Abstract
New heterocyclic derivatives of 8-hydroxyquinoline were prepared and screened as antimicrobial agents. Chemical structures were elucidated and confirmed using different spectroscopic methods such as elemental analysis data, Infrared, Nuclear Magnetic Resonance Spectroscopy. In order to explore their potential biological activity, the “in vitro” antibacterial activity was investigated against [E. coli (ATCC35218), S. aureus (ATCC29213), V. parahaemolyticus (ATCC17802), and P. aeruginosa (ATCC27853)]. The studied compounds exhibited a remarkable antibacterial activity superior to the standard antibiotic (Penicillin G). These new heterocyclic derivatives of 8-hydroxyquinoline, which proved to be potentially effective, can be used as alternative chemical antimicrobial agents applications. It was very interesting to observe that POM (Petra/Osiris/Molinspiration) bioinformatic analyses of the 8-hydroxyquinoline derivative (5) exhibited more important antibacterial activity (MIC = 10−6 mg/mL against V.p and S.a bacteria) and good drug score (DS = 0.71) when compared with Penicillin (DS = 0.33; MIC = 10−3 mg/mL).
Keywords: Biochemistry, Microbiology, 8-hydroxyquinoline, Characterization, Antimicrobial activity, Antibacterial pharmacophore site, POM (Petra/Osiris/Molinspiration) analyses
Biochemistry; Microbiology; 8-hydroxyquinoline; Characterization; Antimicrobial activity; Antibacterial pharmacophore site; POM (Petra/Osiris/Molinspiration) analyses
1. Introduction
In the recent years, heterocyclic compounds based on 8-hydroxyquinoline become important because of their potential properties in a wide range of fields such as biological activity (Sravanthi and Manju, 2016; Shangguan et al., 2005), anticorrosion (Rbaa et al., 2017, 2019; Rbaa and Lakhrissi, 2019), and complexing properties of metals (Mladenova et al., 2002). The increase of factories and industrial companies will cause serious diseases for man and environment which led to the fact that the use of antibiotics has become indispensable. Moreover, the 8-hydroxyquinoline derivatives are marked several biological properties such as antibiotic, antifungal, antitumor and anticancer (El Faydy et al., 2017). Therefore, the 8-hydroxyquinoline skeleton is an interesting entity for the development and the synthesis of novel biologically active heterocyclic compounds. However, many side effects have been declared by the users of penicillin as antibiotic (Adamkiewicz et al., 2003). Thus, it is urgent to make some changes in the medicinal treatment of bacterial diseases.
In this study, we have synthesized novel heterocyclic compounds based on 8-hydroxyquinoline. Their structures have been fully characterized using standard spectroscopic methods such as elemental analysis data, Infrared, and Nuclear Magnetic Resonance Spectroscopy. All the synthesized compounds 1–6 (Fig. 1) have been evaluated "in vitro" for their antimicrobial activity using the following bacterial strains: E. coli, S. aureus, V. parahaemolyticus and P. aeruginosa. The novelty of POM analyses in this work is the identification of antibacterial (N---HO)-pharmacophore site and elucidation the reason why the (8-hydroxyquinolin-5-yl) methyl-4-chlorobenzoate (5) constitutes a better hits than Penicillin itself.
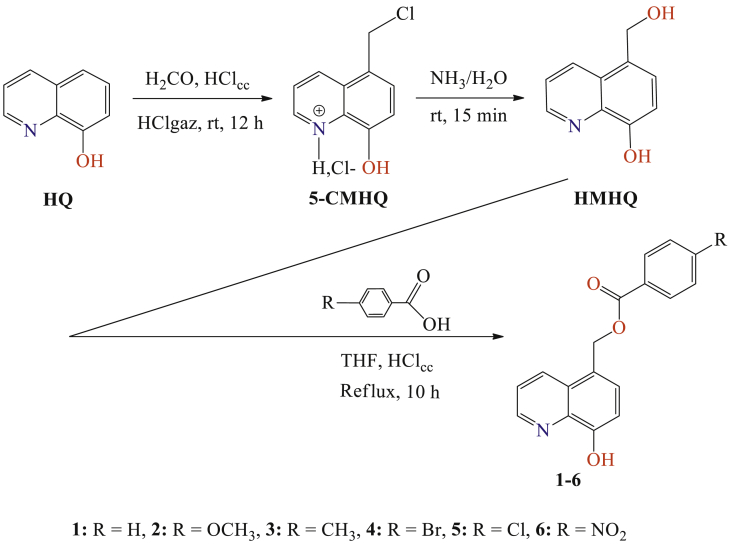
Fig. 1.
Synthetic route for the preparation of 8-hydroxyquinoline derivatives 1–6.
2. Materials & methods
2.1. Chemicals and apparatus
All reagents and solvents used in this study were discussed in a previous work (Feng et al., 2008).
2.2. Synthesis of organic compounds
2.2.1. Synthesis of compounds 1–6
Our strategy was to develop a simple and acceptable yielding procedure in a very short time, to prepare the desired 8-hydroxyquinoline derivatives 1–6 (Fig. 1).
2.2.2. Synthesis of compounds 5-CMHQ and 5-HMHQ
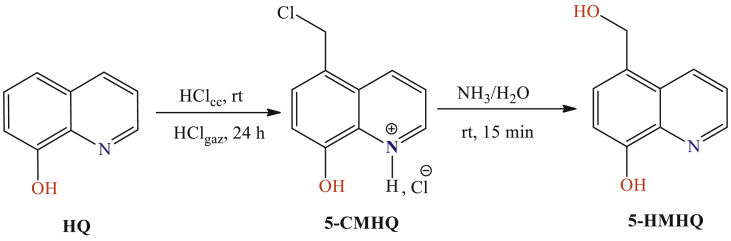
5-Hydroxymethyl-8-hydroxyquinoline (5-HMHQ) was prepared by treating 5-chloromethyl-8-hydroxyquinoline hydrochloride (5-CMHQ) with ammonia at room temperature for 15 minutes (Fig. 2). The compound 5-CMHQ was prepared from 8-hydroxyquinoline (HQ) according to a method described in a previous work (Rbaa et al., 2019).
Fig. 2.
The reaction for conversion of 5-CMHQ to 5-HMHQ.
2.2.3. Synthesis of 8-hydroxyquinoline derivatives 1–6
2.2.3.1. General procedure
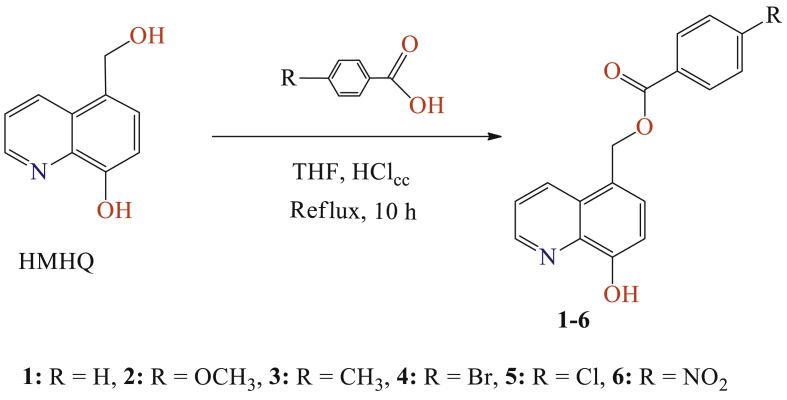
A solution of 5-hydroxymethyl-8-quinolinol (0.01 mol), para-substituted benzoic acid (0.01 mol) in absolute THF (50 ml) and a solution of concentrated HCl (37 %), was refluxed under magnetic stirring for 10 h. The reaction was monitored by thin layer chromatography using hexane-acetone (6:4, v/v) as the mobile phase. After completion, the reaction mixture was cooled to room temperature, diluted with water (20 mL), and extracted with dichloromethane (3 × 20 mL). The combined organic layers were dried over anhydrous MgSO4, filtered and the solvent was removed by rotary evaporation under vacuum. The obtained crude residue was washed by acetone, and purified by chromatography on a silica gel column using dichloromethane/hexane (85/15: v/v), and then recrystallized from absolute ethanol (Fig. 3).
Fig. 3.
Synthesis of 8-hydroxyquinoline derivatives 1-6.
2.3. Antimicrobial activity
2.3.1. Bacterial strains
The bacteria selected for this study are E. coli responsible for food poisoning and infections (Monod et al., 1952). S. aureus S. aureus causes serious life-threatening complications, such as the infection of the blood, bones or lungs (Meylan et al., 2017). P. aeruginosa is a human pathogen which is more often responsible for nosocomial infections (Appelbaum, 2006) and V. parahaemolyticus (Bej et al., 1999) represents a serious and global threat to human health. They were all provided by the Laboratory of Agro Physiology, Biotechnology, Environment and Quality, Faculty of Sciences, Ibn Tofail University, Kenitra - Morocco. Each bacterium was inoculated into the Mueller-Hinton agar culture medium.
2.3.2. Antibacterial test
Using the agar disk diffusion assay, the antibacterial activity of our products was determined while a bacterial culture of 24 hours was spread on the surface of the Muller-Hinton agar plate. A disc of sterile 6 mm whatmann paper was saturated with 10 μL of 8-hydroxyquinoline derivatives solution under investigation in dimethylsulfoxide as solvent. After 1 h of diffusion, the Petri dishes were incubated at 37 °C for 24 hours and the areas of inhibition of development were measured and compared with those of the Penicillin G reference discs.
3. Results and discussions
3.1. Chemical study
3.1.1. Procedure
All products were synthesized by conventional and simple methods, we have mixed an equimolar amount of 5-hydroxymethyl-8-hydroxyquinoline and 4-substituted benzoic acids in absolute THF in the presence of HClcc. The reaction mixture was refluxed for 10 h under magnetic stirring. The reaction was monitored by a silica thin layer plate and the purification of all the products were obtained and purified by silica gel column chromatography [CH2CH2/hexane,85/15: v/v], and (Fig. 1). The pure product obtained was then triturated via diethyl ether and recrystallized from absolute methanol to yield the (8-hydroxyquinolin-5-yl) methyl-4-alkylbenzoate derivatives 1–6.
3.1.2. Data value, and validation
3.1.2.1. Preparation of (8-hydroxyquinolin-5-yl) methylbenzoate (1)
Yield 38 %, Aspect White solid, Mp 185-187 °C, Fr 0.35 (C6H14/CH2Cl2, 50/50: v/v), Mm 279.29 g/mol. IR: 1627 (C=C), 3423 (OH), 1798 (C=O). 1H NMR (300 MHz, DMSO-d6): δppm = 4.51 (s, 1H, OH), 4.49 (s, 2H, CH2), 7.56–7.55 (m, 4H, benzene), 7.01-7.32-7.61-8.95-9.87 (m, 5H, Arquinoline). 13C NMR (300 MHz, DMSO-d6): δppm = 63.74 (CH2), 155.79 (ArC-OH), 157.02 (C=O), 129.28–127.78 (ArCHbenzene), 132.00 (ArCbenzene), 110.64-122.15-125.72-153.11 (ArCHquinoline), 129.12-132.51-133.80 (ArCquinoline). Microanalysis (%): Observed, C, (73.71) - H, (5.15) - N, (4.78). Calculated, C, (73.60) - H, (5.30) - N, 4.83.
3.1.2.2. Preparation of (8-hydroxyquinolin-5-yl) methyl-4-methoxybenzoate (2)
Yield 58 %, Aspect Black solid, Mp 179-181 °C, Fr 0.49 (C6H14/CH2Cl2), 50/50: v/v), Mm 309.32 g/mol. IR: 1648 (C=C), 3456 (OH), 1717 (C=O). 1H NMR (300 MHz, DMSO-d6): δppm = 5.91 (s, 1H, OH), 4.50 (s, 2H, CH2), 3.41 (s, 3H, CH3) 7.43–7.44 (m, 4H, benzene), 7.04-7.32-8.62-8.75-8.76 (m, 5H, Arquinoline). 13C NMR (300 MHz, DMSO-d6): δppm = 45.54 (CH2), 153.32 (ArC-OH), 153.99 (C=O), 21.29 (CH3), 126.23–129.11 (ArCHbenzene), 139.16 (ArCbenzene), 111.04-122.42-123.13-132.25-148.70 (ArCHquinoline), 127.57-136.55-140.09 (ArCquinoline). Microanalysis (%): Observed, C, (69.89) -H, (4.89) - N, (4.53). Calculated, C, (68.50) - H, (4.28) - N, (4.87).
3.1.2.3. Preparation of (8-hydroxyquinolin-5-yl) methyl-4-methylbenzoate (3)
Yield 49 %, Aspect Black solid, Mp 176-178 °C, Fr 0.45 (C6H14/CH2Cl2), 50/50: v/v), Mm 293.32 g/mol. IR: 1633 (C=C), 3613.58 (OH), 1799 (C=O). 1H NMR (300 MHz, DMSO-d6): δppm = 6.99 (s, 1H, OH), 4.72 (s, 2H, CH2), 7.56-7.58-8.42 (m, 5H, benzene), 7.02-7.37-7.55-8.85-9.79 (m, 5H, Arquinoline). 13C NMR (300 MHz, DMSO-d6): δppm = 61.10 (CH2), 153.06 (ArC-OH), 155.74 (C=O), 126.11–128.49 (ArCHbenzene), 138.07 (ArCbenzene), 111.62-116.10-123.10-134.58-147.98 (ArCHquinoline), 127.59-128.84-139.26 (ArCquinoline). Microanalysis (%): Calculated for C17H13NO3 (279.29). Observed, C, (73.11) - H, (4.69) - N, (5.02); Calculated, C, (73.65) - H, (4.16) - N, (5.73).
3.1.2.4. Preparation of (8-hydroxyquinolin-5-yl) methyl-4-bromobenzoate (4)
Yield 65 %, Aspect White solid, Mp 201-203 °C, Fr 0.47 (C6H14/CH2Cl2), 50/50: v/v), Mm 358.19 g/mol. IR: 1632 (C=C), 3414 (OH), 1884.41 (C=O). 1H NMR (300 MHz, DMSO-d6): δppm = 5.30 (s, 1H, OH), 3.38 (s, 2H, CH2), 7.59–7.57 (m, 4H, benzene), 7.07-7.43-8.43-8.85-9.84 (m, 5 H, Arquinoline). 13C NMR (300 MHz, DMSO-d6): δppm = 51.42 (CH2), 168.15 (ArC-OH), 169.65 (C=O), 139.28 (C-Br), 129.99–128.56 (ArCHbenzene), 129.50 (ArCbenzene), 110.70-122.14-124.50-148.67 (ArCHquinoline), 127.53-133.10-139.11 (ArCquinoline). Microanalysis (%): Observed, C, (57.00) - H, (3.38) - N, (3.91). Calculated, C, (56.98) - H, (3.24) - N, (3.89).
3.1.2.5. Preparation of (8-hydroxyquinolin-5-yl) methyl 4-nitrobenzoate (5)
Yield 49 %, Aspect Brown solid, Mp 180-182 °C, Fr 0.41 (C6H14/CH2Cl2), 50/50: v/v), Mm 324.29 g/mol. IR: 1614 (C=C), 3372 (OH), 1709 (C=O). 1H NMR (300 MHz, DMSO-d6): δppm = 4.39 (s, 1H, OH), 4.35 (s, 2H, CH2), 8.12–8.56 (m, 4H, benzene ring), 7.05-7.49-7.56-8.59-8.61 (m, 5H, Ar-quinoline). 13C NMR (300 MHz, DMSO-d6): δppm = 59.84 (CH2), 146.04 (ArC-OH), 152.26 (C=O), 146.48 (C-NO2), 123.66–128.54 (ArCH-benzene ring), 136.2 (ArC-benzene ring), 114.59-119.90-125.92-134.17-145.07 (ArCH-quinoline), 127.84-128.75-139.98 (ArC-quinoline). Microanalysis (%): Observed, C, 62.96 - H, 3.73 -N, 9.64. Calculated, C, 62.87 - H, 3.77 - N, 9.66.
3.1.2.6. Preparation of (8-hydroxyquinolin-5-yl) methyl 4-chlorobenzoate (6)
Yield 57 %, Aspect Yellow solid, Mp 199-201 °C, Fr 0.37 (C6H14/CH2Cl2), 50/50: v/v), Mm 313.74 g/mol. IR: 1621 (C=C), 3407 (OH), 1736 (C=O). 1H NMR (300 MHz, DMSO-d6): δppm = 5.05 (s, 1H, OH), 4.19 (s, 2H, CH2), 7.63–7.73 (m, 4H, benzene ring), 7.07-7.43-7.58-8.58-8.86 (m, 5H, Ar-quinoline). 13C NMR (300 MHz, DMSO-d6): δppm = 49.38 (CH2), 153.90 (ArC-OH), 167.26 (C=O), 139.32 (C-Cl), 131.82–128.34 (ArCH-benzene ring), 129.50 (ArC-benzene ring), 110.85-122.56-123.36-148.48 (ArCH-quinoline), 127.35-132.46-142.14 (ArC-quinoline). Microanalysis (%): Observed, C, 65.08; H, 3.86; N, 4.46. Calculated, C, 65.02; H, 3.70; N, 4.70.
3.1.2.7. Preparation of 5-hydroxymethyl-8-hydroxyquinoline (5-HMHQ)
Yield 90 %, Aspect White solid, Mp 138 °C, Fr 0.60 (C6H14/CH2Cl2), 50/50: v/v), Mm 175.18 g/mol. IR: 2022 (C=N), 1636 (C=C), 3496 (OH), 1881.41 (CH2). 1H NMR (300 MHz, DMSO-d6): δppm = 4.66 (s, 2H, CH2), 7.51-7.55-8.44-8.83-8.84 (m, 5 H, ArH). 13C NMR (300 MHz, DMSO-d6): δppm = 23.92 (CH2), 152.40 (C-OH), 111.07-122.13-126.87-127.76-148.24 (Ar-CH), 128.26-133.35-139.33 (Ar-C).
3.1.2.8. Preparation of 5-chloromethyl-8-hydroxyquinoline hydrochloride (5-CMHQ)
Yield 98 %, Aspect Green solid, Mp > 260 °C, Fr 0.60 (C6H14/CH2Cl2), 50/50: v/v), Mm 230.09 g/mol. IR: 1743 (C=N), 1624 (C=C), 3248 (OH), 1819.34 (CH2). 1H NMR (300 MHz, DMSO-d6): δppm = 3.48 (s, 2H, CH2), 7.64-7.67-8.03-8.06-8.39 (m, 5H, ArH). 13C NMR (300 MHz, DMSO-d6): δppm = 53.88 (CH2), 162.63 (C-OH), 111.72-122.29-127.95-132.92-148.59 (Ar-CH), 129.95-130.60-136.50 (Ar-C).
The synthesis of the target compounds in their stable conjugated bicyclic fused rings form was carried out from 5-(hydroxymethyl)-8-hydroxyquinoline (5-HMHQ) and para-substituted-benzoic acids. The esterification reaction was carried out in two steps, the first one consists in the activation of the carboxylic acid function by HCl in absolute THF as a solvent, and the second one consists in the nucleophilic attack of the primary hydroxyl group on the carbonyl group to yield compounds 1–6.
3.2. Bioactivity of compounds 1-6
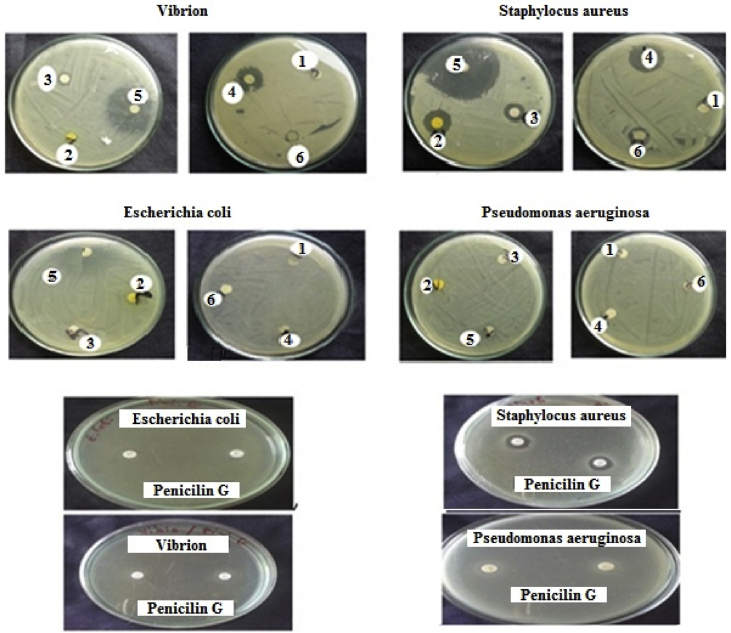
The compounds were screened for their “in vitro” antimicrobial activity at 10−3 g/mL on four bacterial strains. The bacterial growth inhibition outcome is shown in Fig. 4 and summarized in Table 1. The result obtained for this study showed that all the tested products exhibit an exceptional antimicrobial effect against the bacterial strains compared to antibiotic standard. Compound 1 was an exception and it shows no antimicrobial effect on all the tested bacterial strains. Studies of antibacterial activity in the last decade have demonstrated the importance of quinoline-based heterocyclic compounds as potent antibacterial agents (Broch et al., 2010; Fakhfakh et al., 2003). For this purpose, in order to improve the antibacterial activity of 8-hydroxyquinoline, we grafted it with a benzene ring which bears electron donor substituents such as chlorine, bromine, methyl, methoxy and an electron-withdrawing such as the nitro group. From the literature, we have found that the compounds which have electron-withdrawing substituents have a lower activity against Gram-positive and Gram-negative bacteria, than those with electron-donor substituents (Sánchez-Moreno, 2002).
Fig. 4.
The antibacterial activity of compounds 1–6 against bacterial strains compared to Penicillin G after 24 h of incubation at 37 °C.
Table 1.
Inhibition zone in (mm) of the synthesized compounds 1–6 compared with standard antibiotic Penicillin G against Gram-positive and Gram-negative bacteria in 10−3 g/ML.
| Compound | Inhibition zone diameter (mm) |
|||
|---|---|---|---|---|
| Gram-positive bacteria |
Gram-negative bacteria |
|||
| S. a | V. | E. c | P. a | |
| 1 | — | — | — | — |
| 2 | 17 | 07 | — | — |
| 3 | 15 | 7 | — | — |
| 4 | 19 | 14 | — | — |
| 5 | 32 | 30 | 18 | — |
| 6 | 08 | 05 | — | — |
| Penicillin G | 11 | 5 | 12 | 9 |
(—): No zone.
In this series, compounds 2 to 6 substituted on the phenyl ring exhibit excellent antibacterial activity over unsubstituted compound 1. The compound 5 which bears the chlorine in benzene ring shows a good activity against Gram-positives and Gram-negatives strains.
These varieties in the antibacterial activity are probably due to the nature of the substituent, the structure of the tested molecules and the bacteria sensitivity. In addition, these products are more active against the Gram-positive bacteria than Gram-negative bacteria. This can be explained by the presence of cytoplasmic membrane in Gram-negative bacteria that block the transfer of compounds through it. These results go in line with the findings of (Sánchez-Moreno, 2002), who showed that the “in vitro” antibacterial activity of the grafted quinolines was more active against Gram-positive than the Gram-negative bacteria (Table 2).
Table 2.
MIC of the synthesized compounds 1-6.
| Compound | MIC (mg/mL) |
|||
|---|---|---|---|---|
| V. p. | S. a. | P. a. | E. c. | |
| 1 | NA | NA | NA | NA |
| 2 | 10–3 | 10–3 | NA | NA |
| 3 | 10–3 | 10–3 | NA | NA |
| 4 | 10–4 | 10–4 | NA | NA |
| 5 | 10–6 | 10–6 | NA | 10–4 |
| 6 | 10–4 | 10–3 | NA | NA |
| SD | 10–3 | 10–4 | 10–3 | 10–3 |
SD: Standard Drug (Penicillin G). V. p.: Vibrio parahaemolyticus (ATCC17802); S. a.: Staphylococcus aureus (ATCC29213); P. a.: Pseudomonas aeruginosa (ATCC27853); E. c.: Escherichia coli (ATCC35218).
Interestingly, in contrast to encouraging activity of compounds 2–6 against the two Gram-positive bacteria (V. p and S. a), we have noted that they are not active (NA) against any one of the two tested Gram-negative bacteria (P. a, and E. c) (Table 2). This indicates that the inhibition of Gram-negative bacteria needs more lipo-soluble antibacterial agents. To get more insights on this point, Petra/Osiris/Molinspiration (POM) analysis has been executed.
3.3. POM analyses
The analyses of the physicochemical properties of the compounds 1–6 by using a bioinformtic POM platform-2018 is necessary and useful to identify the type of pharmacophore sites, to evaluate and to predict hits and their efficacy as leading candidates against various diseases (Al-Maqtari et al., 2017; Youssoufi et al., 2015; Lahsasni et al., 2015). The POM physicochemical calculations considered a partition coefficient (cLogP), aqueous solubility, donor hydrogen-bond, and drug-likeness. The Lipinski's rule-of-five was used to evaluate the calculations. To ensure that the oral bioavailability of these products is in a good quality, the topological polar surface (TPSA) should be ˂ 140 Å2. The results of POM physicochemical analyses of compounds 1–6 are shown in Tables 3 and 4. These compounds demonstrated good oral bioavailability (TPSA range = 59–105). Drug score (DS) and Drug-Likeness (DL) analyses were also given for comparison as are shown in Tables 3 and 4.
Table 3.
Osiris calculations of toxicity risks of compounds 1–6 and Penicillin G as Standard Drug.
| Compound | MW |
Toxicity Risks[a] |
Osiris calculations[b] |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MUT | TUM | IRRI | REP | cLogP | cLogS | DL | DS | ||
| 1 | 279 | +++ | +++ | — | +++ | 2.96 | -3.49 | 0.37 | 0.40 |
| 2 | 309 | +++ | +++ | +++ | +++ | 2.89 | -3.51 | -1.84 | 0.47 |
| 3 | 293 | +++ | +++ | +++ | +++ | 3.30 | -3.83 | -0.54 | 0.54 |
| 4 | 357 | +++ | +++ | +++ | +++ | 3.69 | -4.32 | -2.74 | 0.36 |
| 5 | 313 | +++ | +++ | +++ | +++ | 3.57 | -4.23 | 3.38 | 0.71 |
| 6 | 324 | +++ | +++ | +++ | +++ | 2.04 | -3.95 | -14.3 | 0.41 |
| SD[c] | 334 | — | — | +++ | +++ | 1.54 | -2.04 | 11.28 | 0.33 |
[a]Highly toxic: (—), Slightly toxic: (+), Not toxic (+++).[a]MUT: Mutagenic, TUM: Tumorigenic, IRRIT: Irritant, RE: Reproductive effective.[b]Sol: Solubility, DL: Druglikness, DS: Drug-Score.[c]SD: Standard Drug (Penicillin G).
Table 4.
Molinspiration calculations of compounds 1–6 and Penicillin G (SD).
| Compound |
Molinspiration calculations[a] |
Drug-likeness[b] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TPSA | NONH | NV | VOL | GPCRL | ICM | KI | NRL | PI | EI | |
| 1 | 59 | 1 | 0 | 248 | -0.02 | 0.03 | -0.01 | 0.01 | -0.08 | 0.12 |
| 2 | 69 | 1 | 0 | 274 | -0.08 | -0.04 | -0.01 | 0.03 | -0.07 | 0.06 |
| 3 | 59 | 1 | 0 | 265 | -0.05 | -0.06 | -0.04 | 0.01 | -0.11 | 0.05 |
| 4 | 59 | 1 | 0 | 266 | -0.11 | -0.06 | -0.04 | -0.08 | -0.17 | 0.03 |
| 5 | 59 | 1 | 0 | 262 | -0.00 | 0.02 | -0.01 | 0.02 | -0.09 | 0.08 |
| 6 | 105 | 1 | 0 | 271 | -0.14 | -0.04 | -0.13 | -0.05 | -0.15 | -0.02 |
| SD[c] | 87 | 2 | 0 | 288 | 0.10 | -0.42 | -0.71 | -0.37 | 0.86 | 0.30 |
[a]TPSA: Total molecular polar surface area; NONH: number of OH—N and O—NH interaction, NV: number of violation of five Lipinsky rules; VOL: volume.[b]GPCRL: GPCR ligand; ICM: Ion Channel Modulator; KI: Kinase Inhibitor; NRL: Nuclear Receptor Ligand; PI: Protease Inhibitor; EI: Enzyme Inhibitor.[c]SD: Standard Drug (Penicillin G).
4. Conclusion
The analysis of the results obtained by the disk diffusion method shows that the compounds substituted in phenyl ring are more active compared to the non-substituted compound and also compared to the standard antibiotic. The antibacterial activity of the chlorinated compound 5 is more important against Gram-positive bacteria than Gram-negative bacteria such as the S. aureus strain, which is the most sensitive.
The bioactivity scores of the series of 1-6 have undergone a comparative POM analyses with Penicillin G indicates that compound 5 needs to be tested in free ligand as antibacterial agent and as potential ligand for ruthenium (II) complexes as more efficient and selective Mycobacterium Tuberculosis Inhibitors (Ben Hadda et al., 2009).
Declarations
Author contribution statement
Mohamed Rbaa, Siham Jabli: Performed the experiments.
Faisal Almalki: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Mohamed Ouhssine, Brahim Lakhrissi, Taibi Ben Hadda, Younes Lakhrissi, Saida Messgo-Moumene, Abbelkader Zarrouk: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the “National Center for Scientific and Technical Research” and the ‘‘Moroccan Ministry of Higher Education’’ for providing all the facilities and for supporting this work.
Contributor Information
Taibi Ben Hadda, Email: taibi.ben.hadda@gmail.com.
Brahim Lakhrissi, Email: brahim.lakhrissi@uit.ac.ma, b.lakhrissi2012@gmail.com.
References
- Adamkiewicz T.V., Sarnaik S., Buchanan G.R., Iyer R.V., Miller S.T., Pegelow C.H. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J. Pediatr. 2003;143:438–444. doi: 10.1067/S0022-3476(03)00331-7. [DOI] [PubMed] [Google Scholar]
- Al-Maqtari H.M., Jamalis J., Ben Hadda T., Sankaranarayanan M., Chander S., Ahmad N.A., Sirat H.M., Althagafi I.I., Mabkhot Y.N. Synthesis, characterization, POM analysis and antifungal activity of novel heterocyclic chalcone derivatives containing acylated pyrazole. Res. Chem. Intermed. 2017;43:1893–1907. [Google Scholar]
- Appelbaum P.C. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2006;12 doi: 10.1111/j.1469-0691.2006.01344.x. 16-2. [DOI] [PubMed] [Google Scholar]
- Bej A.K., Patterson D.P., Brasher C.W., Vickery M.C., Jones D.D., Kaysner C. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J. Microbiol. Methods. 1999;36:215–225. doi: 10.1016/s0167-7012(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Ben Hadda T., Akkurt M., Filali Baba M., Daoudi M., Bennani B., Kerbal A., Chohan Z.H. Anti-tubercular activity of ruthenium (II) complexes with polypyridines. J. Enzyme. Inhib. Med. 2009;24:457–463. doi: 10.1080/14756360802188628. [DOI] [PubMed] [Google Scholar]
- Broch S., Aboab B., Anizon F., Moreau P. Synthesis and in vitro antiproliferative activities of quinoline derivatives. Eur. J. Med. Chem. 2010;45:1657–1662. doi: 10.1016/j.ejmech.2010.01.003. [DOI] [PubMed] [Google Scholar]
- El Faydy M., Djassinra T., Haida S., Rbaa M., Ounine K., Kribii A., Lakhrissi B. Synthesis and investigation of antibacterial and antioxidants properties of some new 5-subsituted-8-hydroxyquinoline derivatives. Mater. J. Environ. Sci. 2017;8:3855–3863. [Google Scholar]
- Fakhfakh M.A., Fournet A., Prina E., Mouscadet J.F., Franck X., Hocquemiller R., Figadère B., Bioorg F. Synthesis and biological evaluation of substituted quinolines: potential treatment of protozoal and retroviral co-infections. Med. Chem. 2003;11:5013–5023. doi: 10.1016/j.bmc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Feng L., Wang X., Chen Z. Synthesis and photophysics of novel 8-hydroxyquinoline aluminum metal complex with 1,3,4-oxadiazole units. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008;71:312–316. doi: 10.1016/j.saa.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Lahsasni S., Ben Hadda T., Masand V., Pathan N.B., Parvez A., Warad I., Shaheen U., Bader A., Aljofan M. POM analyses of raltegravir derivatives: a new reflection enlightening the mechanism of HIV-integrase inhibition. Res. Chem. Intermed. 2015;41:5121–5136. [Google Scholar]
- Meylan S., Porter C.B., Yang J.H., Belenky P., Gutierrez A., Lobritz M.A., Collins J.J. Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Chem. Biol. 2017;24:195–206. doi: 10.1016/j.chembiol.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenova R., Ignatova M., Manolova N., Petrova T., Rashkov I. Preparation, characterization and biological activity of Schiff base compounds derived from 8-hydroxyquinoline-2-carboxaldehyde and Jeffamines EDR. Eur. Polym. J. 2002;38:989–999. [Google Scholar]
- Monod J., Pappenheimer A.M., Jr., Cohen-Bazire G. La cinétique de la biosynthèse de la β-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim. Biophys. Acta. 1952;9:648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- Rbaa M., Galai M., El Kacimi Y., Ouakki M., Touir R., Lakhrissi B., Ebn Touhami M. Adsorption properties and inhibition of carbon steel corrosion in a hydrochloric solution by 2-(4,5-diphenyl-4,5-dihydro-1h-imidazol-2-yl)-5-methoxyphenol. Port. Electrochim. Acta. 2017;35:323–338. [Google Scholar]
- Rbaa M., Benhiba F., Obot I.B., Oudda H., Warad I., Lakhrissi B., Zarrouk A. Two new 8-hydroxyquinoline derivatives as an efficient corrosion inhibitors for mild steel in hydrochloric acid: synthesis, electrochemical, surface morphological, UV–visible and theoretical studies. J. Mol. Liq. 2019;276:120–133. [Google Scholar]
- Rbaa M., Lakhrissi B. Novel oxazole and imidazole based on 8-hydroxyquinoline as a corrosion inhibition of mild steel in HCl Solution: insights from Experimental and Computational Studies. Surfaces and Interfaces. 2019;15:43–59. [Google Scholar]
- Sánchez-Moreno C. Review: methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002;8:121–137. [Google Scholar]
- Shangguan G., Xing F., Qu X., Mao J., Zhao D., Zhao X., Ren J. DNA binding specificity and cytotoxicity of novel antitumor agent Ge132 derivatives. Chem. Lett. 2005;15:2962–2965. doi: 10.1016/j.bmcl.2005.04.053. [DOI] [PubMed] [Google Scholar]
- Sravanthi T.V., Manju S.L. Indoles - a promising scaffold for drug development. Eur. J. Pharm. Sci. 2016;91:1–10. doi: 10.1016/j.ejps.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Youssoufi M.H., Sahu P.K., Sahu P.K., Agarwal D.D., Ahmad M., Messali M., Lahsasni S., Ben Hadda T. POM analyses of antimicrobial activity of 4H-pyrimido [2,1-b] benzothiazole, pyrazole, and benzylidene derivatives of curcumin. Med. Chem. Res. 2015;24:2381–2392. [Google Scholar]