Abstract
This study was undertaken to examine the clinical utility of lactate clearance as an indicator of mortality in pediatric septic shock, and to compare the performance of lactate clearance at 6, 12, and 24 h for predicting in-hospital and 60-day mortality. Pediatric patients with septic shock were prospectively studied. Vital signs, laboratory values, Pediatric Risk of Mortality Score, and pediatric logistic organ dysfunction score were obtained at presentation (hour 0), hour 6, hour 24 and over the first 72 h of hospitalization. Lactate clearance was obtained at 6, 12, and 24 h of hospital admission. Therapy received, outcome parameters of mortality, and duration of hospitalization were recorded. The primary outcome variable of 60-day mortality rate was 31.25%. Only lactate clearance at 6 and 24 h was significantly associated with mortality, with odds of 0.97 (95% CI, 0.951–981; p < 0.001) and 0.975 (95% CI, 0.964–0.986; p < 0.001), respectively. Approximately there was a 24% decrease in likelihood of mortality for each 10% increase in lactate clearance at 24 h. At a threshold value of 10% 6-h lactate clearance had a sensitivity of 0.948 and specificity of 0.571, while at a threshold of 20% 24-h lactate clearance had a sensitivity of 0.922 and specificity of 0.629. The comparison of clearance at 6 and 24 h using receiver operating characteristic showed that former was “fair” (area under the curve = 0.753) and later was “good” (area under the curve = 0.81) in predicting mortality in pediatric septic shock.
Conclusion
We concluded that optimal lactate clearance in pediatric septic shock both during the early presentation and after the initial “golden hours” is associated with lower in-hospital and 60-day mortality. Further, 24-h lactate clearance appears superior to 6 h lactate clearance in predicting mortality in such patients.
Keywords: Pediatric sepsis, septic shock, lactate clearance, Pediatric Risk of Mortality Score, vasoactive-inotropic score, lactate
Introduction
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection.1 The third international consensus defined septic shock as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with an increased risk of mortality than with sepsis alone.1 Early recognition of patients at risk and aggressive treatment within the first few hours after presentation may prevent the invariable progression and poor outcome, manifested clinically by end-organ damage, failure of multiple organ systems, and death.2,3
Elevated serum lactate levels reflect the anaerobic metabolism related to cellular hypoxia and are thought to be an important marker of impaired tissue perfusion in patients with septic shock.4 Small observational studies in adults and children have demonstrated that lactate can correlate with severity of shock and prognosis in sepsis.5,6 Whilst the sensitivity and specificity of single lactate concentrations as markers of tissue hypoperfusion have been debated7,8 studies have shown that serial measurements or lactate clearance (LC) over time may be better prognosticators of organ failure and mortality.9–13 Further, studies in adults have established the use of lactate and LC as a diagnostic, prognostic, and therapeutic marker of global tissue hypoxia in sepsis and septic shock, however literature regarding its possible prognosticator role in pediatric septic shock is scanty.14–16 Further, there is no data available regarding comparison of LC at different intervals during resuscitation of pediatric septic shock. This study was designed to examine the clinical utility of LC as an indicator of mortality in pediatric septic shock, and to compare the efficiency of LC at 6, 12, and 24 h for predicting in-hospital and 60-day mortality. We also defined a cutoff for LC that is associated with improved outcome after 6 and 24 h of intensive care intervention.
Materials and methods
Study design
This prospective observational study was performed in the pediatric intensive care unit (PICU) of Department of Pediatrics at SKIMS, which is an urban, academic medical center in Srinagar. The study was conducted over a period of two years from January 2015 to December 2016. The study was approved by hospital ethical committee. Informed consents were taken from the parents/guardians of the study patients.
Participants
One hundred and twelve consecutive children in the age group of one month and 17 years, diagnosed with septic shock constituted the study group. Sepsis and septic shock were defined as per International pediatric sepsis consensus definitions.17 Included patients were admitted through emergency department and immediately shifted to PICU, where they received central venous and arterial catheterization, and were managed as per the prescribed guidelines for goal directed and stepwise management of hemodynamic support in infants and children.18 Targeted resuscitation end-points were:18
Blood pressure (systolic pressure at least 5th percentile for age: 60 mmHg <1 month of age, 70 mmHg + (2 × age in years) in children 1 month to 10 years of age, 90 mmHg in children 10 years of age or older).
Central and peripheral pulses (strong, distal pulses equal to central pulses).
Normal mental status.
Adequate skin perfusion (warm, and capillary refill <2 s).
Urine output ≥1 mL/kg/h (after effective circulating volume is restored).
Patients were intubated and mechanically ventilated as required. We excluded neonates and patients above 17 years of age, children with diagnosed hepatic disease, diagnosed cases of inborn errors of metabolism, and patients who died within 24 h of hospitalization.
Data collection and data elements
The primary outcome variable was 60-day mortality. Demographic characteristics and admission diagnosis were collected at the baseline. Glasgow Coma Scale (GCS), baseline vital signs (temperature, heart rate, mean arterial pressure, central venous pressure), arterial lactate, laboratory values, and therapy received were recorded. Severity of critical illness was assessed using the Pediatric Risk of Mortality III (PRISM III) score within 24 h of hospital admission. Organ dysfunction was assessed and followed using pediatric logistic organ dysfunction (PELOD) score at 0 (at presentation), 6, 24, 48, and 72 h while in the hospital. Vasopressor use was assessed using vasoactive inotropic score (VIS). The VIS was calculated using the following formula: dopamine (mg/kg/min) × 1 + dobutamine (mg/kg/min) × 1 + epinephrine (mg/kg/min) × 100 + norepinephrine (mg/kg/min) × 100 + phenylephrine (mg/kg/min) × 100 + vasopressin (U/kg/min) × 10,000 + milrinone (mg/kg/min) × 10.19
Lactate levels were measured on admission to PICU along with other baseline investigations and septic work-up, and repeat lactate levels were taken at 6, 12, and 24 h post-admission. Lactate measurements were done using GEM Premier 3000 Bedford (USA).
LC definition
LC (%) was defined as: lactate at presentation minus lactate at follow-up, divided by lactate at presentation, then multiplied by 100. A positive value denotes a fall in serum lactate, whereas a negative value denotes a rise in lactate
Lactate clearance = (initial lactate − follow-up lactate) / initial lactate (1)
We obtained LC at 6 h (LC0–6), 12 h (LC0–12), and at 24 h (LC0–24) of hospital admission.
Statistical analysis
The statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 20 and XLSTAT 2016. Univariate in-hospital mortality comparisons between survivors and non-survivors were done using either Student’s t tests or Wilcoxon rank sum tests for the continuous variables and either chi-square tests or Fisher’s exact tests for the categorical variables. The variables with significant univariate comparison (p < 0.05) were then included in a multivariate logistic regression model. Calibration of logistic model was done using the Hosmer–Lameshow goodness-of-fit test.20 Results were presented as percent (%), median, interquartile range (IQR), and 95th percent confidence interval (95% CI). Optimal LC cutoffs were devised using receiver operating characteristic (ROC) curve. The ROC curves were compared by using Hanley’s method.21 Kaplan–Meier estimation was done to obtain 60-day survival curves for LC below and above the devised cutoff, which were then compared using the log-rank (Mantel-Cox) test for survival data.
Results
Patient characteristics
During the two-year study period, 112 pediatric patients with septic shock met the eligibility criteria. Respiratory disease was the most common etiology, and pneumonia was the most common underlying infection, accounting for 46% of cases microbiological cultures were positive in 52 (46.4%) patients and organisms isolated were: Staphylococcus aureus 15 (28.85), Klebsiella pneumonia 10 (19.23), Streptococcus pneumonia 8 (15.38), Escherichia coli 8 (15.38), Enterococcus faecalis 6 (11.54), Enterobacter aerogens 2 (3.85), Acenetobacter bauminii 2 (3.85), and Candida albicans 1 (1.92). The primary outcome variable of 60-day mortality rate was 31.25%, and the total mechanical ventilation rate was 49.1%. Patients had a median (IQR) baseline PRISM-III score of 9 (8–16) and lactate of 6.1 (4.6–8.4) mmol/L at presentation (Table 1).
Table 1.
Baseline characteristics, therapy received, and univariate comparisons between survivors and non-survivors.
| Characteristic | All subjects (n = 112) | Survivors (n = 77) | Non-survivors (n = 35) | p value |
|---|---|---|---|---|
| Age (months), median (IQR) | 22 (8–72) | 24.5 (10.5–66) | 22.5 (7–78) | 0.12 |
| Male, n (%) | 57 (45.97) | 36 (45) | 21 (47.7) | 0.86 |
| Weight (kg), median (IQR) | 10.4 (5–18.6) | 11.6 (7.6–16) | 11.2 (5.5–18.2) | 0.82 |
| Respiratory disease, n (%) | 71 (63.4) | 53 (68.8) | 18 (51.4) | 0.40 |
| Hemato-oncologic disease, n (%) | 14 (12.5) | 8 (10.4) | 6 (17.1) | 0.38 |
| Neurologic disease, n (%) | 11 (9.8) | 5 (6.5) | 6 (17.1) | 0.18 |
| Renal disease, n (%) | 11 (9.8) | 7 (9.0) | 4 (11.4) | 0.7 |
| Gastrointestinal disease, n (%) | 5 (4.5) | 4 (5.2) | 1 (2.8) | 1.0 |
| Vital signs, median (IQR) | ||||
| GCS | 9 (6–14) | 9 (5–14) | 7 (4–13) | 0.64 |
| Temperature, ℃ | 36.2 (32.2–39.8) | 36.6 (31.8–39.1) | 37 (36.6–40.1) | 0.87 |
| Heart rate, beats/min | 165 (132–212) | 166 (130–188) | 174 (138–204) | 0.58 |
| CVP, mmHg | 3.3 (3–6.2) | 3.6 (3.2–5.5) | 4.1 (3.3–6.1) | 0.62 |
| MAP, mmHg | 36.4 (34.5–68.6) | 52.4 (38.2–66.1) | 45.5 (40.2–64.2) | 0.53 |
| PRISM-III score, median (IQR) | 9 (5–18) | 10 (6–14) | 10 (8–16) | 0.12 |
| PELOD score, median (IQR) | 14 (8.2–18) | 13 (8.5–17.8) | 14.2 (10–18.6) | 0.31 |
| Laboratory values, median (IQR) | ||||
| Hematocrit, % | 29.4 (22.2–56.6) | 32.5 (24–52.2) | 36.3 (25.4–47.4) | 0.34 |
| Total leukocyte count, per mm3 | 4.2 (2.3–19.6) | 5.5 (1.8–18.6) | 2.8 (2.2–12.4) | 0.02 |
| Platelet count, per mm3 | 110 (16–365) | 124 (36–285) | 82 (33–302) | 0.13 |
| pH | 7.21 (6.9–7.32) | 7.24 (7.0–7.28) | 7.10 (7.0–7.18) | 0.016 |
| Creatinine, mg/dL | 1.1 (0.32–3.66) | 0.9 (0.42–2.2) | 1.14 (0.66–3.8) | 0.072 |
| Total bilirubin, mg/dL | 1.12 (0.5–4.8) | 0.82 (0.44–3.6) | 1.3 (0.62–3.88) | 0.059 |
| Albumin, mg/dL | 3.2 (1.45–4.2) | 3.8 (1.8–4.4) | 2.6 (1.2–4.2) | 0.011 |
| Prothrombin time, s | 22.2 (13.4–52.4) | 18.2 (13–42) | 25 (14.3–54.6) | 0.021 |
| Duration of hospital stay, days | 17 (8.75–28) | 22 (14–31) | 11 (4.5–17) | <0.001 |
| Lactate, mmol/L, median (IQR) | ||||
| Admission | 6.1 (4.6–8.4) | 6.25 (4.75–8.1) | 5.3 (4.5–10.6) | 0.99 |
| 6 h | 2.8 (1.6–4.3) | 2.45 (1.52–4.9) | 4.5 (2.2–6.8) | 0.01 |
| 12 h | 4.2 (3.2–4.8) | 3.2 (2.2–5.3) | 3.88 (3.25–6.52) | 0.055 |
| 24 h | 3.7 (2.2–7.6) | 3.3 (1.3–4) | 4.6 (3.2–7.5) | 0.047 |
| Lactate clearance, %, median (IQR) | ||||
| LC0–6 | 57.85 (47.5–76.8) | 65.6 (49.5–76.8) | 9.39 (−9.09–77.35) | 0.01 |
| LC0–12 | 35.65 (10.1–50) | 32.7 (10.8–47.9) | 16.6 (4.5–51.5) | 0.062 |
| LC0–24 | 46.7 (24.9–71.7) | 53.4 (44.1–73.8) | 3.2 (−3.03–37.6) | 0.005 |
| Therapy during first 24 h | ||||
| VIS at 6 h, median (IQR) | 60 (10–350) | 66.6 (10–300) | 70.2 (10–320.5) | 0.089 |
| VIS at 24 h, median (IQR) | 40 (5–150) | 40.5 (0–220) | 52.6 (0–235.6) | 0.057 |
| Additional fluids, mL/kg, median (IQR) | 60 (40–100) | 55 (40–90) | 66.55 (45.5–98.6) | 0.076 |
| Mechanical ventilation, n (%) | 36 (32.14) | 36 (46.7) | 19 (54.3) | 0.72 |
| Duration of hospital stay, days | 17 (8.75–28) | 22 (14–31) | 11 (4.5–17) | <0.001 |
CVP: central venous pressure; IQR: interquartile range; LC0–6: lactate clearance at 6 h; LC0–12: lactate clearance at 12 h; LC0–24: lactate clearance at 24 h; MAP: mean arterial pressure; PELOD: pediatric logistic organ dysfunction; PRISM: Pediatric Risk of Mortality; VIS: vasoactive inotropic score.
Survivors versus non-survivors
The comparison of LC in survivors and non-survivors was statistically significant for 6 and 24 h and insignificant for 12 h (LC0–6 65.6 (49.4–76.8) vs. 9.39 (−9.09–57.57), p < 0.05; LC0–24 53.4 (40–73.8) vs. 3.2 (−3.03–37.6), p < 0.05; LC0–12 32.7 (10.8–47.9) vs. 16.6 (4.5–51.5), p = 0.06 respectively; Table 2). Univariate comparisons of age, PRISM-III, PELOD score, duration of hospital stay, serum lactate, LC0–6, LC0–12, laboratory values, and therapy received between survivors and non-survivors were performed (Table 1). There was a statistically significant difference between survivors and non-survivors for total leukocyte count, pH, albumin, LC0–6, LC0–24, and duration of hospital stay. Statistically significant univariate variables were entered into a logistic regression model. The Hosmer-Lameshow goodness-of-fit test showed that the model was well calibrated with a significant p value of 0.32. Only LC0–6 and LC0–24 were significantly associated with mortality, with odds of 0.97 (95% CI, 0.951–981; p = 0.001) and 0.975 (95% CI, 0.964–0.986; p = 0.001) respectively. Approximately there was a 24% decrease in likelihood of mortality for each 10% increase in LC at 24 h.
Table 2.
Baseline characteristics, therapy, and outcome between high and low lactate clearance groups at 6 h.
| Characteristics | Lactate clearance < 10%, n = 24 | Lactate clearance > 10%, n = 88 | p value |
|---|---|---|---|
| Age (years), median (IQR) | 22.8 (9–77) | 24.2 (10.5–66) | 0.14 |
| Vital signs, median (IQR) | |||
| GCS | 7 (4–13) | 9 (5–14) | 0.97 |
| Temperature, ℃ | 36.2 (31.3–39.3) | 36.4 (34.6–40.2) | 0.72 |
| Heart rate, beats/min | 178 (130–196) | 167 (138–194) | 0.65 |
| CVP, mmHg | 4.2 (3.3–5.4) | 3.8 (3.2–6.3) | 0.88 |
| MAP, mmHg | 48.5 (33.2–64.2) | 54.4 (37.2–66.5) | 0.76 |
| PRISM-III, median (IQR) | 9 (8–16) | 10 (6–13) | 0.67 |
| Laboratory values, median (IQR) | |||
| Hematocrit, % | 35.5 (25.2–46.8) | 32.5 (24.2–52.1) | 0.44 |
| Total leukocyte count, per mm3 | 2.4 (2.1–12.5) | 5.5 (1.9–18.6) | 0.023 |
| Platelet count, per mm3 | 76 (32–263) | 118 (36–282) | 0.058 |
| pH | 7.08 (6.9–7.18) | 7.20 (7.0–7.28) | 0.011 |
| Creatinine, mg/dL | 1.18 (0.76–3.8) | 0.9 (0.52–3.2) | 0.082 |
| Total bilirubin, mg/dL | 1.4 (0.66–3.80) | 0.88 (0.46–3.6) | 0.079 |
| Albumin, mg/dL | 2.4 (1.2–3.2) | 3.3 (1.6–4.4) | 0.015 |
| Prothrombin time, s | 26 (15.3–56.4) | 17.2 (13–46.7) | 0.014 |
| Lactate, mmol/L, median (IQR) | 4.5 (4.4–6.4) | 3.9 (3.5–5.8) | 0.130 |
| Therapy during first 72 h | |||
| Additional fluids, mL/kg, median (IQR) | |||
| 0–6 | 60 (40–100) | 60 (20–90) | 0.38 |
| 7–72 | 20 (20–40) | 10 (0–40) | 0.088 |
| VIS, median (IQR) | |||
| 6 h | 66 (18–310) | 60.5 (10–286.5) | 0.13 |
| 24 h | 55.5 (10–230) | 40 (0–210) | 0.22 |
| 72 h | 30.5 (0–150) | 20 (0–100) | 0.039 |
| Mechanical ventilation, n (%) | |||
| 0–6 | 13 | 22 | 0.075 |
| 7–72 | 8 | 10 | 0.072 |
| Outcome | |||
| PELOD score, h, median (IQR) | |||
| 0 | 14.6 (10.2–18.4) | 12.6 (10.5–17.2) | 0.24 |
| 6 | 14.5 (10.2–17.3) | 12 (10–16.6) | 0.18 |
| 24 | 13.2 (9.3–16.2) | 11.6 (8.2–14.6) | 0.63 |
| 48 | 11.4 (8.2–16.1) | 8.8 (6.8–14.2) | 0.045 |
| 72 | 10.2 (7.4–15.4) | 8.5 (6.6–13.8) | 0.056 |
| Duration of hospital stay, days | 10 (4.75–16) | 22 (12.5–31.25) | <0.001 |
| Mortality, n (%) | |||
| In-hospital | 16 (66.7) | 13 (14.8) | <0.001 |
| 60-day | 20 (83.3) | 15 (17.05) | <0.001 |
CVP: central venous pressure; GCS: Glasgow Coma Scale; IQR: interquartile range; LC0–6: lactate clearance at 6 h; LC0–12: lactate clearance at 12 h; LC0–24: lactate clearance at 24 h; MAP: mean arterial pressure; PELOD: pediatric logistic organ dysfunction; PRISM: Pediatric Risk of Mortality; VIS: vasoactive inotropic score.
Defining threshold and comparison of LC at different time intervals
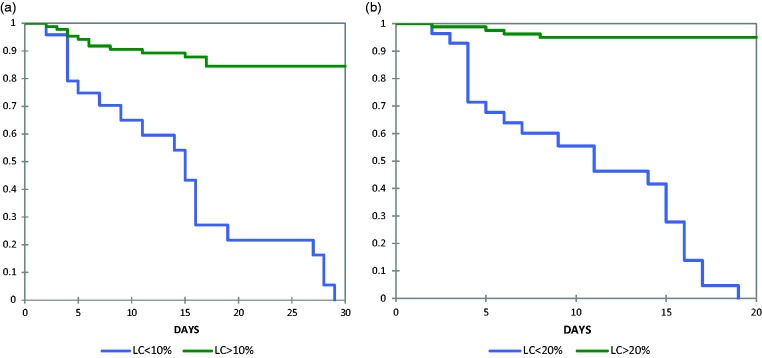
Since there was no previous study in children defining LC after 6, 12, and 24 h, we devised optimal cutoffs (thresholds) using ROC curve. An optimal LC cutoff was defined as the LC with the maximum sum of sensitivity and specificity for predicting 60-day mortality (Figure 1). Based on LC cutoff we categorized the patients into two groups: low clearance group (<10% for 6 h and < 20 for 24 h) and high clearance group (>10% clearance and > 20 for 24 h). We did not categorize 12 h LC as it was not significant between survivors and non-survivors. Both the groups were comparable in terms of baseline characteristics like age, PRISM-III score, PELOD score, vital signs, serum lactate at presentation, laboratory values, and therapy given (Tables 2 and 3). However, the high clearance group had lower in-hospital and 60-day mortality, but a longer duration of hospital stay, compared with low clearance group (p < 0.05). The comparison of LC0–6 and LC0–24 using ROC showed that former was fair (AUC = 753) while as latter was good (AUC = 0.81) in predicting 60-day mortality (Table 4).22 Kaplan–Meier estimation was used to obtain 60-day survival curves for LC below and above the threshold values (Figure 2). The comparison of the survival curves using the log-rank test for survival data was significant for both 6 and 24 h LC (p < 0.05).
Figure 1.
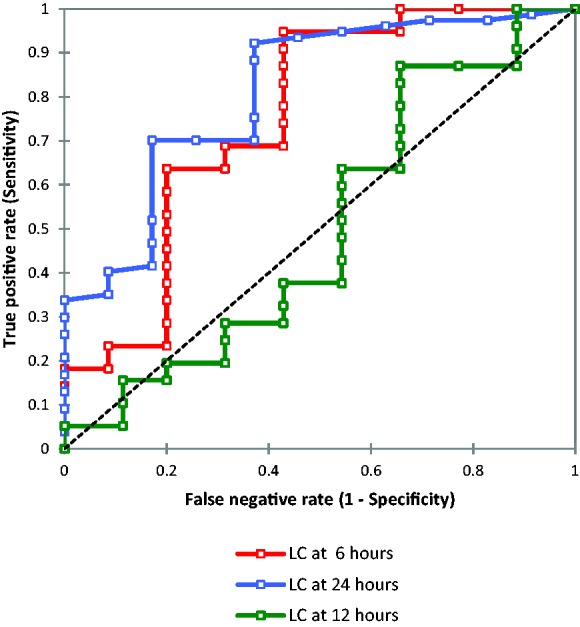
ROC curve to determine threshold level for lactate clearance at 6, 12, and 24 h.
ROC: receiver operating characteristics; AUC: area under the curve.
Table 3.
Baseline characteristics, therapy, and outcome between high and low lactate clearance groups at 24 h.
| Characteristics | Lactate clearance <20%, n = 28 | Lactate clearance >20%, n = 84 | p value |
|---|---|---|---|
| Age (years), median (IQR) | 24.8 (8–80) | 22.2 (10.5–76) | 0.11 |
| Vital signs, median (IQR) | |||
| GCS | 7 (4–13) | 9 (5–13) | 0.91 |
| Temperature, ℃ | 35.8 (31.3–39.4) | 36.2 (34.6–40.4) | 0.62 |
| Heart rate, beats/min | 182 (130–210) | 167 (139–192) | 0.23 |
| CVP, mmHg | 4.4 (3.3–5.5) | 3.6 (3.2–6.4) | 0.89 |
| MAP, mmHg | 48.5 (33–64.4) | 54.6 (37.6–66.4) | 0.74 |
| PRISM-III, median (IQR) | 9 (8–18) | 10 (7–14) | 0.45 |
| Laboratory values, median (IQR) | |||
| Hematocrit, % | 34.5 (24.4–48.8) | 32.5 (26.2–52.2) | 0.72 |
| Total leukocyte count, per mm3 | 2.6 (1.2–12.5) | 5.6 (2.8–18.2) | 0.020 |
| Platelet count, per mm3 | 66 (32–260) | 120 (36–292) | 0.12 |
| pH | 7.06 (6.9–7.18) | 7.20 (7.0–7.28) | 0.010 |
| Creatinine, mg/dL | 1.18 (0.72–3.9) | 0.9 (0.50–3.3) | 0.087 |
| Total bilirubin, mg/dL | 1.4 (0.60–3.82) | 0.88 (0.44–3.6) | 0.082 |
| Albumin, mg/dL | 2.1 (1.2–3.4) | 3.2 (2.1–4.5) | 0.011 |
| Prothrombin time, s | 26 (16–58.4) | 17.2 (13–44.5) | 0.012 |
| Lactate on admission, mmol/L, median (IQR) | 4.6 (4.2–6.6) | 4.1 (3.5–5.9) | 0.12 |
| Therapy during first 72 h | |||
| Additional fluids, mL/kg, median (IQR) | |||
| 0–6 | 60 (40–100) | 60 (20–90) | 0.42 |
| 7–72 | 40 (20–40) | 10 (0–40) | 0.054 |
| VIS, median (IQR) | |||
| 6 h | 66 (20–320) | 60 (10.4–280) | 0.21 |
| 24 h | 58.2 (10–230) | 40 (0–210) | 0.24 |
| 72 h | 28.5 (0–150) | 20 (0–105) | 0.12 |
| Mechanical ventilation, n (%) | |||
| 0–6 | 16 | 22 | 0.062 |
| 7–72 | 7 | 9 | 0.13 |
| Outcome | |||
| PELOD score, h, median (IQR) | |||
| 0 | 14.7 (10.2–18.6) | 12.2 (10.5–17.4) | 0.22 |
| 6 | 14.5 (10.2–17.4) | 12 (10–16.8) | 0.19 |
| 24 | 13.2 (9.4–16.2) | 11.4 (8.2–14.4) | 0.56 |
| 48 | 11.8 (8.4–16.2) | 8.6 (6.8–14.4) | 0.040 |
| 72 | 10.4 (7.4–15.6) | 8.5 (6.4–13.4) | 0.051 |
| Duration of hospital stay, days | 8 (4–15) | 23.5 (15–32) | <0.001 |
| Mortality, n (%) | |||
| In-hospital | 24 (85.7) | 6 (7.2) | <0.001 |
| 60-day | 28 (100) | 7 (8.3) | <0.001 |
CVP: central venous pressure; GCS: Glasgow Coma Scale; IQR: interquartile range; LC0–6: lactate clearance at 6 h; LC0–12: lactate clearance at 12 h; LC0–24: lactate clearance at 24 h; MAP: mean arterial pressure; PELOD: pediatric logistic organ dysfunction; PRISM: Pediatric Risk of Mortality; VIS: vasoactive inotropic score.
Table 4.
Comparison of lactate clearance at different time intervals.
| LC0–6 | LC0–12 | LC0–24 | |
|---|---|---|---|
| AUC | 0.753 | 0.503 | 0.810 |
| [95% CI] | 0.647–0.860 | 0.379–0.627 | 0.726–0.895 |
| Optimal cutoff | 10 | 10 | 20 |
| Sensitivity | 0.948 | 0.831 | 0.922 |
| Specificity | 0.571 | 0.343 | 0.629 |
| PPV | 0.830 | 0.736 | 0.845 |
| NPV | 0.833 | 0.480 | 0.786 |
| +LR, −LR | 2.21, 0.091 | 1.26, 0.49 | 2.48, 0.124 |
| Accuracy | 0.83 | 0.68 | 0.83 |
AUC: area under the curve; CI: confidence interval; LC0–6: lactate clearance at 6 h; LC0–12: lactate clearance at 12 h; LC0–24: lactate clearance at 24 h; LR: likelihood ratio; NPV: negative predictive value PPV: positive predictive value.
Figure 2.
Kaplan–Meier survival analysis between patients with low and high lactate clearance (a) at 6 h and (b) 24 h lactate clearance. LC: lactate clearance.
Discussion
Severity of critical illness and organ dysfunction assessed using the PRISM III and PELOD score, and lactate levels at presentation were similar in survivors and non-survivors. However, the LC0–6 and LC0–24 were higher in survivors compared to non-survivors. Our observations suggest that LC, as defined by the percentage of lactate cleared over a period of time after disease presentation, is an independent variable associated with decreased mortality rate. Assessment of the utility of serum lactate in critically ill patients has shown that both in the emergency department and in the intensive care setting, lactate levels have a role in risk-stratification.23–25 Besides serial measurements, the duration and area under the curve of increased lactate levels are related to both morbidity and mortality in different patient groups.26,27 Studies have shown that during the most proximal stage of resuscitation, lactate levels seem to be more closely related to outcome than frequently used hemodynamic measurements, including oxygen delivery and oxygen consumption.27 We observed that mortality was high in both sets of patients, those with LC0–6 of <10% and the ones with LC0–24 of <20%. However, LC0–24 was a better predictor of mortality than LC0–6 on comparison (AUC of 0.81 vs. AUC of 0.753 respectively). Several studies in adults, in severe sepsis, pointed out the value of blood LC in the first 6 h of resuscitation for the prediction of day-28 survival,6,28 but no data are available for longer duration. Few studies reported that septic patients with the lowest lactate value at 24 h, even with the same initial lactate concentration, had the highest survival rate.2,29 Some authors further observed that calculation and interpretation of LC appeared useful even after the initial “golden hours” and enabled detection of patients with a high risk of death.30 To the best of our knowledge this is the first study in pediatric setting that compares LC at different intervals, and reports superiority of LC0–24 over LC0–6 during the management of pediatric septic shock. Comparing univariate variables in multivariate logistic regression model, we observed that there was an approximately 24% decrease in likelihood of mortality for each 10% increase in LC at 24 h. However, this observation was not consistent with LC0–12. In this study LC0–12 besides being insignificant between survivors and non-survivors was poor (AUC = 503) in predicting 60-day mortality. We attributed this observation to the fact that early goal-directed therapy targeted at improving hemodynamic parameters enhances LC0–6, but increase is consistent only in those patients who have sustained improvement in perfusion at 24 h. Further, studies have shown that sustained improvement in perfusion at 24 h independently predict survival in patient with septic shock.31
Interpretation of the literature dealing with lactate in adults is complicated by variation in the choice of the “optimal” cutoff in clearance value with maximum efficiency for predicting in-hospital and 60-day mortality.5,6,9,10 In our study we defined a “cutoff” value of 10% for LC0–6 and 20% for LC0–24 using ROC curve. We observed that patients with higher LC (>10% at 6 h and >20% at 24 h) had decreased in-hospital and 60-day mortality rates. Munde et al. in their study in a pediatric setting observed that LC < 30% at 6 h predicted mortality with sensitivity of 75%, specificity of 97%, positive predictive value of 90%, and negative predictive value of 91.42%.32 They concluded that LC in first 6 h of hospitalization was related to in-hospital mortality and PRISM score.32 Choudhary et al. observed that a LC rate of <10% at 24 h had a sensitivity and specificity of 78.7% and 72.2%, respectively and a positive predictive value of 83.1% for death.33 They concluded that failure to achieve a LC of more than 10% at 24 h was associated with greater risk of mortality (likelihood ratio + 2.83; 95% CI = 1.82–4.41).33 In our study the longer duration of hospital stay in improved LC groups was attributed to the more number of survivors in these groups, and needed longer in-hospital care for complete recovery.
There are certain limitations to our study. First, it was an observational analysis whose results support an association and not necessarily causation, and does not necessarily mean that LC can be used as a therapeutic target at the studied intervals. Further the data were from a single medical center and the sample size was small, so the findings may not be generalized. Large, multicenter studies will be needed in order to confirm and generalize our results.
We concluded that optimal LC in pediatric septic shock both during the early presentation and after the initial “golden hours” is associated with lower in-hospital and 60-day mortality. Further, 24 h LC appears superior to 6 h LC in predicting mortality in such patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent
Informed consent was obtained from parents/guardians of all individual participants included in the study.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017; 43: 304. [DOI] [PubMed] [Google Scholar]
- 4.Kim YA, Ha EJ, Jhang WK, et al. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med 2013; 39: 1818–1823. [DOI] [PubMed] [Google Scholar]
- 5.Arnold RC, Shapiro NI, Jones AE, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009; 32: 35. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004; 32: 1637–1642. [DOI] [PubMed] [Google Scholar]
- 7.Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care 2013; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorgis N, Asselin JM, Fontana C, et al. Evaluation of the association of early elevated lactate with outcomes in children with severe sepsis or septic shock. Pediatr Emerg Care. Epub ahead of print 9 January 2017. doi: 10.1097/PEC.0000000000001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott HF, Brou L, Deakyne SJ, et al. Lactate clearance and normalization and prolonged organ dysfunction in pediatric sepsis. J Pediatr 2016; 170: 149–55. e1-4. [DOI] [PubMed] [Google Scholar]
- 10.Dettmer M, Holthaus CV, Fuller BM. The impact of serial lactate monitoring on emergency department resuscitation interventions and clinical outcomes in severe sepsis and septic shock: an observational cohort study. Shock 2015; 43: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertoff J, Chisum M, Simmons L, et al. Prognostic utility of plasma lactate measured between 24 and 48 h after initiation of early goal-directed therapy in the management of sepsis, severe sepsis, and septic shock. J Intensive Care 2016; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 2010; 182: 752–761. [DOI] [PubMed] [Google Scholar]
- 13.Dettmer M, Holthaus CV, Fuller BM. The impact of serial lactate monitoring on emergency department resuscitation interventions and clinical outcomes in severe sepsis and septic shock: an observational cohort study. Shock 2015; 43: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin TG, Jo IJ, Hwang SY, et al. Comprehensive interpretation of central venous oxygen saturation and blood lactate levels during resuscitation of patients with severe sepsis and septic shock in the emergency department. Shock 2016; 45: 4–9. [DOI] [PubMed] [Google Scholar]
- 15.Gorgis N, Fontana C, Asselin J, et al. 1033: is initial lactate a reliable predictor of outcome in pediatric severe sepsis and septic shock? Crit Care Med 2015; 43: 260. [Google Scholar]
- 16.Miescier MJ, Lane RD, Sheng X, et al. Association Between Initial Emergency Department Lactate and Use of Vasoactive Medication in Children With septic Shock. Pediatr Emerg Care. 2019; 35: 455–460. [DOI] [PubMed]
- 17.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6: 2–8. [DOI] [PubMed] [Google Scholar]
- 18.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009; 37: 666–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010; 11: 234–238. [DOI] [PubMed] [Google Scholar]
- 20.Hosmer DW and Lemeshow D. Applied logistic regression. Vol. 81. John Wolfley Sons, 1989, pp.8–20.
- 21.McNeil BJ, Hanley JA. Statistical approaches to the analysis of receiver operating characteristic (ROC) curves. Med Decis Making 1984; 4: 137–150. [DOI] [PubMed] [Google Scholar]
- 22.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 2007; 96: 644–647. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Xu X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2014; 42: 2118. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009; 37: 1670. [DOI] [PubMed] [Google Scholar]
- 25.Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014; 17: 76. [DOI] [PubMed] [Google Scholar]
- 26.Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med 2009; 37: 2827–2839. [DOI] [PubMed] [Google Scholar]
- 27.Jansen TC, van Bommel J, Woodward R, et al. Association between blood lactate levels, sequential organ failure assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med 2009; 37: 2369–2374. [DOI] [PubMed] [Google Scholar]
- 28.Tian HH, Han SS, Lv CJ, et al. The effect of early goal lactate clearance rate on the outcome of septic shock patients with severe pneumonia. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2012; 24: 42–45. [PubMed] [Google Scholar]
- 29.Rivers EP. Early goal-directed therapy in severe sepsis and septic shock: converting science to reality. Chest 2006; 129: 217–8. Erratum in: Chest 2006; 129: 1393. [DOI] [PubMed] [Google Scholar]
- 30.Marty P, Roquilly A, Vallée F, et al. Lactate clearance for death prediction in severe sepsis or septic shock patients during the first 24 hours in intensive care unit: an observational study. Ann Intensive Care 2013; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westphal GA, Gonçalves AR, Caldeira Filho M, et al. Guidelines for treatment of severe sepsis/septic shock: tissue perfusion assessment. Rev Bras Ter Intensiva 2011; 23: 6–12. [PubMed] [Google Scholar]
- 32.Munde A, Kumar N, Beri RS, et al. Lactate clearance as a marker of mortality in pediatric intensive care unit. Indian Pediatr 2014; 51: 565–567. [DOI] [PubMed] [Google Scholar]
- 33.Choudhary R, Sitaraman S, Choudhary A. Lactate clearance as the predictor of outcome in pediatric septic shock. J Emerg Trauma Shock 2017; 10: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]