Introduction
Psoriasis vulgaris is a chronic inflammatory skin disorder presenting in genetically predisposed individuals. In the last decades, numerous biologics were approved for the treatment of psoriasis. These include tumor necrosis factor (TNF)-α inhibitors, therapeutic antibodies that inhibit the p40 subunit shared by interleukin (IL)-12 and -23, and IL-17 inhibitors. A recent asset in the therapeutic arsenal for psoriasis is guselkumab (Tremfya; Janssen-Cilag, Beerse, Belgium), a high-affinity inhibitor of the p19 subunit of IL-23 with clinically proven superiority compared with ustekinumab. It was approved in 2017 (by the US Food and Drug Administration and the European Medicines Agency) for the treatment of adults with moderate-to-severe plaque psoriasis and is the first available molecule with this mode of action.1
Case report
A 47-year old man with a history of psoriasis vulgaris was referred to our hospital for evaluation of exacerbating erythematosquamous plaques despite treatment with guselkumab. Previous psoriasis treatments were topical steroids, phototherapy, methotrexate, cyclosporine, and apremilast, all failing to achieve clearance, except for cyclosporine, which was stopped because of side effects. Other medications (atorvastatin, 20 mg/d, desloratadine, 5 mg/d, levocetirizine, 10 mg/d, and lercanidipine, 10 mg/d) were given on a stable dose for more than a year. There was no family history of eczema, but the patient had a history of atopic dermatitis as a child.
Treatment with subcutaneous guselkumab was started 3 months before consultation in our hospital (first administration on September 27, 2018) and was administered according to label (100 mg at weeks 0 and 4 and every 8 weeks thereafter). Initially, there was a manifest improvement, achieving a Psoriasis Area and Severity Index (PASI) score of 100 at week 7, which sustained for several weeks. At week 10, erythematosquamous plaques started developing, associated with severe itching. No new topical or systemic treatments were initiated. Because of progressive complaints and exacerbating skin lesions not responding to topical steroids, the patient was referred to our hospital on December 20, 2018. The third dose (scheduled on December 21, 2018) was not administered.
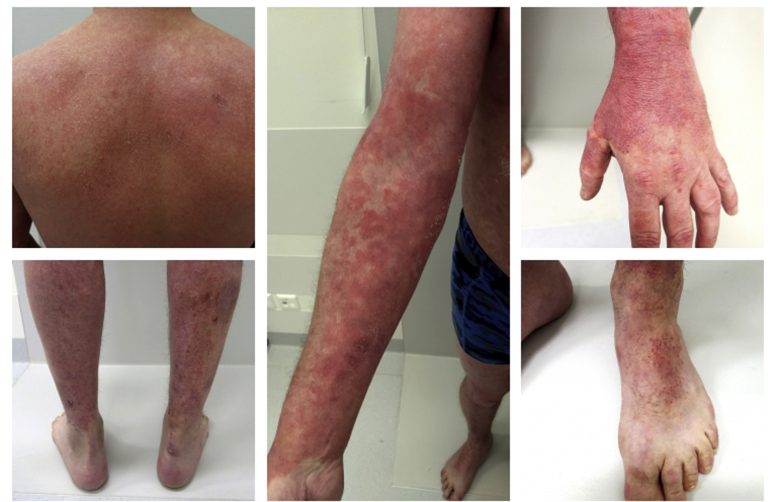
On physical examination, diffuse erythema was observed, most pronounced on the back of the hands and flexor side of both arms. On the trunk and lower limbs, we saw extensive erythematosquamous plaques with multiple excoriations. The face and eyelids were edematous and showed diffuse erythema with pronounced scaling (Fig 1). Body surface area involved was approximately 38%.
Fig 1.
Clinical presentation.
Skin biopsy specimens were taken from the shoulder and the back of the hand. Histologic examination found irregular acanthosis, spongiosis, and maturation into a confluent parakeratotic layer. In the papillary dermis was a lymphocytic inflammatory infiltrate mixed with several eosinophils, locally attacking the epidermis. Histology of the hand showed a regular acanthosis of the epidermis with extension to a confluent parakeratotic layer. These findings were compatible with diagnosis of eczema.
Laboratory examination found mild eosinophilia (0.5 × 109/L [normal, ≤0.4]), and total IgE was 347 kU/L (normal, ≤114). Serum drug concentration at this time was determined using an in-house–developed assay and was 1.4 μg/mL (±0.1). The clinical, histologic, and laboratory findings are consistent with diagnosis of eczema with features of atopic dermatitis.
The patient was unresponsive to topical steroids. He was hospitalized on January 9, 2019 for treatment with tar preparation (betamethasone dipropionate 0.05% in Pix 3% in salicylic acid 1% - Propylene glycol 10% - unguentum simplex) twice daily, resulting in rapid resolution.
Discussion
Psoriasis is a chronic inflammatory skin disorder resulting from complex interactions between immunologic mechanisms, environmental stimuli, and genetic susceptibility. Dendritic cells secrete IL-23, driving Helper T cell (TH) differentiation toward TH17 cell line. These T cells produce various cytokines (eg, IL-17) and are key drivers in the development of psoriasis. Through these cytokines, a chronic inflammatory state—epidermal hyperproliferation, apoptosis, and neoangiogenesis—is induced, resulting in the cutaneous findings observed in psoriasis.2
Real world data for guselkumab are sparse. Reported adverse effects in clinical trials involving skin are contact dermatitis, erysipelas, herpes simplex, tinea, and pruritus.3
Eczematous reactions in patients treated with biologics are frequently reported in the literature. Plenty of reports refer to the development of eczematous reactions associated with TNF-α inhibition when used for psoriasis or extracutaneous indications (inflammatory bowel disease, rheumatology).4 For IL-17 inhibitors, only 4 cases are described. Interestingly, all these patients had a positive personal history of atopy.5 For biologics targeting IL-12/-23, even fewer cases are described. We found 1 case report in the literature for ustekinumab6 and 1 case report describing the onset of nummular eczema under treatment with guselkumab.7
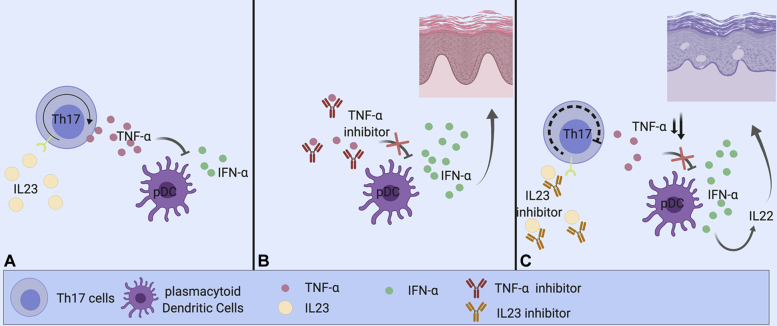
To the best of our knowledge, we are the first to report an eczematous skin eruption with characteristics of atopic dermatitis after administration of guselkumab. It has been hypothesized that paradoxical skin reaction under TNF-α inhibition may be explained by the following mechanism: TNF-α inhibits plasmacytoid dendritic cell secretion of interferon (IFN)-α, a known inducer of psoriasiform-eczematous lesions. Therefore, inhibition of TNF-α can lead to an unopposed increase in IFN-α, resulting in psoriasiform-eczematous skin lesions. As IL-23 induces upregulation of TNF-α through TH17 cells, one could argue that guselkumab partially acts as a TNF-α inhibitor, resulting in increased IFN-α production. It has been shown that IFN-α induces the expression of IL-22 receptors in keratinocytes.8 Furthermore, IL-22 is suggested as a central cytokine in atopic dermatitis.8,9 We hypothesize that through this pathway, a paradoxical eczematous skin reaction can occur in predisposed patients (Fig 2).
Fig 2.
Effect of IL-23 inhibition on TNF-α and IFN-α. (Created with BioRender.com.) A, IL-23 induces upregulation of TNF-α through TH17 cells. TNF-α inhibits plasmacytoid dendritic cell (pDC) secretion of IFN-α, a known inducer of inflammatory skin lesions. B, Inhibition of TNF-α by a TNF-α inhibitor can lead to an unopposed increase in IFN-α, resulting in inflammatory skin lesions. C, As IL-23 induces upregulation of TNF-α through TH17 cells, one could argue that an IL-23 inhibitor partially acts as a TNF-α inhibitor. Through this mechanism, it could increase IFN-α, possibly explaining the paradoxical skin reaction in our patient.
This case report might contribute to a better understanding of the underlying pathophysiologic mechanisms of paradoxical inflammatory reactions. As our antipsoriatic treatments gain affinity for their respective targets, a more potent inhibition of inflammatory pathways can be expected. It is conceivable that in predisposed individuals, this shift in production of a specific TH cell line leads to a change in inflammatory phenotype.
Especially when targeting pivotal cytokines, the role of therapeutic drug monitoring can become more prominent in the future. It is conceivable that dose adjustments based on serum drug concentrations not only positively influence the health economics balance but might also prevent certain side effects.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Wechter T., Cline A., Feldman S.R. Targeting p19 as a treatment option for psoriasis: an evidence-based review of guselkumab. Ther Clin Risk Manag. 2018;14:1489–1497. doi: 10.2147/TCRM.S177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H.J., Lebwohl M.G. Biologics and psoriasis: the beat goes on. Dermatol Clin. 2019;37:29–36. doi: 10.1016/j.det.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Litt’s drug eruption database. http://www.drugeruptiondata.com/drug/id/4732 Available from:
- 4.Nakamura M., Lee K., Singh R. Eczema as an adverse effect of anti-TNFα therapy in psoriasis and other Th1-mediated diseases: a review. J Dermatolog Treat. 2017;28:237–241. doi: 10.1080/09546634.2016.1230173. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano M., Megna M., Fabbrocini G. Eczematous eruption during anti-interleukin 17 treatment of psoriasis: an emerging condition. Br J Dermatol. 2019;181:604–606. doi: 10.1111/bjd.17779. [DOI] [PubMed] [Google Scholar]
- 6.Pernet C., Guillot B., Bessis D. Eczematous drug eruption after ustekinumab treatment. Arch Dermatol. 2012;148:959–960. doi: 10.1001/archdermatol.2012.586. [DOI] [PubMed] [Google Scholar]
- 7.Truong A., Le S., Kiuru M., Maverakis E. Nummular dermatitis on guselkumab for palmoplantar psoriasis. Dermatol Ther. 2019;32:e12954. doi: 10.1111/dth.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tohyama M., Yang L., Hanakawa Y., Dai X., Shirakata Y., Sayama K. IFN-α enhances IL-22 receptor expression in keratinocytes: a possible role in the development of psoriasis. J Invest Dermatol. 2012;132:1933–1935. doi: 10.1038/jid.2011.468. [DOI] [PubMed] [Google Scholar]
- 9.Brunner P.M., Pavel A.B., Khattri S. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol. 2019;143:142–154. doi: 10.1016/j.jaci.2018.07.028. [DOI] [PubMed] [Google Scholar]