Summary
Computed tomography is a powerful medical imaging modality for longitudinal studies in cancer to follow neoplasia progression and evaluate anticancer therapies. Here, we report the generation of a photon-counting micro-computed tomography (PC-CT) method based on hybrid pixel detectors with enhanced sensitivity and precision of tumor imaging. We then applied PC-CT for longitudinal imaging in a clinically relevant liver cancer model, the Alb-R26Met mice, and found a remarkable heterogeneity in the dynamics for tumors at the initiation phases. Instead, the growth curve of evolving tumors exhibited a comparable exponential growth, with a constant doubling time. Furthermore, longitudinal PC-CT imaging in mice treated with a combination of MEK and BCL-XL inhibitors revealed a drastic tumor regression accompanied by a striking remodeling of macrophages in the tumor microenvironment. Thus, PC-CT is a powerful system to detect cancer initiation and progression, and to monitor its evolution during treatment.
Subject Areas: Optics, Optical Imaging, Cancer
Graphical Abstract
Highlights
-
•
Development of photon-counting micro-computed tomography (PC-CT) with hybrid pixels
-
•
PC-CT allows longitudinal imaging of tumor dynamics in mouse cancer models
-
•
RTK-driven tumors are heterogeneous at onset, but grow steadily during progression
-
•
MEK + BCL-XL targeting leads to tumor regression and microenvironment remodeling
Optics; Optical Imaging; Cancer
Introduction
In vivo non-invasive longitudinal monitoring in cancer mouse models is a powerful strategy to follow over time the progression of tumors in the same animal (Anton et al., 2017). Among available methods, X-ray micro-computed tomography (CT) has gained significant interest for a series of advantages it offers (Ashton et al., 2015, Martiniova et al., 2010, Schambach et al., 2010, Wathen et al., 2013). Besides the relatively fast and easy process required, the high spatial resolution achieved (~10–100 μm) provides detailed anatomical information of tumors in the host organ. Micro-CT allows generating tomographic data that can be subsequently processed for a 3D reconstruction of the tumor area. The availability of several formulations of contrast agents has further implemented the micro-CT imaging approach in cancer animal models, permitting visualization of tumors and metastasis in different organs (Boll et al., 2011, Mannheim et al., 2016). In particular, a set of contrast agents has been developed for micro-CT imaging of tumors in the liver for mouse preclinical studies (Anton et al., 2017, Boll et al., 2011, Rothe et al., 2015, Willekens et al., 2009). Among these contrast agents, the commercially available ExiTron nano 12000 (Miltenyi Biotec GmbH, Germany), which can be injected in small volumes, exhibits high amount of X-ray absorption being an alkaline earth metal nanoparticle, thus offering excellent attenuation and contrast enhancement (Boll et al., 2013, Liu et al., 2019, Wathen et al., 2013). ExiTron nano 12000 is phagocytized and accumulated by macrophages in the spleen and Kupffer cells in the reticuloendothelial system of the liver. Kupffer cells are resident liver macrophages essential for tissue physiology and homeostasis (Krenkel and Tacke, 2017), ensuring as well host defense through phagocytosis of particles such as the contrast ExiTron nano 12000 that we used in our studies. Consequently, both spleen and liver are highly contrasted by micro-CT imaging. Anatomical evaluations of the spleen and the liver in normal and regenerative processes using ExiTron nano 12000 have been reported (Das et al., 2016, Will et al., 2017). Importantly, ExiTron nano 12000 does not cause hepatotoxicity and does not lead to pro-inflammatory cytokine release in the liver or serum, thus ensuring a relatively safe approach for longitudinal pharmacology and toxicology studies (Boll et al., 2011, Boll et al., 2013, Liu et al., 2019, Mannheim et al., 2016). This approach turned out to be very powerful for imaging primary tumors or metastases in the liver, which appear in negative because they lack contrast agents, as opposed to the liver, which is highly contrasted because of the high proportion of macrophages in the reticuloendothelial system (Bour et al., 2014, Pandit et al., 2013). However, several limitations should still be overcome, including the quality required for quantitative image processing, radiation doses, and small tumor detectability.
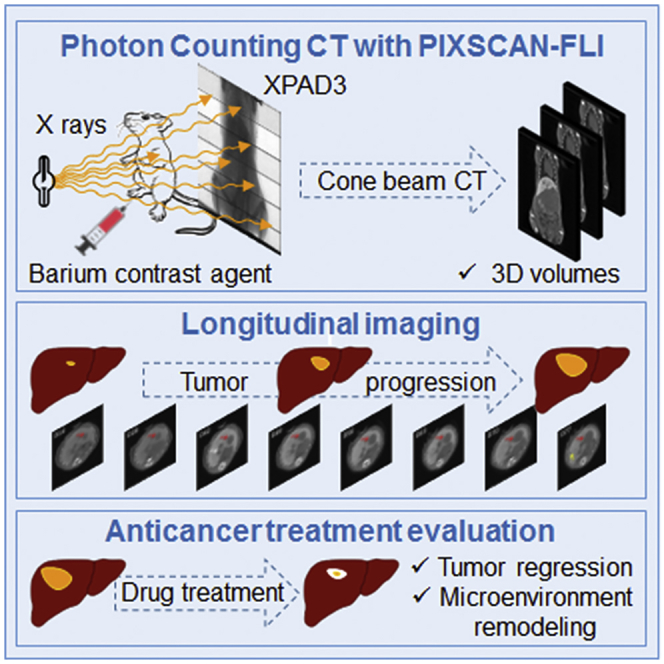
In the present study, we report the development of a micro-CT scanner prototype named PIXSCAN-FLI based on hybrid pixel detectors (Ballabriga et al., 2016, Cassol et al., 2009, Delpierre, 2014, Wermes, 2005) to perform photon-counting (PC)-CT scans of mice (Cassol et al., 2016, Taguchi and Iwanczyk, 2013). PC-CT allows reducing the required dose of radiation received by the mice, thus limiting the damaging effects after multiple scans that might affect tissue homeostasis and the mouse life. We then applied this system for longitudinal studies in a clinically relevant liver tumor mouse model, the Alb-R26Met mice. We have previously reported that Alb-R26Met mice, carrying a slight upregulation of the receptor tyrosine kinase (RTK) MET in the liver, spontaneously develop liver tumors overtime, further progressing into hepatocellular carcinoma (HCC) (Fan et al., 2015, Fan et al., 2017, Fan et al., 2019, Genestine et al., 2011, Tonges et al., 2011). Importantly, the Alb-R26Met HCC model recapitulates the “proliferative-progenitor” HCC patient subgroup (Arechederra et al., 2018, Fan et al., 2017). Therefore the Alb-R26Met liver cancer model offers the unique possibility to follow initiation, latency, and evolution of spontaneous tumors, in contrast to other preclinical systems based on single-tumor formation following experimental implantation of HCC cells in the liver of nude or syngeneic mice. We show that the PIXSCAN-FLI prototype can be used efficiently to perform qualitative analyses and quantitative measurements of spontaneous liver tumors. Furthermore, high contrast and spatial resolution was achieved with radiation doses that did not induce noticeable side effects, thus allowing longitudinal studies with multiple scans up to 3 months. We highlighted the temporal dynamics of tumor growth in normal condition, during and after combinatorial drug treatments. Combined imaging and marker analysis provided insights into a remodeling process involving macrophages in the tumor microenvironment occurring during drug-triggered tumor regression.
Results
The PIXSCAN-FLI PC-CT Prototype Permitted Long-Term Longitudinal Imaging with High Contrast and Spatial Resolution of Spontaneous Liver Tumors
We developed a micro-CT scanner prototype for non-invasive imaging of small animals named PIXSCAN-FLI (Figure S1). This scanner was equipped with a hybrid pixel XPAD3 camera (Cassol et al., 2009, Pangaud et al., 2007). Hybrid pixels consist of a pixelated sensor wherein each pixel is physically bound to its readout electronics integrated in a chip. Their principal innovative characteristic relies on the digital conversion of the X-ray photons that give a signal higher than a predefined threshold. Hence, hybrid pixels provide PC instead of integrating the overall signal charge generated by the X-ray beam. This feature allowed a linear detector response free from electronic noise (Cassol et al., 2009), thus minimizing the X-ray flux and the dose delivered to the animal (Cassol et al., 2016). We optimized the scanner acquisition parameters (50 kV/500 μA, 0.6 mm Al filtering, 720 projections) to reach a low radiation dose (180 mGy/scan), thus preventing radiobiological effects along repeated scans and maximizing contrast-to-noise ratio observed for a contrast phantom QRM-microCT-HA (QRM GmbH, Germany). Notably, the PIXSCAN-FLI prototype allows performing a full-body scan of adult mice, in contrast with other micro PC-CT scanners. For in vivo imaging, mice were placed in vertical position on the PC-CT rotation table (Figure S1) and remained under isoflurane anesthesia during the whole scan, which lasted for 7.5 min.
The PIXSCAN-FLI prototype was used for longitudinal PC-CT imaging of liver tumors. For this purpose, the Alb-R26Met liver cancer genetic model was particularly appropriate, as spontaneous tumor formation and progression to HCC occurs in these mice, with a frequency ranging from 42% to 79% at the age of 40–48 weeks and >67 weeks, respectively (Fan et al., 2017). Consequently, the Alb-R26Met model allows detecting endogenous tumors at initiation stages and following their progression overtime, in contrast to other models previously used for longitudinal micro-CT imaging based on the HCC cell implantation into the liver. Small tumor detectability is affected by the noise of the system (Cassol et al., 2016, Hanson, 1979), which in the case of PC is only related to the counting statistics, and hence to the delivered dose. Moreover, it depends on the intrinsic contrast of the lesion and on its dimension. Therefore, to improve the detectability of small tumors, we used a contrast agent, which increased the intrinsic contrast between the tumors and the healthy liver tissue. To maximize visualization and detection of soft tissues in parenchymal organs, we selected the ExiTron nano 12000, a long-lasting contrast agent based on barium nanoparticles. This approach ensures great parenchymal contrast in the liver and spleen as the high metal load particles are taken up by macrophages. ExiTron nano 12000 increased by a factor of 5 the liver tissue X-ray attenuation coefficient, with a maximum tissue contrast 24 h after a 4 μL/g mouse of injected agent (Figure S2A). With a single injection, we were able to perform longitudinal imaging for 4 to 6 weeks, depending on the animal. As we performed imaging analyses for longer time (up to 84 days), a second injection of ExiTron nano 12000 contrast agent was required. After a second injection, in livers we observed a contrast deviation, coherent with an additional accumulation of the contrast agent in Kupffer cells (Figure S2B). Unlike, no significant variations were observed in tumors and the contrast was stable over time (Figure S2C).
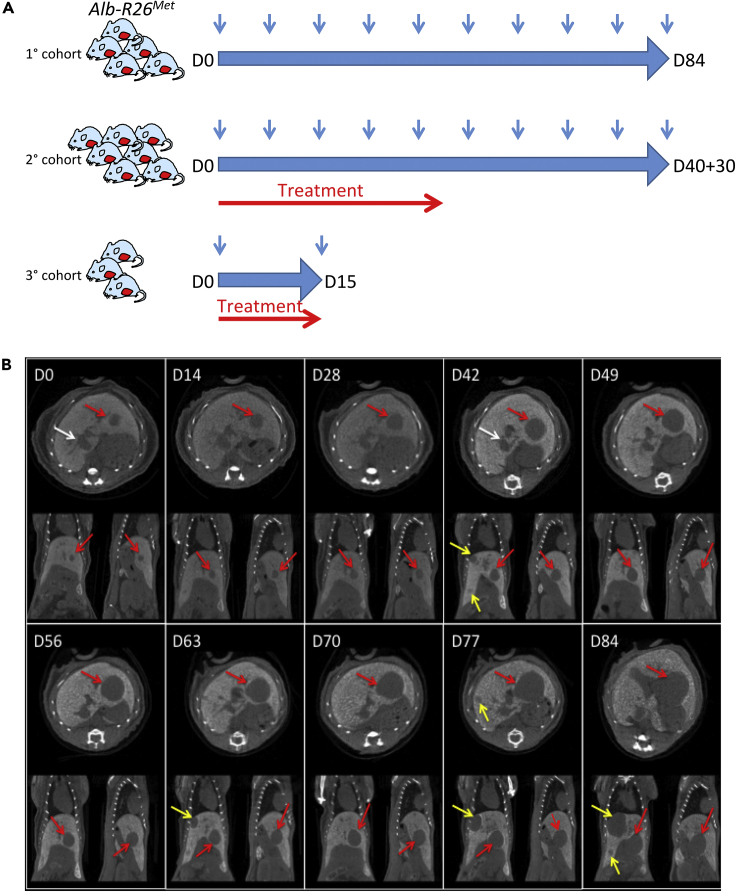
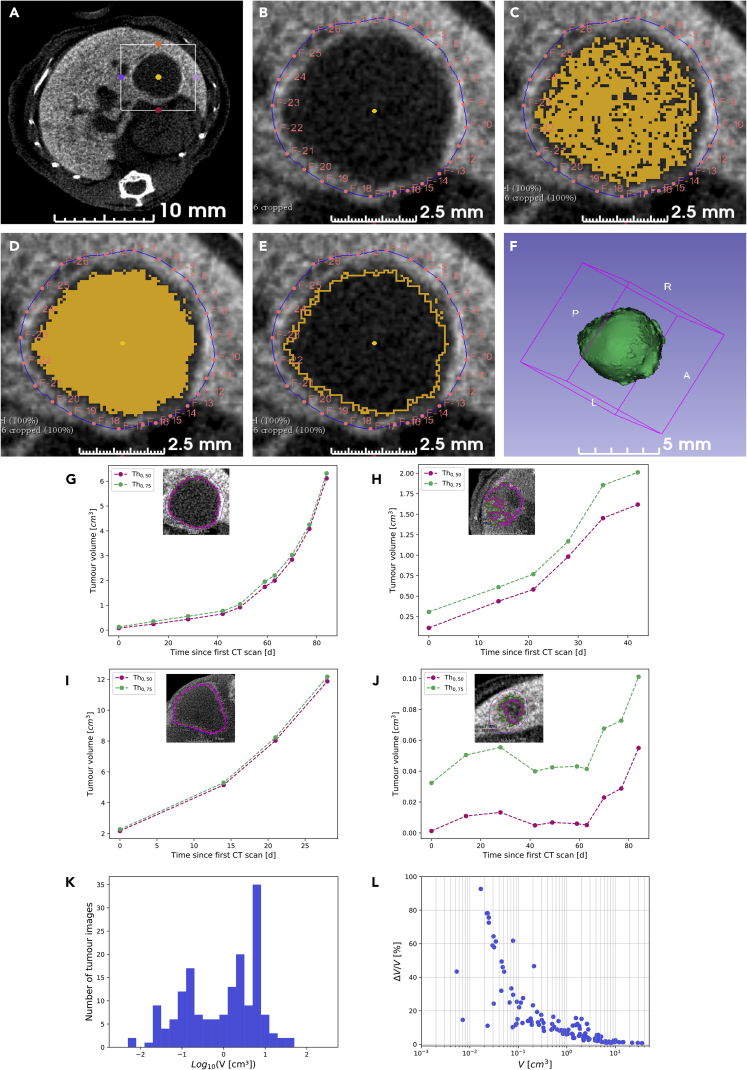
The mouse cohorts used in this study and the overall described procedures are illustrated in Figure 1A (see Table S1 for details). A first cohort of six Alb-R26Met mice carrying liver tumors was selected for longitudinal imaging to follow the evolution over time of a total of 15 tumors of different size. PC-CT images acquired with the PIXSCAN-FLI prototype allowed clear detection of neoplastic areas, which appeared without contrast agent with respect to the liver tissue (Figure 1B). Next, we performed a series of processing steps of acquired images for qualitative and quantitative analyses. To quantify the tumor volume, we elaborated a semiautomatic segmentation protocol using the open software 3D Slicer. This segmentation protocol is based on contrast thresholds, in Hounsfield units (HU), defined relative to the liver CT value (HUL) and the tumor CT value (HUT): Thq = HUT + q (HUL- HUT), with 0 < q < 1. The liver CT value was measured at each scan, being variable with time, whereas the tumor value was constant and assumed equal to 130 HU, which corresponds to the liver CT value without contrast agent, as estimated in a control mouse sample (Figures S2B and S2C). After a manual selection of the region including the tumor (Figures 2A–2F), the volumes V0.50 and V0.75 below the thresholds at q = 0.50 and q = 0.75, respectively, were automatically extracted using the segmentation tools provided by 3D Slicer (Figures 2G–2J). These tools permitted to select pixels below a given threshold (Threshold Effect) and to smoothen the segmented volume by discarding isolated pixels and connecting adjacent segmented regions (Remove Island Effect; Figures 2A–2F). The threshold at q = 0.5 corresponds to the mid-CT value between healthy high-contrasted liver tissue and tumor tissue, which can be considered as the natural threshold to select between the two densities. However, as the tumor boundaries appeared to be slightly irregular, as illustrated by a partial inclusion of the contrast agent, we have also considered a more inclusive threshold at q = 0.75. The volumes V0.75 and V0.50 differed significantly for small tumors only, which appeared less uniform than bigger tumors, furthermore, with an unfavorable surface to volume ratio (Figure S3). Then, the volume was defined as V = (V0.75 + V0.50)/2 and the volume uncertainty as ΔV = (V0.75 – V0.50)/2, which actually reflects the tumor contrast inhomogeneity, hence the uncertainty associated to the volume estimation based on the threshold method. We were able to detect tumors starting from about 2.5 mm diameter (corresponding to 0.5 × 10−2 cm3 of tumor volume; Figure 2K). The volume uncertainty was larger for small tumors (Figure 2L), which were less contrasted (Figure 2J), although it decreased below 20% for volumes higher than 0.1 cm3.
Figure 1.
Longitudinal In Vivo PC-CT Imaging of Spontaneous Liver Tumor Dynamics in the Alb-R26Met Model
(A) Schematic representation of longitudinal in vivo imaging studies that we performed to follow tumor initiation and evolution over time in the Alb-R26Met mouse model. Three cohorts were used for imaging of untreated mice (1° cohort), mice treated with drug combinations (2° cohort), and mice with a short treatment with drug combinations to perform histological studies (3° cohort).
(B) Example of longitudinal monitoring of liver tumor growth in an Alb-R26Met mouse (M1) with the PIXSCAN-FLI PC-CT prototype. For this representative mouse, image monitoring was performed at D0, D14, D28, D42, D49, D56, D63, D70, D77, and D84. For each day of monitoring, transversal (top), coronal (left bottom), and sagittal (right bottom) slices are shown. The red arrows indicate tumor already detected at D0. The white arrows at D0 and D42 point to the venae cava. The yellow arrows indicate additional spontaneous tumors detected starting from D42.
See also Figures S1 and S2.
Figure 2.
Establishment of a Processing Protocol for Quantitative Measurement of Tumor Volume
(A–F) Images exemplifying the semiautomatic segmentation protocol we followed for tumor volume measurements. The region of interest enclosing the tumor to analyze for quantifications (A) was processed with manual demarcation of the area of segmentation by means of red fiducial markers (B) and application of the predefined contrast threshold to select voxels with a value inferior to the contrast threshold (C). Morphological smoothing to connect the area of voxels was then applied (D), and segmented area was defined according to the predefined contrast threshold (E). Images corresponding to the whole tumor were processed, and values were merged for a 3D reconstruction of the tumor volume (F).
(G–J) Graphs reporting representative examples of longitudinal follow-up of tumor volume growth, corresponding to the largest tumor reported in Figure 1B (G; tumor M1-T4), a medium tumor (H; tumor M5-T1), a big tumor (I; tumor M4-T1), and a small tumor (J; tumor M1-T3). Tumor volume measurements are shown for the two defined contrast thresholds, Th0.50 (pink) and Th0.75 (green). Images shown in each panel correspond to transverse slices at the tumor maximal diameter acquired at D49 (G and J), D21 (H), and D15 (I); the two-contrast thresholds applied are reported on images.
(K) Histogram of the overall measured tumor volumes, defined as V = (V0.50 + V0.75)/2.
(L) Graph reporting the tumor volume uncertainty, defined as ΔV = (V0.75 - V0.50)/2. Note that major uncertainties were observed mainly for tumors with a volume less than 10−1 cm3.
See also Figure S3.
Long-Term Longitudinal PC-CT Imaging Applied to the Alb-R26Met Genetic Model Revealed Distinct Kinetics in Liver Tumor Growth at Initiation and Evolution Phases
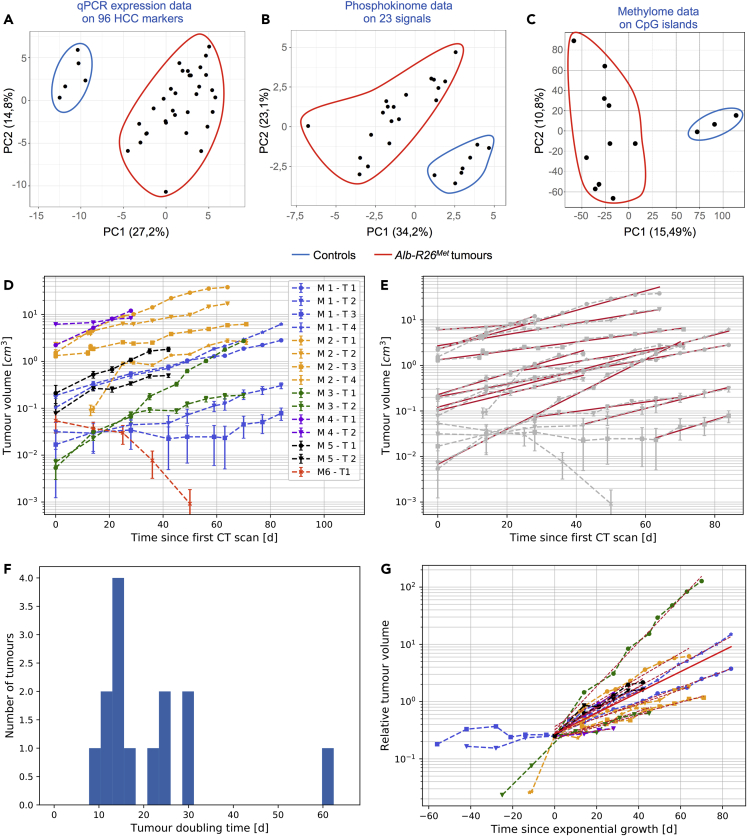
As reported above, the Alb-R26Met HCC model corresponds to the “proliferative-progenitor” HCC patient subgroup (Arechederra et al., 2018, Fan et al., 2017). We revisited a set of data from the Alb-R26Met tumors related to (1) qPCR expression of 96 HCC markers (Fan et al., 2017), (2) phosphokinome analysis of 23 signals (Fan et al., 2017), and (3) methylome of CpG islands (Arechederra et al., 2018). These analyses revealed that even though the Alb-R26Met tumors clearly segregate from control livers, they belong to a broad cluster (Figures 3A–3C). This may reflect the heterogeneity reported among patients with HCC even when belonging to the same subgroup (Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm.edu; Cancer Genome Atlas Research Network, 2017, Llovet et al., 2016, Zucman-Rossi et al., 2015). We therefore reasoned that the Alb-R26Met cancer model could be an appropriate system to evaluate the dynamics of tumors from the initiation stage toward their progression.
Figure 3.
Molecular Heterogeneity and Growth Dynamics of Liver Tumors Modeled in the Alb-R26Met Mice
(A–C) Graphs reporting Principal Component Analysis (PCAs) of control livers and Alb-R26Met tumors using qPCR expression data on 96 HCC markers (A), phosphokinome data on 23 signaling proteins (expression and phosphorylation levels; B), and methylome data on CpG islands (C). Note that tumor samples, distinct from the control samples, form a wider cluster, recapitulating the molecular heterogeneity observed in HCC patient subgroups.
(D) Graph reporting longitudinal quantitative measurements of tumor volumes for the 15 monitored tumors in analyzed mice (see Table S1). Tumor volume was determined according to the semiautomatic protocol illustrated in Figure 2. Tumors with the corresponding mice are indicated on the right. Bars are the maximal volume variation estimated as the half of the difference between volumes calculated for the contrast thresholds Th0.50 and Th0.75.
(E) Graph reporting the exponential growth modeled by V(t) = V0exp(λt), λ being the tumor growth constant. In (D and E) note the spontaneous partial regression of tumor M6-T1.
(F) Histogram of the tumor doubling time for the 15 tumors analyzed by longitudinal measurement. The tumor doubling time was calculated as τD = ln2/λ. The median of distribution amounts to 15.9 ± 5.3 days.
(G) Graph reporting the relative tumor volume since exponential growth (time zero in the graph).
In (D and E) the data are defined as V = (V0.50 + V0.75)/2 and ∆V = (V0.75 – V0.50)/2, represented as mean ± SEM.
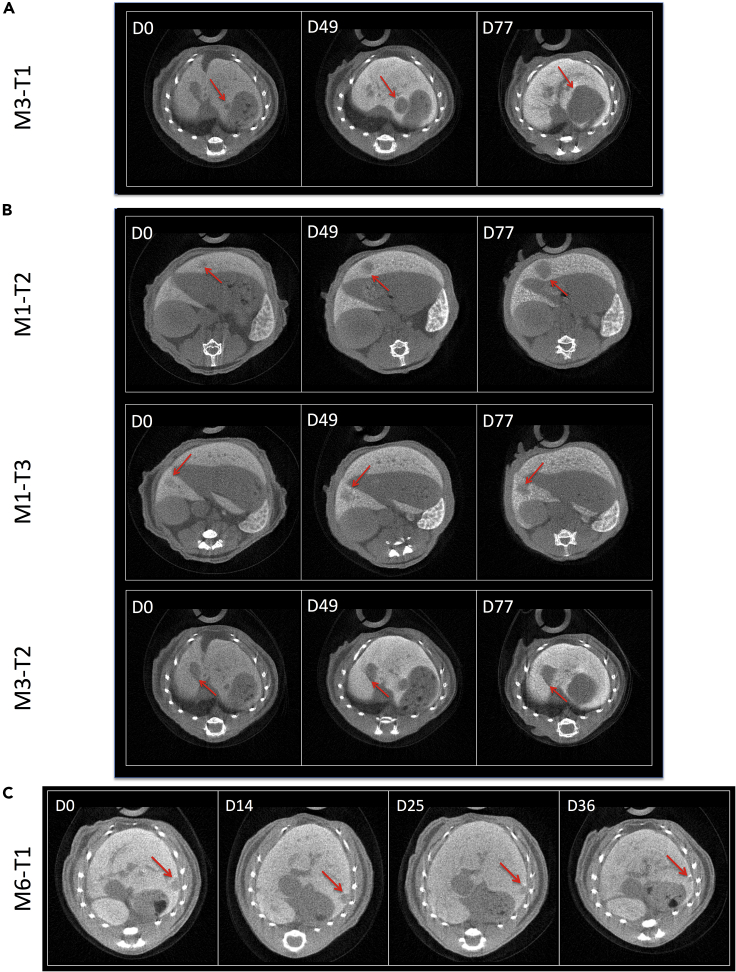
We processed imaging data to establish the kinetics of growth of each individual tumor in Alb-R26Met mice (Figure 3D). Concerning tumors above 10−1 cm3, we observed a comparable trend in tumor volume increase over time, corresponding to an exponential growth with a tumor doubling time ranging from 10 to 30 days (Figures 3E–3G). The only exception was M4-T2, a rather big tumor (0.5 × 101 cm3 at day 0), for which the doubling time was about 60 days. Overall, the median tumor doubling time amounted to 15.9 ± 5.3 days. Concerning small tumors (below 0.5 × 10−1 cm3 at day 0), their behavior over time was rather heterogeneous. Of five tumors we observed an increase in size in one tumor since its detection (M3-T1), whereas for the other four tumors the size remained unchanged for several weeks (Figures 3D and 4). Intriguingly, whereas M1-T2, M1-T3, and M3-T2 tumors started to grow after about 42, 63, and 28 days, respectively, we observed a spontaneous partial regression of M6-T1 tumor (Figures 4B and 4C). Spontaneous regressions of liver tumors are occasionally observed in patients (Saito et al., 2014).
Figure 4.
Detection of Liver Tumors Starting from 0.5 × 10−2 cm3 of Tumor Volume and Assessment of Their Evolution with the PIXSCAN-FLI PC-CT Prototype
(A) Tumor with fast progressive growth in size.
(B) Tumors with very slow progression in size.
(C) Tumor with spontaneous partial regression.
Longitudinal PC-CT Imaging Provided Insight on Anticancer Treatment Effects and the Associated Modifications of the Tumor Environment
Next, we aimed at assessing through longitudinal imaging the dynamics of tumor evolution during anticancer drug treatments. We have previously reported that the Alb-R26Met HCC model recapitulates resistance to sorafenib (Fan et al., 2017), a multispecific kinase inhibitor approved as a first-line therapy for HCC (Llovet et al., 2008, Xie et al., 2012). Combining phosphokinome screen outcomes with bioinformatics and with an “educated guess” drug screen, we previously identified new synthetic lethal interactions. One of those involved the combined inhibition of MEK and BCL-XL (Fan et al., 2017). This drug combination treatment is effective on Alb-R26Met HCC cells both in vitro and in xenografts of nude mice (Fan et al., 2017). Therefore, we first performed PC-CT imaging on a group of mice to identify a cohort of six Alb-R26Met mice carrying endogenous liver tumors (for a total of seven tumors), which were then treated with the MEK plus BCL-XL inhibitors. We carried out longitudinal imaging over a period of 40 days during which mice were treated with this drug combination (Figure 5A). Overall, we gathered a complete set of data corresponding to five tumors in four mice, as two mice died before the treatment was completed, likely due to the overall complexity of the manipulation (Table S1). Qualitative and quantitative analyses revealed a progressive regression of tumor volume in Alb-R26Met-treated mice (Figures 5B and 6). Remarkably, the tumor volume regressed about 80% as soon as after 10 days of treatment, reaching a regression between 92% and 100% at the end of treatment (Figures 5B and 6). Concerning tumor M10-T1, with a volume of 0.15 × 102 cm3 before treatment (day 0), combined MEK and BCL-XL inhibition blocked its progression, with a 35% reduction 10 days after treatment and then stabilized at 15% in subsequent measurements (Figures 6A–6C). Intriguingly, at the first time point of imaging after treatment (D10), we observed a hyper-contrasted zone inside the tumor and in the surrounding region, which became more pronounced at D20 and remained intense until the end of the treatment (Figures 5B and 6; orange volume in 6B and C). The corresponding signal quantification revealed an apparent correlation between the hyper-contrasted volume and the amplitude of tumor regression (Figures 6B and 6C). Collectively, these results showed how PC-CT longitudinal imaging allows quantitative evaluations of drug effects on endogenous tumors. Moreover, our findings revealed that MEK + BCL-XL inhibition is effective not only on HCC xenografts in nude mice (Fan et al., 2017) but also on endogenous tumors in Alb-R26Met mice.
Figure 5.
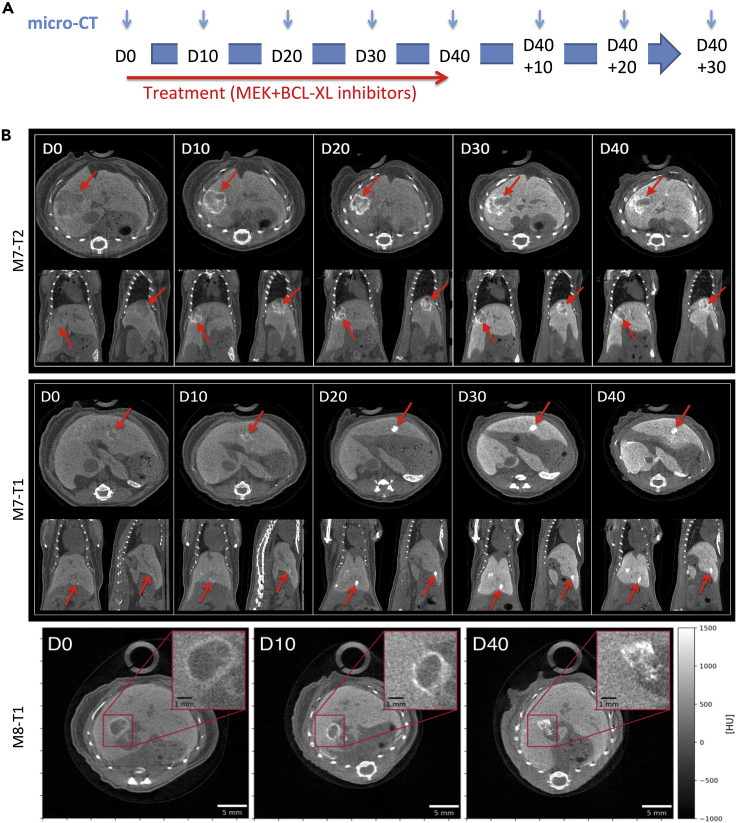
Longitudinal PC-CT In Vivo Imaging of Liver Tumor Dynamics in Alb-R26Met Mice Treated with a Combination of MEK and BCL-XL Inhibitors
(A) Schematic representation of the longitudinal in vivo imaging studies that we performed in Alb-R26Met mice to measure tumor response following MEK and BCL-XL targeting.
(B) Examples of longitudinal monitoring of liver tumor regression in Alb-R26Met mice treated with MEK and BCL-XL inhibitors. Three tumor examples are reported: tumors M7-T2, M7-T1, and M8-T1. For each day of monitoring, transversal (top), coronal (left bottom), and sagittal (right bottom) slices are shown. The red arrows indicate the tumor during treatment. Once treatment started, note a hyper-contrasted area appearing already at D10 inside and around the tumor, with an enhanced intensity at later time point of treatment. The insets in the lower panels show an enlargement of the tumor, illustrating the highly contrasted area.
Figure 6.
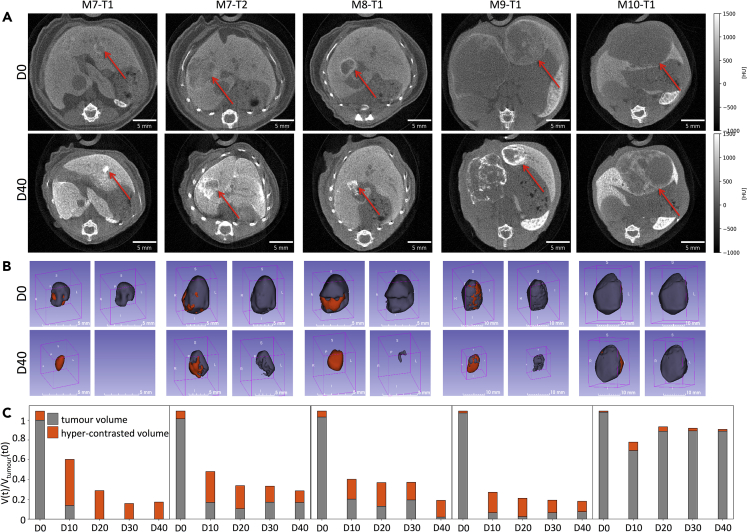
Qualitative and Quantitative PC-CT Measurements of Alb-R26Met Liver Tumors in Mice Treated with MEK and BCL-XL Inhibitors
(A) Images corresponding to transverse slices at maximum diameter of five independent tumors before (D0) and at the end of the treatment with MEK + BCL-XL inhibitors (D40).
(B) 3D reconstructions of tumor volumes before (D0) and at the end of the treatment with MEK + BCL-XL inhibitors (D40). On the left, tumor (gray) and high-contrasted (orange) volumes are reported. On the right, images correspond to tumor volumes (gray).
(C) Graphs reporting longitudinal imaging measurements of tumor (gray) and high-contrasted (orange) volumes before (D0) and during drug treatments. Note the dramatic reduction of tumor volumes after treatment in four tumors (M7-T1, M7-T2, M8-T, M9-T1). For a big tumor (M10-T1), treatment stopped its growth, with only a slight regression during treatment. In this tumor, a very small high-contrasted area was observed.
Next, we extended PC-CT longitudinal imaging studies with the PIXSCAN-FLI prototype to evaluate the dynamics of tumors in Alb-R26Met mice once treatment was stopped (Figure 5A). Concerning M7-T1 tumor, we found a complete regression and no relapse was observed even 30 days after discontinuation of treatment (Figures 7 and 8). Concerning the other four tumors, in which regression was not complete after treatment, we observed a progressive increase in tumor volume over time after cessation of treatment, although with different kinetics (Figures 7 and 8). M8-T1 and M9-T1 tumors did not reach the volume observed before beginning of the treatment. In contrast, 30 days after discontinuation of the treatment, the M7-T2 tumors has returned to their original value (measured before treatment), and M10-T1, the less responding tumor, rapidly increased in volume (Figure 8). Concerning the hyper-contrasted region inside the tumor and in the surrounding area, we recorded a progressive decrease of its volume when tumor started to relapse (Figure 8; orange volume in 8B and C). Together, these longitudinal in vivo imaging analyses performed over a 70-day period spanning an initial treatment phase followed by a resting phase, exemplify the relevance of evaluating treatment effectiveness in preclinical assays with a powerful imaging system like the PIXSCAN-FLI PC-CT prototype. Of note, this equipment offers the possibility of performing multiple measurements over time due to the limited X-ray dose used for the imaging session.
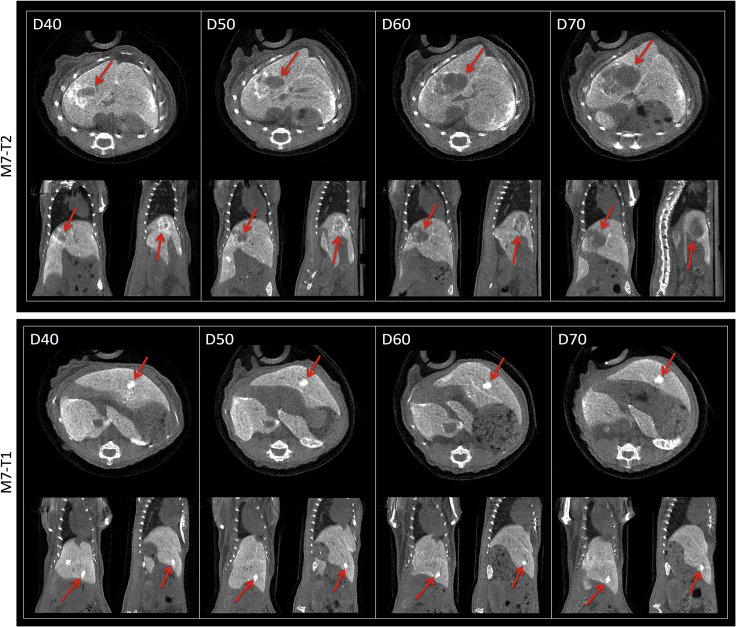
Figure 7.
Longitudinal PC-CT In Vivo Imaging of Liver Tumor Dynamics in Alb-R26Met Mice Once Treatment with Targeted Drug Combination Was Discontinued
Examples of longitudinal monitoring of liver tumor growth in Alb-R26Met mice from the last day of treatment (D40) over time (D50, D60, D70). M7-T2 tumor started to grow (top), whereas M7-T1 tumor growth was not observed and a high-contrasted area persisted over time. For each day of monitoring, transversal (top), coronal (left bottom), and sagittal (right bottom) slices are shown. The red arrows indicate the corresponding tumor. Note that these two tumors recorded in the same mouse were both sensitive to the treatment, although the M7-T2 relapsed, whereas the M7-T1 did not.
Figure 8.
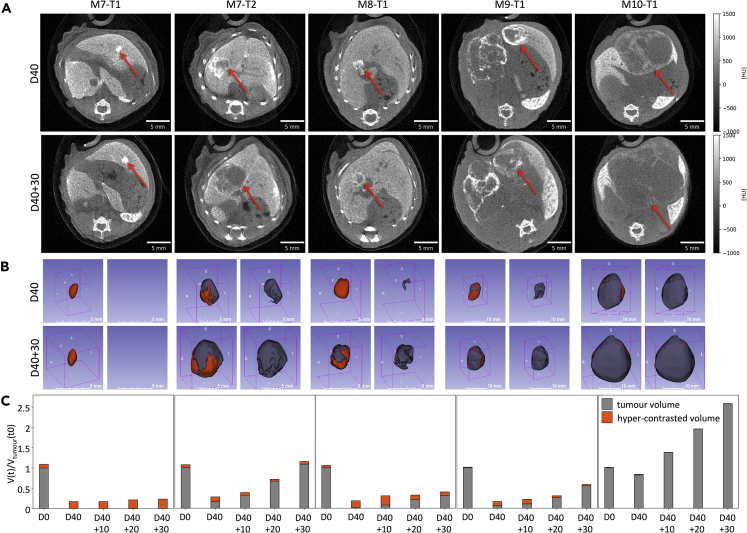
Qualitative and Quantitative PC-CT Measurements of Alb-R26Met Liver Tumors in Mice after Treatment with Targeted Drug Combinations
(A) Images corresponding to transverse slices at tumor maximum diameter of five independent tumors at the end of treatment (D40) and 30 days later (D40 + 30).
(B) 3D reconstructions of tumor volumes at D40 (end of treatment) and after 30 days (D40 + 30). On the left, tumor (gray) and high-contrasted (orange) volumes are reported. On the right, images correspond to tumor volumes (gray).
(C) Graphs reporting longitudinal imaging measurements of tumor (gray) and high-contrasted (orange) volumes at D0 (before treatment), at the end of treatment (D40), and during the following 30 days. Complete regression was observed for M7-T1 tumor, whereas regrowth occurred for the other four tumors, although with a variable speed and kinetics. Images of tumors at D40, already shown in Figure 5A, are also shown here for direct comparison with tumor images at D40 + 30.
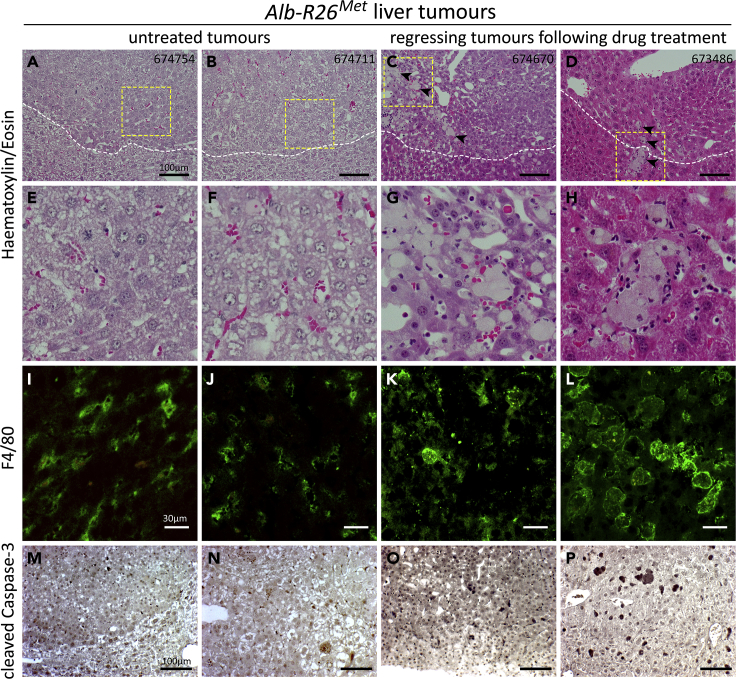
As reported above, our imaging studies highlighted that regression of tumors during drug treatment was accompanied by the appearance of highly contrasted zones within the tumor and in the surrounding region. As ExiTron nano 12000 is phagocytized and accumulated by macrophages, we hypothesized that these highly contrasted zones could correspond to accumulation of macrophages in the environment of these regressing tumors. We explored this possibility by performing histological studies of Alb-R26Met tumors during the drug-driven regression phase. In particular, a third cohort of tumor-bearing Alb-R26Met mice were treated for 15 days with MEK plus BCL-XL inhibitors, imaging was performed before (D0) and at the end of the treatment (D15), then mice where sacrificed for histological analyses. H&E staining of Alb-R26Met liver tumor sections confirmed the presence of HCC and revealed an enrichment of macrophage hyperplasia in the microenvironment of regressing tumors in contrast to tumors from untreated mice (Figures 9A–9H). Immunohistological analyses using anti-F4/80 antibodies, which recognize macrophages, evidenced the presence of highly enlarged macrophages surrounding the regressing tumors in Alb-R26Met-treated mice in contrast to tumors from untreated animals (Figures 9I–9L). Anti-cleaved Caspase-3 immunostaining revealed the presence of dying cells in tumors from Alb-R26Met-treated mice in contrast to untreated animals (Figures 9M–9P). Thus, highly contrasted regions in the microenvironment of regressing tumors, revealed by PC-CT imaging, correspond to an enrichment of highly enlarged macrophages.
Figure 9.
Enlarged Macrophages Accumulate in the Microenvironment of Alb-R26Met Regressing Tumors during MEK plus BCL-XL Inhibition
(A–H) H&E staining of tumor sections from Alb-R26Met mice either untreated (A, B, E, and F) or treated with MEK and BCL-XL inhibitors (C, D, G, and H). In (C and D) note extensive peritumoral macrophage hyperplasia (arrowheads) surrounding locally invasive well-differentiated HCC cells. The dotted lines depict the border between the tumor and the normal parenchyma. (E–H) High magnifications of the area indicated by the yellow squares in (A–D).
(I–L) Immunohistological staining showing anti-F4/80-positive macrophages in the peritumoral area of tumors. Note highly enlarged macrophages in the microenvironment of drug-driven regressing tumors (K and L) compared with tumors from untreated Alb-R26Met mice (I and J).
(M–P) Immunohistological staining with anti-cleaved Caspase-3 in the peritumoral area of untreated (M and N) and treated (O and P) Alb-R26Met mice.
The mice IDs are indicated on the top right corners of (A–D). Scale bars are indicated.
Discussion
Hybrid pixel technology was originally invented for charged particle tracking in high-energy physics experiments. At the end of the nineties, this technology started to be applied also to X-ray imaging both for material science and biomedical imaging (Delpierre, 2014, Wermes, 2005). In this study, we exploited for the first time the hybrid pixel camera XPAD3 for in vivo imaging in a biomedical context. The PC-CT PIXSCAN-FLI prototype, equipped with a XPAD3 camera, permitted to provide optimized imaging acquisition parameters (50 kV/500 μA, 0.6 mm Al filtering, 720 projections) and to reach a good compromise between tumor detectability and the radiation dose. Indeed, the achieved dose of 180 mGy per scan allowed performing longitudinal studies over several months without observing any major consequences in mice. Moreover, the short acquisition time (7.5 min/mouse) was compatible with a simultaneous follow-up of a relatively large mouse cohort. For future developments, the use of CdTe hybrid pixels instead of Si hybrid pixels (Buton et al., 2014) would allow to reach similar imaging results with a reduced dose of about 60 mGy, benefitting of a much higher X-ray detection efficiency of the CdTe sensors (Cassol et al., 2015). Concerning detectability of small objects such as tumors in animal models, a critical factor is the intrinsic contrast and the image noise. Image noise could be reduced at the photon statistics level by the PC property of the XPAD3 camera, whereas optimal intrinsic contrast was achieved using targeted contrast agents such as the ExiTron nano 12000. We chose this agent for several properties previously reported, including (1) the in vivo stability over time, (2) the superior performance compared with other contrast agents, (3) the small volume of injection required, and (4) the lack of hepatotoxicity and of pro-inflammatory cytokine release (Boll et al., 2011, Boll et al., 2013, Liu et al., 2019, Mannheim et al., 2016, Wathen et al., 2013). In our studies, we showed that the ExiTron nano 12000 not only induced a significant increase in X-ray attenuation in the liver (about a factor of 5) but also permitted to reveal unexpected changes in the environment of treatment tumors. Thus, the combination of PC-CT with suitable contrast agents can provide both anatomical and functional information, which deserves further exploitation for medical and pre-clinical imaging. Future longitudinal PC-CT analyses of liver tumors, like those in the Alb-R26Met mouse model, using other contrast agents, such as non-toxic nanodroplets loaded with iodine with high-contrast properties specific for hepatocytes and with strong liver persistence (Anton et al., 2017), will widen this approach for quantitative follow-up of tumor evolution and regression while testing anticancer treatments. Furthermore, in a perspective of clinical application, this imaging approach could be particularly relevant for the early diagnosis and monitoring of liver tumors in human patients, according to guidelines of the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver.
The application of longitudinal PC-CT imaging with the PIXSCAN-FLI prototype on the Alb-R26Met cancer model with spontaneous liver tumor formation allowed us to explore the dynamics of tumors at distinct steps of the oncogenic program: the initiation, the evolution, the response to treatments, and the later follow-up. The high performance and quality of our PC-CT detection was particularly appropriate to determine the behavior of tumors at the very first initiation phase (with a size of about 0.5 × 10−2 cm3) when they spontaneously originate in the liver. We found that whereas one tumor rapidly evolved by increasing its size, with a growth rate comparable to those of larger tumors, three others remained in an apparent latent phase, as illustrated by an unchanged size for 1–2 months. It is tempting to speculate that such heterogeneity in the behavior of small tumors might reflect the severity of alterations at the root of the tumorigenic events. Indeed, different combinatorial alterations lead to distinct “fitness”: strong alterations can lead to a rapid tumor growth, whereas weak alterations may result in a latency state whose evolution is conditioned by the acquisition of additional modifications. Differences in “fitness” on tumorigenic events have been recently exemplified by a forward genetic screening in mice, exploring genetic specificity in cooperation for tumorigenesis (Rogers et al., 2018, Wangensteen et al., 2018). Another example comes from a transposon mutagenesis screen we performed in the Alb-R26Met genetic model, which uncovered distinct modes of cooperation with RTKs (Fan et al., 2019). Nevertheless, alterations of distinct genes appeared to not have equivalent strengths in tumorigenesis acquisition, as illustrated by the variety of tumor size formed following targeting distinct tumor suppressors. Future studies using a large cohort of mice would permit extending these findings and corroborate them with statistical analysis to determine whether the behaviors of small tumors are equally distributed between long-term latency versus rapid growth and to clarify the frequency of spontaneous tumor regression we observed. It would be particularly interesting as well to combine imaging data with screen outcomes of distinct tumors to uncover how tumor latency/growth matches with (epi)genetic/transcriptome/proteome signatures.
Concerning the evolution of growing tumors modeled by the Alb-R26Met mice, we recorded an overall trend of tumor doubling time ranging between 10 and 30 days, with a median of 15.9 ± 5.3 days. Indeed, we observed that the doubling time for each tumor remained constant over time. It is tempting to speculate that the sets of alterations ensuring tumor growth at early phases might determine the speed of tumor growth over time. Concerning our longitudinal studies performed by treating mice with a drug combination targeting MEK and BCL-XL, a synthetic lethal interaction in HCC cells we previously reported in vitro and in nude mice xenografts (Fan et al., 2017), our studies showed its remarkable effects also on Alb-R26Met endogenous tumors. The only exception was for the rather big tumor, for which MEK + BCL-XL inhibition did not lead to significant regression, although it clearly blocked its growth. These results support the relevance to further explore the MEK + BCL-XL drug combination through preclinical studies in a translational perspective for human HCC patient subsets. In addition, beside complete regression for a relatively small tumor, our results showed that once the treatment was stopped, the other three medium-sized tumors regrew after an approximately 90% reduction of their volume. Future studies combining imaging data with phosphoproteomic profiles of distinct tumors would be instrumental to clarify whether and to what extent tumors maintain sensitivity to MEK + BCL-XL treatment over time. These approaches may also reveal resistance mechanisms, which can be targeted by subsequent treatments.
Our longitudinal imaging in mice treated with MEK and BCL-XL inhibitors also uncovered a remarkable remodeling of the tumor microenvironment, as illustrated by the accumulation of macrophages in regressing tumors. Such accumulation of macrophages is coherent with a process of removing dying cancer cells caused by drug treatment. Nevertheless, it is well known that macrophages can contribute to tumor destruction or facilitate tumor growth and metastasis, depending on their phenotype (Ostrand-Rosenberg, 2008). Macrophages are a primary source of several mediators such as cytokines, chemokines, and growth factors (Chawla et al., 2011, Stienstra et al., 2010). These factors can influence the cross talk between cancer and immune cells, the presence of cytotoxic T-cells targeting the tumor and/or of tumor-promoting immune cells fostering cancer cell proliferation and invasion (Becht et al., 2016), as well as angiogenesis (Ehling et al., 2014). As our studies were performed in tumor-bearing immunocompetent mice, future studies will determine whether tumor regression targeting MEK and BCL-XL can be further potentiated by an immunotherapy to achieve maximal anticancer response. Immunotherapy is gaining tremendous attention for its potential as anticancer treatment. Current cancer immunotherapies focus on overcoming ineffective immune reactivity against solid tumors, either by global activation of the immune system or by manipulation of immune-regulatory molecules in the tumor microenvironment, including the so-called immune checkpoints. The latter aims at increasing the ratio of cytotoxic T cells over suppressive cells. Several new treatment possibilities are currently under investigation to exploit better the immunotherapy potential in clinics.
Among these, the programmed death-1 (PD-1) system received tremendous attention and several preclinical/clinical studies support the potential of its modulation for therapy, including for HCC treatment (Foerster et al., 2018, Greten and Sangro, 2017, Inarrairaegui et al., 2018, Ruiz de Galarreta et al., 2019, Tacke, 2017, Wu et al., 2009). As a subset of patients with HCC still showed relapse after anti-PD-1 treatment (Ruiz de Galarreta et al., 2019), combining toxic drugs to target cancer cells and checkpoint blockade might maximize the therapeutic responses (Champiat et al., 2014, Harding, 2018). It would be relevant to assess whether the detrimental effects of MEK plus BCL-XL targeting on HCC cells accompanied by an increased tumor clearance by macrophages could be potentiated upon immune checkpoint modulation. Whether these tumor-loaded macrophages conjointly acquire increased immunogenic properties will require further investigations.
Future effective targeted molecular therapies will likely emerge using agents combined to target simultaneously cancer and immune cells (Champiat et al., 2014). Nevertheless, this requires implementing knowledge on (1) remodeling processes of immune cell types occurring when cancer cells are targeted by drugs, (2) optimal timing of simultaneous drug administration, and (3) beneficial constant versus pulses of treatments over time to eradicate cancer, or transform it into a chronic disease. The PIXSCAN-FLI prototype for longitudinal PC-CT imaging of spontaneous liver tumors like those modeled by the clinically relevant Alb-R26Met mice represents a valuable system to explore these aspects and to design new therapeutic interventions combining cancer cell targeting agents with immunotherapy for achieving maximal anticancer responses.
Limitations of the Study
As the Alb-R26Met model recapitulates the molecular characteristics of the “proliferative-progenitor” HCC patient subgroup as well as the molecular heterogeneity that characterizes these patients, we would predict that knowledge acquired through these studies is relevant to the human HCC pathology. However, it would be necessary to corroborate findings by analyzing a large cohort of mice (for example, composed of about 50 animals) for longitudinal measurement of several parameters at distinct phases of the oncogenic program. Moreover, the combination of imaging data with screen outcomes for individual tumors will be essential to elucidate how tumor dynamics matches with molecular signatures.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank: all members of our laboratories for helpful discussions and comments, A. Abdouni and F. Helmbacher for valuable feedback on the study, and people at the IBDM mouse facility platform for excellent help with animal husbandry. This work was funded by FdF (Fondation de France; 2014_00051580 and 2016_00067080), ARC (Association pour la Recherche contre le Cancer; SFE2011_1203807 and PJA20181208172), GEFLUC – Les Entreprises contre le Cancer, and SATT Sud-Est to F.M. M.A. was supported by an FdF fellowship. The contribution of the Region Provence Alpes Côtes d’Azur and of the Aix-Marseille Univ to the IBDM animal facility is also acknowledged. The development of the PC-CT PIXSCAN-FLI prototype was partly supported by France Life Imaging (grant ANR-11-INBS-44-0006 from the French “Investissement d’Avenir” program) and by the Cancéropôle Provence-Alpes-Côte d’Azur. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
F.C. conceived this interdisciplinary study; designed, supervised, and performed imaging analysis; performed data analysis and interpretation; and contributed to write the manuscript. L.P. performed imaging, data analysis, and interpretation and provided input on studies and on the manuscript. S.R. contributed to imaging studies and histological analysis. M.D. contributed to imaging acquisition and reconstruction. Y.B. contributed to imaging reconstruction. M.A. performed computational work with Alb-R26Met screen outcomes, data analysis, and interpretation and provided inputs on studies and on the manuscript. N.A-A. supervised histological studies and contributed to data interpretation. L.C. performed histological studies, data analysis, and interpretation. C.L. contributed to interpret histological analysis and staining and provided inputs on pathological studies. S.F. performed imaging studies, data analysis, and interpretation. L.B. performed imaging studies, data analysis, and interpretation. F.L. contributed to experimental work for histological studies and interpretation and provided inputs on studies. R.D. contributed to establishing the Alb-R26Met mouse model and provided inputs on studies. B.G. contributed to supervise imaging studies. T.L. provided inputs on studies. C.M. designed and supervised imaging studies, analyzed and interpreted data; ensured financial support; and contributed to write the manuscript. F.M. conceived this interdisciplinary study; designed and supervised the biological studies, contributed to experimental work, analyzed and interpreted data; ensured financial support; and wrote the manuscript.
Declaration of Interests
The authors declare no competing interest.
Published: November 22, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.10.015.
Contributor Information
Christian Morel, Email: morel@cppm.in2p3.fr.
Flavio Maina, Email: flavio.maina@univ-amu.fr.
Supplemental Information
References
- Anton N., Parlog A., Bou About G., Attia M.F., Wattenhofer-Donze M., Jacobs H., Goncalves I., Robinet E., Sorg T., Vandamme T.F. Non-invasive quantitative imaging of hepatocellular carcinoma growth in mice by micro-CT using liver-targeted iodinated nano-emulsions. Sci. Rep. 2017;7:13935. doi: 10.1038/s41598-017-14270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechederra M., Daian F., Yim A., Bazai S.K., Richelme S., Dono R., Saurin A.J., Habermann B.H., Maina F. Hypermethylation of gene body CpG islands predicts high dosage of functional oncogenes in liver cancer. Nat. Commun. 2018;9:3164. doi: 10.1038/s41467-018-05550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton J.R., West J.L., Badea C.T. In vivo small animal micro-CT using nanoparticle contrast agents. Front. Pharmacol. 2015;6:256. doi: 10.3389/fphar.2015.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabriga R., Alozy J., Campbell M., Frojdh E., Heijne E.H.M., Koenig T., Llopart X., Marchal J., Pennicard D., Poikela T. Review of hybrid pixel detector readout ASICs for spectroscopic X-ray imaging. J. Instrum. 2016;11:1–31. [Google Scholar]
- Becht E., Giraldo N.A., Dieu-Nosjean M.C., Sautes-Fridman C., Fridman W.H. Cancer immune contexture and immunotherapy. Curr. Opin. Immunol. 2016;39:7–13. doi: 10.1016/j.coi.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Boll H., Nittka S., Doyon F., Neumaier M., Marx A., Kramer M., Groden C., Brockmann M.A. Micro-CT based experimental liver imaging using a nanoparticulate contrast agent: a longitudinal study in mice. PLoS One. 2011;6:e25692. doi: 10.1371/journal.pone.0025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll H., Figueiredo G., Fiebig T., Nittka S., Doyon F., Kerl H.U., Nolte I., Forster A., Kramer M., Brockmann M.A. Comparison of Fenestra LC, ExiTron nano 6000, and ExiTron nano 12000 for micro-CT imaging of liver and spleen in mice. Acad. Radiol. 2013;20:1137–1143. doi: 10.1016/j.acra.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Bour G., Martel F., Goffin L., Bayle B., Gangloff J., Aprahamian M., Marescaux J., Egly J.M. Design and development of a robotized system coupled to microCT imaging for intratumoral drug evaluation in a HCC mouse model. PLoS One. 2014;9:e106675. doi: 10.1371/journal.pone.0106675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buton C., Dawiec A., Graber-Bolis J., Arnaud K., Bérar J.-F., Blanc N., Boudet N., Clémens J.-C., Debarbieux F., Delpierre P. Comparison of three types of XPAD3.2/CdTe single chip hybrids for hard X-ray applications in material science and biomedical imaging. Nucl. Instr. Methods Phys. Res. A. 2014;758:44–56. [Google Scholar]
- Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm.edu; Cancer Genome Atlas Research Network Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341.e23. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol F., Clemens J.C., Hemmer C., Morel C. Imaging performance of the hybrid pixel detectors XPAD3-S. Phys. Med. Biol. 2009;54:1773–1789. doi: 10.1088/0031-9155/54/6/024. [DOI] [PubMed] [Google Scholar]
- Cassol F., Portal L., Graber-Bolis J., Perez-Ponce H., Dupont M., Kronland-Martinet C., Boursier Y., Blanc N., Bompard F., Boudet N. K-edge imaging with the XPAD3 hybrid pixel detector, direct comparison of CdTe and Si sensors. Phys. Med. Biol. 2015;60:5497–5511. doi: 10.1088/0031-9155/60/14/5497. [DOI] [PubMed] [Google Scholar]
- Cassol F., Dupont M., Kronland-Martinet C., Ouamara H., Dawiec H., Boursier Y., Bonissent A., Clémens J.-C., Portal L., Debarbieux F. Characterization of the imaging performance of a micro-CT system based on the photon counting XPAD3/Si hybrid pixel detectors. Biomed. Phys. Eng. Express. 2016;2:025003. [Google Scholar]
- Champiat S., Ileana E., Giaccone G., Besse B., Mountzios G., Eggermont A., Soria J.C. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J. Thorac. Oncol. 2014;9:144–153. doi: 10.1097/JTO.0000000000000074. [DOI] [PubMed] [Google Scholar]
- Chawla A., Nguyen K.D., Goh Y.P.S. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N.M., Hatsell S., Nannuru K., Huang L., Wen X., Wang L., Wang L.H., Idone V., Meganck J.A., Murphy A. In vivo quantitative microcomputed tomographic analysis of vasculature and organs in a normal and diseased mouse model. PLoS One. 2016;11:e0150085. doi: 10.1371/journal.pone.0150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre P. A history of hybrid pixel detectors, from high energy physics to medical imaging. J. Instrum. 2014;9:1–12. [Google Scholar]
- Ehling J., Bartneck M., Wei X., Gremse F., Fech V., Mockel D., Baeck C., Hittatiya K., Eulberg D., Luedde T. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960–1971. doi: 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Richelme S., Avazeri E., Audebert S., Helmbacher F., Dono R., Maina F. Tissue-specific gain of RTK signalling uncovers selective cell vulnerability during embryogenesis. PLoS Genet. 2015;11:e1005533. doi: 10.1371/journal.pgen.1005533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.N., Arechederra M., Richelme S., Daian F., Novello C., Calderaro J., Di Tommaso L., Morcrette G., Rebouissou S., Donadon M. A phosphokinome-based screen uncovers new drug synergies for cancer driven by liver-specific gain of nononcogenic receptor tyrosine kinases. Hepatology. 2017;66:1644–1661. doi: 10.1002/hep.29304. [DOI] [PubMed] [Google Scholar]
- Fan Y.N., Bazai S.K., Daian F., Arechederra M., Richelme S., Temiz N.A., Yim A., Habermann B.H., Dono R., Largaespada D.A. Evaluating the landscape of gene cooperativity with receptor tyrosine kinases in liver tumorigenesis using transposon-mediated mutagenesis. J. Hepatol. 2019;70:470–482. doi: 10.1016/j.jhep.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Foerster F., Hess M., Gerhold-Ay A., Marquardt J.U., Becker D., Galle P.R., Schuppan D., Binder H., Bockamp E. The immune contexture of hepatocellular carcinoma predicts clinical outcome. Sci. Rep. 2018;8:5351. doi: 10.1038/s41598-018-21937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestine M., Caricati E., Fico A., Richelme S., Hassani H., Sunyach C., Lamballe F., Panzica G.C., Pettmann B., Helmbacher F. Enhanced neuronal Met signalling levels in ALS mice delay disease onset. Cell Death Dis. 2011;2:e130. doi: 10.1038/cddis.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten T.F., Sangro B. Targets for immunotherapy of liver cancer. J. Hepatol. 2017 doi: 10.1016/j.jhep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K.M. Detectability in computed tomographic images. Med. Phys. 1979;6:441–451. doi: 10.1118/1.594534. [DOI] [PubMed] [Google Scholar]
- Harding J.J. Immune checkpoint blockade in advanced hepatocellular carcinoma: an update and critical review of ongoing clinical trials. Future Oncol. 2018;14:2293–2302. doi: 10.2217/fon-2018-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inarrairaegui M., Melero I., Sangro B. Immunotherapy of hepatocellular carcinoma: facts and hopes. Clin. Cancer Res. 2018;24:1518–1524. doi: 10.1158/1078-0432.CCR-17-0289. [DOI] [PubMed] [Google Scholar]
- Krenkel O., Tacke F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- Liu C.N., Morin J., Dokmanovich M., Bluette C.T., Goldstein R., Manickam B., Bagi C.M. Nanoparticle contrast-enhanced micro-CT: a preclinical tool for the 3D imaging of liver and spleen in longitudinal mouse studies. J. Pharmacol. Toxicol. Methods. 2019;96:67–77. doi: 10.1016/j.vascn.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Zucman-Rossi J., Pikarsky E., Sangro B., Schwartz M., Sherman M., Gores G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- Mannheim J.G., Schlichthaerle T., Kuebler L., Quintanilla-Martinez L., Kohlhofer U., Kneilling M., Pichler B.J. Comparison of small animal CT contrast agents. Contrast Media Mol. Imaging. 2016;11:272–284. doi: 10.1002/cmmi.1689. [DOI] [PubMed] [Google Scholar]
- Martiniova L., Schimel D., Lai E.W., Limpuangthip A., Kvetnansky R., Pacak K. In vivo micro-CT imaging of liver lesions in small animal models. Methods. 2010;50:20–25. doi: 10.1016/j.ymeth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr. Opin. Genet. Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit P., Johnston S.M., Qi Y., Story J., Nelson R., Johnson G.A. The utility of micro-CT and MRI in the assessment of longitudinal growth of liver metastases in a preclinical model of colon carcinoma. Acad. Radiol. 2013;20:430–439. doi: 10.1016/j.acra.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangaud P., Basolo S., Boudet N., Berar J.F., Chantepie B., Delpierre P., Dinkespiler B., Hustache S., Menouni M., Morel C. XPAD3: a new photon counting chip for X-ray CT-scanner. Nucl. Instrum. Meth. A. 2007;571:321–324. [Google Scholar]
- Rogers Z.N., McFarland C.D., Winters I.P., Seoane J.A., Brady J.J., Yoon S., Curtis C., Petrov D.A., Winslow M.M. Mapping the in vivo fitness landscape of lung adenocarcinoma tumor suppression in mice. Nat. Genet. 2018;50:483–486. doi: 10.1038/s41588-018-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe J.H., Rudolph I., Rohwer N., Kupitz D., Gregor-Mamoudou B., Derlin T., Furth C., Amthauer H., Brenner W., Buchert R. Time course of contrast enhancement by micro-CT with dedicated contrast agents in normal mice and mice with hepatocellular carcinoma: comparison of one iodinated and two nanoparticle-based agents. Acad. Radiol. 2015;22:169–178. doi: 10.1016/j.acra.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Ruiz de Galarreta M., Bresnahan E., Molina-Sanchez P., Lindblad K.E., Maier B., Sia D., Puigvehi M., Miguela V., Casanova-Acebes M., Dhainaut M. beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Naito M., Matsumura Y., Kita H., Kanno T., Nakada Y., Hamano M., Chiba M., Maeda K., Michida T. Spontaneous regression of a large hepatocellular carcinoma with multiple lung metastases. Gut Liver. 2014;8:569–574. doi: 10.5009/gnl13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambach S.J., Bag S., Schilling L., Groden C., Brockmann M.A. Application of micro-CT in small animal imaging. Methods. 2010;50:2–13. doi: 10.1016/j.ymeth.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Stienstra R., Saudale F., Duval C., Keshtkar S., Groener J.E., van Rooijen N., Staels B., Kersten S., Muller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- Tacke F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Taguchi K., Iwanczyk J.S. Vision 20/20: single photon counting x-ray detectors in medical imaging. Med. Phys. 2013;40:100901. doi: 10.1118/1.4820371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonges L., Ostendorf T., Lamballe F., Genestine M., Dono R., Koch J.C., Bahr M., Maina F., Lingor P. Hepatocyte growth factor protects retinal ganglion cells by increasing neuronal survival and axonal regeneration in vitro and in vivo. J. Neurochem. 2011;117:892–903. doi: 10.1111/j.1471-4159.2011.07257.x. [DOI] [PubMed] [Google Scholar]
- Wangensteen K.J., Wang Y.J., Dou Z., Wang A.W., Mosleh-Shirazi E., Horlbeck M.A., Gilbert L.A., Weissman J.S., Berger S.L., Kaestner K.H. Combinatorial genetics in liver repopulation and carcinogenesis with a in vivo CRISPR activation platform. Hepatology. 2018;68:663–676. doi: 10.1002/hep.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen C.A., Foje N., van Avermaete T., Miramontes B., Chapaman S.E., Sasser T.A., Kannan R., Gerstler S., Leevy W.M. In vivo X-ray computed tomographic imaging of soft tissue with native, intravenous, or oral contrast. Sensors (Basel) 2013;13:6957–6980. doi: 10.3390/s130606957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermes N. Pixel detectors for tracking and their spin-off in imaging applications. Nucl. Instrum. Meth. A. 2005;541:150–165. [Google Scholar]
- Will O.M., Damm T., Campbell G.M., von Schonfells W., Acil Y., Will M., Chalaris-Rissmann A., Ayna M., Drucker C., Gluer C.C. Longitudinal micro-computed tomography monitoring of progressive liver regeneration in a mouse model of partial hepatectomy. Lab. Anim. 2017;51:422–426. doi: 10.1177/0023677216678824. [DOI] [PubMed] [Google Scholar]
- Willekens I., Lahoutte T., Buls N., Vanhove C., Deklerck R., Bossuyt A., de Mey J. Time-course of contrast enhancement in spleen and liver with Exia 160, Fenestra LC, and VC. Mol. Imaging Biol. 2009;11:128–135. doi: 10.1007/s11307-008-0186-8. [DOI] [PubMed] [Google Scholar]
- Wu K., Kryczek I., Chen L.P., Zou W.P., Welling T.H. Kupffer cell suppression of CD8(+) T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B., Wang D.H., Spechler S.J. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig. Dis. Sci. 2012;57:1122–1129. doi: 10.1007/s10620-012-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman-Rossi J., Villanueva A., Nault J.C., Llovet J.M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–1239.e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.