be generalisable to other intensive care populations where differing health systems, barriers, patient characteristics and priorities towards nutrition might present.22
So far there has been no study in the United Kingdom (UK) that addresses whether VBF is a safe and more effective method than RBF in improving energy and protein delivery in mechanically ventilated ICU patients. We hypothesised that VBF would improve energy and protein delivery without deleterious effects on glycaemic control or gastrointestinal tolerance and subsequently, may improve clinical outcome.
Methodology
Study design and setting
We conducted a single centre study in an adult, mixed medical and surgical ICU in England, UK between January 2015 and March 2017. This is a cohort study, comparing the usual RBF protocol (cohort 1) to a newly implemented VBF Protocol (cohort 2). Retrospective data were used for RBF participants and prospective data were collected for VBF participants, before and after VBF was introduced. An application to both City, University of London's Senate Research Ethics Committee (Reference number MRes/15-16/40) and UK's Health Research Authority advised that ethical approval was not required for this service evaluation, in that these patients would not undergo any additional intrusive procedure to their normal attention, the data collected were part of their routine care and further patient consent was not required.
Participants
Eligible patients were mechanically ventilated adults (>18 years), requiring EN for >48 h at any point during their first 12 days of stay. Consecutive patients were assessed and selected by a registered dietitian for both cohorts. Patients were excluded for the following reasons: contraindications to EN including bowel obstruction, complex bowel surgery (not including post-operative, uncomplicated colonic resections), proximal enterocutaneous fistula, short bowel, bowel ischaemia or paralytic ileus; pre-existing or onset of GI intolerance including profuse diarrhoea (five stools or >750 ml/24 h), nausea, vomiting, abdominal distension (based on nursing assessment), one episode or more of gastric residual volume (GRV) > 250 ml; receiving parenteral nutrition; aspiration of feed within 48 h; pregnancy.
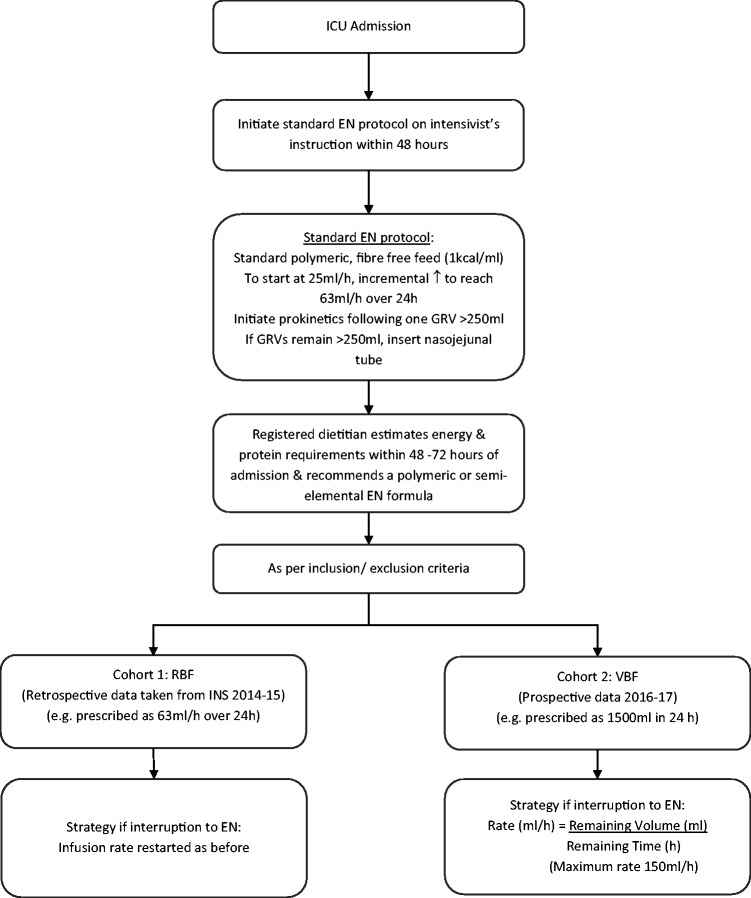
Figure 1 outlines the feeding protocol for our study. GRVs were monitored every 4–6 h and in the absence of a GRV > 250 ml, feed rates were advanced every 4–6 h. If there was one or more GRV >250 ml, feeds were initially reduced to a previously tolerated rate or subsequently reduced to 10–25 ml/h and prokinetic agents were prescribed. EN was stopped if GRVs were excessive (>500 ml).
Figure 1.
Feeding protocol. EN: enteral nutrition; GRV: gastric residual volume; ICU: intensive care unit; RBF: rate based feeding; VBF: volume based feeding.
Recruitment of RBF patients (cohort 1)
Data for RBF patients were collected retrospectively between January and April 2015 as part of an INS (Critical Care Nutrition, INS Study Protocol, 2014/15). Of the 48 participants recruited for the INS, 27 met the inclusion criteria for this study.
Recruitment of VBF patients (cohort 2)
Consecutive patient data were collected prospectively between March 2016 and March 2017. Patients that were established on the standard EN protocol or RBF regimen were assessed by the dietitian for VBF.
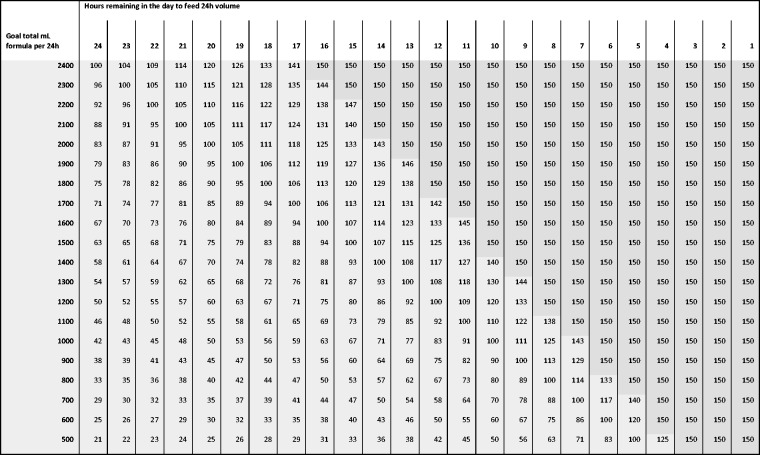
A previously reported VBF protocol18 was modified and adopted for this study, including using a maximum rate of 150 ml/h17 and the pre-calculated algorithm in which the remaining volume has been rounded to 100 ml volumes (instead of 50 ml) to simplify calculations (Figure 2). Education before, during and after the implementation of VBF protocol was provided for ICU staff by the unit dietitian.
Figure 2.
Volume based feeding schedule.
If patients subsequently developed a poor tolerance to EN, presenting with a single GRV > 250 ml, vomiting, blood glucose concentration (>18 mmol/l) or profuse diarrhoea (defined as five stools or 750 ml/24 h period), the nurses were permitted to reduce the rate back to a previously tolerated rate or to 25 ml/h, after the accepted treatments such as prokinetics for high GRV, change of enteral formula for diarrhoea or insulin treatment were unsuccessful.
Data collection
Patient characteristics, demographics, anthropometry, Acute Physiology and Chronic Health Evaluation II (APACHE II)23 score and admission details (date and type of admission, i.e. medical/surgical and aetiology) were recorded on ICU admission. The goals for requirements were determined by the unit dietitian using predictive formulas such as 25 kcal/kg and 1.2–1.5 g/kg for protein10 or Penn State equation.24
The primary outcome measures were the percentage of energy and protein requirements delivered over the patients' ICU stay and included non-nutritious energy from medications such as Propofol. Data were collected until ICU discharge, death or for a maximum of 12 days, whichever came first.
Secondary outcome measures included the number of vomiting episodes, GRV >250 ml, prokinetic use, morning and highest daily blood glucose concentrations in addition to insulin usage. Mechanical ventilation days, ICU and hospital LOS, ICU and hospital mortality were also collected for 60 days during and post ICU admission or until discharge/death.
Statistical analysis
Statistical analysis was completed using IBM SPSS version 22.0 (UK version). The power calculation was based on a similar study18 which demonstrated improvement in the delivery of EN on percentage means of energy delivered for RBF (n = 20) at 80.9% (SD = 18.9%) and VBF (n = 37) 92.9% (SD = 16.8%) of goal energy requirements (P < 0.01), with a medium to high effect size of 0.67. A priori analysis with G*Power for a two-tailed t test of the difference between these independent means (RBF versus VBF), using this effect size, and α error level of 0.05 with 80% power resulted in a sample size of 36 patients per group (total 72). The tests used to compare cohort 1 (RBF) and cohort 2 (VBF) were Mann–Whitney U for continuous variables with skewed distributions and independent t tests for normally distributed variables. Chi Square test or Fisher's Exact test were used for the categorical data as appropriate. Some differences in patient characteristics between groups were adjusted for using regression methods. Continuous outcomes were analysed using linear regression, with a log transformation performed before analysis for those outcomes with positively skewed distributions. Logistic regression was used to analyse binary outcomes. Subsequently, multiple regression was used to adjust for factors found to vary between the two groups from the initial analyses.
Results
Recruitment and demographics
A total of 82 patients met the eligibility criteria and were enrolled into the study. Twenty-seven from 48 patients were enrolled pre-VBF implementation from the INS study for the RBF group. Of the 21 patients excluded from the study, 15 required parenteral nutrition and 6 received no nutrition prior to extubation. Fifty-five out of 56 patients were enrolled for the VBF group. One patient was excluded from the VBF group after enrolment due to the development of a gastrointestinal disorder which required parenteral nutrition.
There was a significant difference in APACHE II score (RBF 23.4 versus VBF 19.4; p = 0.02), type of admission (p = 0.02) and reason for admission diagnoses (p = 0.04) between the groups (see Table 1). Surgical admissions were less common in the VBF group (9% versus 30%; p = 0.02). The majority of patients were admitted for respiratory conditions in both RBF (22.2%) and VBF (59.3%) groups. The VBF group (n = 31, 56%) had a higher number of patients with a medical respiratory diagnosis than the RBF group (p = 0.004).
Table 1.
Demographics and other baseline characteristics.
| Characteristics | Rate based feeding (n=27) | Volume based feeding (n=55) | P-value |
|---|---|---|---|
| Male sex, no. (%) | 15 (56%) | 31 (56%) | 0.95 |
| Age, median [IQR], years | 63 [51, 75] | 63 [43, 75] | 0.57 |
| APACHE II score, mean ±SD | 23.4 ± 6.4 | 19.4 ± 6.7 | 0.02 |
| Weight, median [IQR], kg | 76 [57, 90] | 68 [58, 85] | 0.37 |
| BMI, median [IQR], kg/m2 | 26.2 [24.0, 28.4] | 25.0 [21.3, 29.1] | 0.34 |
| Type of admission | |||
| Medical, no. (%) | 19 (70%) | 50 (91%) | 0.02 |
| Surgical, no. (%) | 8 (30%) | 5 (9%) | |
| Admission diagnosis Medical, no. (%) | 0.04 | ||
| Cardiovascular/vascular | 5 (19%) | 7 (13%) | 0.48 |
| Respiratory | 6 (22%) | 31 (56%) | 0.004 |
| Neurological | 5 (19%) | 8 (15%) | 0.65 |
| Sepsis | 0 (0%) | 2 (4%) | N/A |
| Other | 3 (11%) | 2 (4%) | 0.22 |
| Surgical | |||
| Respiratory | 1 (4%) | 1 (2%) | 0.59 |
| Gastrointestinal | 1 (4%) | 1 (2%) | 0.59 |
| Head and neck | 4 (15%) | 2 (4%) | 0.79 |
| Other | 2 (7%) | 1 (2%) | 0.26 |
| Estimated energy requirements mean ± SD, kcal | 1645 ± 255 | 1702 ± 279 | 0.38 |
| Estimated protein requirements median [IQR], g | 90 [76, 97] | 90 [73, 104] | 0.66 |
| Start of EN median [IQR], days | 2 [1, 2] | 1 [1, 2] | 0.01 |
| Start of VBF mean ±SD, days | 4.5 ± 2.5 | ||
| Patients with interruptions to feed, no. (%) | 26 (96%) | 53 (96%) | 1.00 |
| Interruptions to feed (h/day) | 2.7 | 2.2 | 0.77 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; BMI: body mass index; EN: enteral nutrition; IQR: interquartile range; N/A: not applicable; no.: number; VBF: volume based feeding.
Data are reported as mean ± standard deviation (SD), or median and interquartile range (IQR).
Gastric feeding occurred in most patients; only two patients had post pyloric feeding, both in the VBF group. Enteral feeding was interrupted at least once in 96% of patients for both cohorts. The primary reason for these interruptions was fasting for endotracheal intubation or extubation. The mean hours of all daily interruptions between the RBF and VBF was 2.7 versus 2.2 h per day, respectively (p = 0.233). The average time to start EN was significantly different with a median of two days (interquartile range (IQR) 1, 2) for RBF and one day (IQR 1, 2) for VBF (p = 0.01). The number of days in which patients started VBF from date of admission was 4.5 ± 2.5 days.
Primary outcome
Delivery of energy and protein
Table 2 reports the difference in energy and protein delivered between the groups. The VBF patients received a significantly greater percentage of prescribed energy, including non-nutritious energy from Propofol (82% versus 52%, p < 0.001) and protein (73% versus 40%, p < 0.001) compared to RBF patients. There was also a significant difference in percentage energy delivery from EN alone (78% versus 46%, p < 0.001). The daily mean energy and protein calculated over ≤12 days indicated that the RBF group received 11 kcal/kg and 0.4 g protein/kg in contrast to 20 kcal/kg and 1.0 g protein/kg for the VBF group.
Table 2.
Mean daily delivery of energy and protein from rate based and volume based feeding.
| Outcome | Analysis | Rate based feeding (n = 27) | Volume based feeding (n = 55) | Difference Mean (95% CI) | P value |
|---|---|---|---|---|---|
| Energy (kcal) | Unadjusted | 737 ± 282 | 1308 ± 239 | 570 (452, 689) | <0.001 |
| received | Adjusteda | – | – | 488 (318, 629) | <0.001 |
| % Energy | Unadjusted | 46.1 ± 19.7 | 77.8 ± 13.4 | 31.7 (24.4, 39.1) | <0.001 |
| requirements | Adjusteda | – | – | 25.2 (15.0, 35.5) | <0.001 |
| Energy (kcal) | Unadjusted | 826 ± 256 | 1383 ± 245 | 557 (441, 674) | <0.001 |
| receivedb | Adjusteda | – | – | 492 (327, 666) | <0.001 |
| % Energy | Unadjusted | 51.6 ± 18.6 | 82.2 ± 13.8 | 30.6 (23.3, 37.9) | <0.001 |
| requirementsb | Adjusteda | – | – | 26.2 (16.1, 36.2) | <0.001 |
| Protein (g) received | Unadjusted | 33.4 ± 14.1 | 64.7 ± 15.0 | 31.2 (24.4, 38.1) | <0.001 |
| Adjusteda | – | – | 25.3 (15.7, 34.9) | <0.001 | |
| % Protein requirements | Unadjusted | 40.1 ± 18.9 | 72.9 ± 15.0 | 32.8 (25.2, 40.5) | <0.001 |
| Adjusteda | – | – | 25.2 (14.5, 35.9) | <0.001 | |
| Energy delivered (kcal/kg) | 10.8 | 20.3 | |||
| Protein delivered (g/kg) | 0.44 | 0.95 |
Summary statistics are mean ± standard deviation or number (percentage) in each category.
Adjusted for APACHE II score, admission type, method of estimated energy requirement, time to start enteral nutrition.
Including energy from Propofol.
Secondary outcomes
Safety outcomes
After adjusting for the differences in patient characteristics, there was no significant difference in glycaemic control, units of insulin administered, episodes of GRV >250 ml and prokinetic use between the two groups (Table 3). Vomiting was higher in the RBF group, but this difference was non-significant after adjusting for confounding factors, such as APACHE II score, admission type, time to start EN and method of estimated energy requirements (p = 0.08).
Table 3.
Safety and patient outcomes.
| Outcome | Analysis | Rate based feeding (n = 27) | Volume based feeding (n = 55) | Differencea (95% CI) | P value |
|---|---|---|---|---|---|
| Glycaemic control | |||||
| Hypoglycaemic event | Unadjusted | 1 (4%) | 3 (5%) | – | 1.00 |
| Highest blood glucose | Unadjusted | 11.7 ± 3.2 | 11.6 ± 2.8 | −0.2 (−1.5, 1.2) | 0.80 |
| concentrations (mmol/l) | Adjustedb | – | – | 0.1 (−1.9, 2.0) | 0.94 |
| Morning blood glucose | Unadjusted | 8.4 ± 1.9 | 8.6 ± 1.3 | 0.2 (−0.5, 0.9) | 0.57 |
| concentrations (mmol/l) | Adjustedb | – | – | 0.5 (−0.5, 1.6) | 0.33 |
| Insulin (daily units) | Unadjusted | 4 [0, 52] | 18 [0, 53] | 1.83 (0.78, 4.34) | 0.17 |
| Adjustedb | – | – | 1.21 (0.36, 4.10) | 0.75 | |
| Gastrointestinal tolerance | |||||
| Vomiting | Unadjusted | 7 (26%) | 5 (9%) | 0.29 (0.08, 1.01) | 0.05 |
| Adjustedb | – | – | 0.21 (0.04, 1.21) | 0.08 | |
| ≥1 GRVs > 250 ml | Unadjusted | 2 (7%) | 7 (13%) | 1.82 (0.35, 9.44) | 0.47 |
| Adjustedb | – | – | 1.82 (0.18, 18.7) | 0.62 | |
| Prokinetic use | Unadjusted | 5 (19%) | 5 (9%) | 0.44 (0.12, 1.67) | 0.23 |
| Adjustedb | 0.39 (0.05, 3.04) | 0.37 | |||
| Mechanical ventilation | Unadjusted | 6 [4, 10] | 9 [6, 15] | 1.76 (1.23, 2.51) | 0.002 |
| days | Adjustedb | 1.46 (0.91, 2.35) | 0.12 | ||
| Length of ICU stay | Unadjusted | 10 [6, 15] | 11 [7, 19] | 1.24 (0.88, 1.75) | 0.22 |
| (days) | Adjustedb | – | – | 1.02 (0.63, 1.66) | 0.93 |
| Length of hospital stay | Unadjusted | 13 [10, 44] | 23 [11, 48] | 1.14 (0.75, 1.73) | 0.52 |
| (days) | Adjustedb | – | – | 0.90 (0.49, 1.64) | 0.72 |
| Mortality | |||||
| ICU mortality | Unadjusted | 3 (11%) | 10 (18%) | 1.78 (0.45, 7.08) | 0.42 |
| Adjustedb | – | – | 8.67 (0.95, 79.4) | 0.06 | |
| Hospital mortality | Unadjusted | 6 (22%) | 12 (22%) | 0.98 (0.32, 2.96) | 0.97 |
| Adjustedb | – | – | 3.64 (0.66, 20.1) | 0.14 |
GRV: gastric residual volumes; ICU: intensive care unit.
Summary statistics are mean ± standard deviation, median [interquartile range] or number (%) in each category
Difference between groups reported as mean difference (normally distributed continuous variables), ratios (skewed continuous variables) or odds ratios (binary variables).
Adjusted for APACHE II score, admission type, method of estimated energy requirement, time to start enteral nutrition.
Patient outcomes
The results demonstrated a significant difference between groups in the number of days of mechanical ventilation in the unadjusted analysis (p = 0.002), which was no longer statistically significant (p = 0.12) after controlling for APACHE II score, type of admission and time to start EN. There was no significant difference in both ICU and hospital LOS or ICU and hospital mortality.
Rates of EN infusion during VBF
The mean ‘average’ rate of infusion for VBF was 54 ml/h ± 9.0 and the mean ‘maximum’ rate was 85 ml/h ± 32.6. However, in six cases, rates were increased up to a maximum 150 ml/h with no complications observed.
Discussion
This study established that VBF can significantly increase energy and protein delivery in the first 12 days of ICU admission. These findings offer further evidence that VBF is a safe, alternative strategy in achieving target energy and protein goals in both clinical and research settings in spite of frequent interruptions to EN, intending to minimise nutritional deficits which have been associated with improving clinical outcomes.1,4,6,7 VBF has previously been used as part of a multi-strategy protocol17,20,21 and has shown to increase energy and protein delivery but it is difficult to determine if this increase was attributed entirely to VBF. Other contributing components from these studies include the routine use of protein supplementation (≥24 g protein) at initiation of EN, use of a semi-elemental or peptide feed (1.0–1.5 kcal/ml), initiation of EN at target rate, use of prophylactic prokinetics on initiation of EN and higher GRV threshold.17,20,21 While other VBF studies have also successfully improved the delivery of percentage goal energy,18,19 this is the first study to demonstrate an increase in protein delivery from VBF alone.
Previous work has demonstrated that during interrupted EN days, there was a statistically significant difference in goal energy delivered between VBF (78%) and RBF (62%) (p = 0.001).18 Our study epitomises the perpetual interruptions to EN, where 96% (n = 79) of patients experienced routine interruptions of 2.7 h per day (RBF) and 2.2 h per day (VBF), with no significant difference between the two groups. We identified various reasons for interruptions to EN during our study, primarily fasting for endotracheal intubation or extubation, in addition to medical investigations or procedures, drug administration, an inaccessible gastrointestinal tract or enteral tube displacement. The delays in extubation or possible re-intubation, resulted in EN being held for long periods and on consecutive days, leading to difficulties making up for the entirety of EN hours missed.
Observational studies on mechanically ventilated patients have demonstrated that providing at least 80% of energy25 and protein26 target was associated with improved clinical outcomes, in particular patients with a higher nutritional risk.2 However, there is currently debate on the most efficacious dose of energy and protein to optimise patient outcomes, especially in the early phase of critical illness. Current guidelines recommend 20–25 kcal/kg and 1.2–2.0 g protein per day.10,12 Although our VBF group succeeded in meeting 80% of goal energy, this did not translate into improved clinical outcomes, with the study insufficiently powered for such aspects. In addition, despite a significant increase in protein delivery, it fell short at 73% of goal protein. The barriers in providing adequate protein can be related to the additional provision of energy from non-nutritious sources such as Propofol, glucose containing infusions and citrate anticoagulation used in continuous venovenous haemofiltration,27 which often requires a reduction in energy from EN, subsequently reducing protein provision. Patients will benefit from EN formulas modified to avoid overfeeding exogenous energy and using higher protein formulas or protein supplementation together with VBF.17
This is a study exploring the delivery of energy and protein, safety and clinical outcomes of VBF, which is a relatively novel approach to EN delivery in Europe. It measures the impact of VBF on both gastrointestinal tolerance and glycaemic control. Our results suggest that VBF was delivered safely, with no significant difference in gastrointestinal tolerance, including GRV, vomiting, prokinetic use, glycaemic control and insulin use compared to RBF. The intensive monitoring of GRVs for EN tolerance is currently under question but was included as another measure of safety for this study. Holding or reducing EN is common after a GRV >250 ml, contributing to further interruptions and resulting in a reduction in the volume of EN received and an energy deficit.5 Recent research findings28,29 of patients predominantly with medical diagnoses indicate that monitoring GRVs may be unnecessary and that this, in turn, may assist in further reducing EN interruptions. This study found that GRVs were unaffected by VBF despite being perceived as more aggressive and less likely to be tolerated with potentially faster rates than RBF. Similar studies comparing VBF with RBF demonstrated no difference in gastrointestinal tolerance and pulmonary aspiration,18 ventilator acquired pneumonia and urinary tract infections.19 The anticipated concerns relating to the implementation of VBF in ICUs are the higher rates of hourly EN delivery, leading to vomiting and aspiration of EN resulting in an increase in mechanical ventilation days. Our study demonstrated that irrespective of higher respiratory diagnoses in our VBF group (n = 31, 56%) than the RBF group (n = 6, 22.2%) which also might account for the higher number of mechanically ventilated days, VBF strategy had no significant effect (p = 0.12). Our findings together with several studies17–21 suggest vomiting was also not increased (p = 0.08). This is presumably related to VBF patients in this study being selected based on having good gastrointestinal function and previously tolerating EN.
Data relating to nutritional intake and tolerance were collected from day 1 of admission up to day 12 or until discharge from ICU. We recognise that EN delivery in the early acute phase is often difficult and it remains uncertain whether VBF is a suitable strategy at admission.13 However, it is conceivable that VBF may be beneficial when patients are established on EN post-acute phase and in their recovery phase, over a longer ICU stay. The average number of days from admission to start of VBF in this study was 4.5 ± 2.5.
Our study was conducted in a mixed medical and surgical adult ICU in England, UK. The characteristics of patients were broadly representative and, as a pragmatic effectiveness study, probably represent the reality of current nutritional practice in critical care in the UK. It is notable that the mean APACHE II score for VBF and RBF patients recruited to this study was 23.4 ± 6.4 and 19.4 ± 6.7, respectively, similar to the mean APACHE II (20.5 ± 8.5) of intensive care patients in the UK.22 A similar study investigated VBF in intensive care patients in North America with median APACHE II scores of 10 (IQR 8, 16) and 17 (IQR 12, 19) for RBF and VBF groups, respectively.19 The original single centre VBF study by McClave et al.18 confirmed safe and improved energy delivery in patients with a mean Simplified Acute Physiology Score score of 21.7 ± 9.0 19.5 ± 9.3. Our UK study demonstrated that VBF can be tolerated in patients with a higher disease severity. While the practice of critical care medicine is universal in most countries, there can be differences in disease severity and populations in this already heterogeneous patient group22 and these previous VBF studies18,19 might not be generalisable to critical care populations outside North America.
Strengths of this study include a heterogeneous, adult population in a UK single centre ICU that had pre-existing, established protocols and guidelines for managing nutritional support, raised GRVs and glycaemic control, reflecting good mainstream practice.9,10,12 Despite using a convenience sample from the INS data for cohort 1, the same inclusion and exclusion criteria were used for selection for both cohorts.
The non-randomised controlled design, single centre population that had a greater representation of medical rather than surgical patients may limit generalisability. Recent studies using a multi-strategy EN protocol including VBF have demonstrated an improvement of nutrition delivery in medical patients3,17,21 but did not have the same effect in surgical patients.30 The low frequency of gastrointestinal complications for our VBF group could be due to the selection of patients that were already established on EN. Comorbidities and Nutrition Risk in Critically ill (NUTRIC) or other nutrition screening scores were not collected but might have influenced secondary outcomes such as lower mortality and faster time to discharge alive, in that patients with higher nutritional risk may benefit more from optimal provision of energy and protein compared with those with lower risk.2,31,32
Other limitations include, the small sample size and therefore, underpowered to determine statistical significance for secondary outcomes. The regular education sessions held on VBF for ICU nurses and doctors possibly heightened awareness of nutrition on the unit, contributing to better EN delivery in the VBF cohort. The patients for the two cohorts were recruited over a year apart. During that time the ICU updated its GRV threshold to 350 ml (from 250 ml) before VBF was implemented, therefore, to avoid bias, GRVs recorded by nurses at 250 ml or above were considered as ‘high’ for both groups. Protein supplementation was also introduced during this period; however, it was not routine practice. When protein supplementation was prescribed in 19% of the intervention group, nurses did not routinely administer it, therefore having little effect on total protein intake. Finally, indirect calorimetry was not available and predictive equations were used which are less reliable.33,34
In future, a more robust, adequately powered randomised controlled trial, including more surgical patients is recommended to investigate the impact of the VBF protocol on nutrition delivery. The use of body composition analysis, functional or health related quality of life measures as primary outcomes to evaluate nutrition intervention may be more suitable than mortality and infectious complications.35
In conclusion, this study described an alternative strategy to the RBF protocol. It confirmed that compared to RBF, VBF protocol can be successfully implemented to significantly enhance the delivery of EN safely, with no adverse effect on glycaemic control and gastrointestinal tolerance. However, despite this improvement, there was no beneficial effect observed on clinical outcomes, as it was underpowered to do so. This study's findings should encourage the development of a robust, adequately powered randomised controlled trial to investigate the impact of this safe VBF protocol on nutrition delivery and appropriate clinical outcomes.
Acknowledgements
We would like to thank all the staff on the intensive care unit for their support.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to thank Health Education England/National Institute of Health Research for funding this project.
References
- 1.Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med 2009; 35: 1728–1737. [DOI] [PubMed] [Google Scholar]
- 2.Compher C, Chittams J, Sammarco T, et al. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: a multicenter, multinational observational study. Crit Care Med 2017; 45: 156–163. [DOI] [PubMed] [Google Scholar]
- 3.Heyland DK, Dhaliwal R, Wang M, et al. The prevalence of iatrogenic underfeeding in the nutritionally ‘at-risk’ critically ill patient: results of an international, multicentre, prospective study. Clin Nutr 2015; 34: 659–666. [DOI] [PubMed] [Google Scholar]
- 4.Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 2005; 24: 502–509. [DOI] [PubMed] [Google Scholar]
- 5.Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr 2006; 25: 37–44. [DOI] [PubMed] [Google Scholar]
- 6.Tsai J-R, Chang W-T, Sheu C-C, et al. Inadequate energy delivery during early critical illness correlates with increased risk of mortality in patients who survive at least seven days: a retrospective study. Clin Nutr 2011; 30: 209–214. [DOI] [PubMed] [Google Scholar]
- 7.Mault J. Energy balance and outcome in critically ill patients: results of a multi-centre, prospective, randomised trial by the ICU Nutrition Study Group. J Parenter Enteral Nutr 2000; 24: S4. [Google Scholar]
- 8.Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health 2011; 8: 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reintam Blaser A, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 2017; 43: 380–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreymann KG, Berger MM, Deutz NEP, et al. ESPEN Guidelines on enteral nutrition: intensive care. Clin Nutr 2006; 25: 210–223. [DOI] [PubMed] [Google Scholar]
- 11.Dhaliwal R, Cahill N, Lemieux M, et al. The Canadian Critical Care Nutrition Guidelines in 2013. Nutr Clin Pract 2014; 29: 29–43. [DOI] [PubMed] [Google Scholar]
- 12.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. J Parenter Enteral Nutr 2016; 40: 159–211. . [DOI] [PubMed] [Google Scholar]
- 13.Arabi YM, Casaer MP, Chapman M, et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med 2017; 43: 1239–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Critical Care Nutrition. Canadian Clinical Practice Guidelines. Summary of Revisions to the Recommendations, https://www.criticalcarenutrition.com/docs/CPGs%202015/Summary%20CPGs%202015%20vs%202013.pdf (2015, accessed 28 March 2017).
- 15.McClave SA, Sexton LK, Spain DA, et al. Enteral tube feeding in the intensive care unit: factors impeding adequate delivery. Crit Care Med 1999; 27: 1252–1256. [DOI] [PubMed] [Google Scholar]
- 16.Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition. J Parenter Enteral Nutr 2015; 39: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heyland DK, Cahill NE, Dhaliwal R, et al. Enhanced protein-energy provision via the enteral route in critically ill patients: a single center feasibility trial of the PEP uP protocol. Crit Care 2010; 14: R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClave SA, Saad MA, Esterle M, et al. Volume-based feeding in the critically ill patient. J Parenter Enteral Nutr 2015; 39: 707–712. [DOI] [PubMed] [Google Scholar]
- 19.Haskins IN, Baginsky M, Gamsky N, et al. Volume-based enteral nutrition support regimen improves caloric delivery but may not affect clinical outcomes in critically ill patients. J Parenter Enteral Nutr 2017; 41: 607–611. [DOI] [PubMed] [Google Scholar]
- 20.Taylor B, Brody R, Denmark R, et al. Improving enteral delivery through the adoption of the ‘feed early enteral diet adequately for maximum effect (FEED ME)’ protocol in a surgical trauma ICU. Nutr Clin Pract 2014; 29: 639–648. [DOI] [PubMed] [Google Scholar]
- 21.Heyland DK, Murch L, Cahill N, et al. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: results of a cluster randomized trial. Crit Care Med 2013; 41: 2743–2753. [DOI] [PubMed] [Google Scholar]
- 22.Wunsch H, Angus DC, Harrison DA, et al. Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am J Respir Crit Care Med 2011; 183: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 24.Frankenfield DC, Coleman A, Alam S, et al. Analysis of estimation methods for resting metabolic rate in critically ill adults. J Parenter Enteral Nutr 2009; 33: 27–36. [DOI] [PubMed] [Google Scholar]
- 25.Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake!. Crit Care Med 2011; 39: 2619–2626. [DOI] [PubMed] [Google Scholar]
- 26.Nicolo M, Heyland DK, Chittams J, et al. Clinical outcomes related to protein delivery in a critically ill population. J Parenter Enteral Nutr 2016; 40: 45–51. [DOI] [PubMed] [Google Scholar]
- 27.New AM, Nystrom EM, Frazee E, et al. Continuous renal replacement therapy: a potential source of calories in the critically ill. Am J Clin Nutr 2017; 105: 1559–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reignier J, Mercier E, Le Gouge A, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013; 309: 249–256. [DOI] [PubMed] [Google Scholar]
- 29.Montejo JC, Miñambres E, Bordejé L, et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med 2010; 36: 1386–1393. [DOI] [PubMed] [Google Scholar]
- 30.Declercq B, Deane AM, Wang M, et al. Enhanced protein-energy provision via the enteral route feeding (PEPuP) protocol in critically ill surgical patients: a multicentre prospective evaluation. Anaesth Intensive Care 2016; 44: 93–98. [DOI] [PubMed] [Google Scholar]
- 31.Heyland DK, Dhaliwal R, Jiang X, et al. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011; 15: R268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman A, Hasan RM, Agarwala R, et al. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr 2015; 35: 158–162. [DOI] [PubMed] [Google Scholar]
- 33.Walker RN, Heuberger RA. Predictive equations for energy needs for the critically ill. Respir Care 2009; 54: 509–521. [PubMed] [Google Scholar]
- 34.Tatucu-Babet OA, Ridley EJ, Tierney AC. Prevalence of underprescription or overprescription of energy needs in critically ill mechanically ventilated adults as determined by indirect calorimetry. J Parenter Enteral Nutr 2016; 40: 212–225. [DOI] [PubMed] [Google Scholar]
- 35.Bear DE, Wandrag L, Merriweather JL, et al. The role of nutritional support in the physical and functional recovery of critically ill patients: a narrative review. Crit Care 2017; 21: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]