Abstract
Hypothesis
To determine the effect of receiving the visual feedback of the sphygmomanometer on lumbopelvic motor control (LPMC) tests in professional swimmers.
Method
31 professional swimmers to participate in the study. The outcome was maximum absolute mmHg variation in the pressure biofeedback unit's manometer with and without visual feedback on four LPMC tests.
Results
Test scores were significantly affected by visual feedback F = 10.07, p = 0.002, η2p = 0.117 and the type of test F = 32.53, p < 0.001, η2p = 0.300.
Conclusion
Visual feedback has a positive effect on the Active Straight Leg Raise Test (ASLR), the Knee Lift Abdominal Test (KLAT) scores completed by professional swimmers.
Keywords: Motor control tests, Core control, Visual feedback, Proprioception, Swimming
1. Introduction
Within the past decade, the concept of core stabilisation has emerged in the field of physical therapy. The goal of this concept is to prevent musculoskeletal injuries, to rehabilitate and to improve sports performance.1 Core stability has been defined as the ability of the osteoarticular and muscular structures, coordinated by the motor control system,2 to maintain or resume a position in the trunk path when subjected to internal or external forces.3 One of the body parts with a pronounced capacity to enhance core stability is the lumbopelvic (LP) area.4,5 Lumbopelvic motor control (LPMC) plays a critical role in stabilising the spinal system.6 The contraction of core muscles in conjunction with limb movements is performed to facilitate LPMC and respond to forces generated from distal body segments as well as forces generated from expected or unexpected perturbations.7,8 An optimum static and dynamic LPMC is considered important for maintaining the functional and structural integrity of the lumbar region into the neutral zone.9 Deficits in dynamic stability have been shown to damage the passive spine structures and result in chronic low back pain.10, 11, 12
Various methods of LPMC evaluation are currently applied clinically for diagnostic purposes as part of a physiotherapy examination.13, 14, 15, 16, 17, 18 The Pressure Biofeedback Unit (PBU) is a reliable, non-invasive and nonpainful device that has been recently used to assess LPMC.19 The PBU consists of an inflatable bag connected to a pressure gauge and inflation device. It was developed to monitor LP by recording pressure changes during assessment.20 The pressure bag measures 16.7 × 24.0 cm and is made of nonelastic material. The sphygmomanometer scale ranges from 0 to 200 mmHg in 2 mmHg intervals. Movement or a change in position results in volume changes in the pressure bag, which are registered by the device.
The PBU was developed to monitor LP movement by recording pressure changes during assessment and exercise21,22 and has been employed to examine the influence of motor control (MC) on the LP region during hip movements.23
The PBU has demonstrated satisfactory results in terms of intrarater reliability; the intraclass correlation coefficient (ICC) ranges from 0.60 to 0.95, and the interrater reliability with ICC ranges from 0.40 to 0.86. The acceptable construct validity with ICC ranges from 0.48–0.90.18,19,24, 25, 26, 27
Several LPMC tests have been developed using the PBU to assess core control status in subjects with chronic, non-specific low back pain.1,19,26,28
The tests most commonly used to assess MC using the PBU include the Active Straight Leg Raising (ASLR),29 the Bent Knee Fall Out (BKFO), 16 the Knee Lift Abdominal Test (KLAT)22,23 and the PRONE test.19,26 Studies that assessed LPMC using the PBU considered that ‘excessive’ pressure changes during low-load exercise reflected an inability to maintain isometric contraction of the abdominal muscles, resulting in uncontrolled movement of the lumbar spine.21
Published studies have not specified the use of visual feedback of the PBU's sphygmomanometer, and there is no consensus about its use when performing PBU exercises or tests.1,30 Visual feedback has been shown to be useful for adjusting movements while observing one's own limbs.31 Moreover, the dominance of vision over proprioception on motor programming has been reported.32 However, several empirical studies have demonstrated the relevance of visual and proprioceptive information in the temporal control of movements,31, 32, 33 and the specific contribution of visual information remains unclear. Proprioceptive reafferents naturally provide valid and temporally precise information about the execution of movements; additional visual information may not play a primary role in the control of movement in unimpaired or inexperienced individuals.34 In contrast, studies of motor or somatosensory impairments and studies of the acquisition of motor skills have stressed that motor control largely relies on visual information.32,33 Nevertheless, to the best of our knowledge, there has been a lack of studies comparing the effect of PBU manometer visual feedback on LPMC test performance. Therefore, the aim of the present study was to determine the effect of visual feedback on LPMC tests using the PBU in professional swimmers. We hypothesised that visual feedback would significantly improve the LPMC test performance. We also hypothesised that the scores for different tests, watching would be significantly different.
2. Methods
2.1. Design
A randomised, repeated-measures cross-sectional design was used to determine the difference in LPMC test performance with and without visual feedback conditions. The study was designed according to the STROBE publishing guidelines.
2.2. Participants
A total of39 participants (20 males, 19 females; mean age: 20.3 ± 3.6 years; mean mass: 67.9 ± 9.9 kg; mean height: 1.7 ± 0.1 m; mean body mass index [BMI]: 22.3 ± 1.8 kg m-2) voluntarily participated in the study. The inclusion criteria selected professional swimmers with at least 2 years of experience in national competitions who were willing and able to participate in a core control assessment session. The participants also had to be free of physical impairments that could limit their participation during the testing day. We excluded participants who were pregnant, who had a BMI of 25 kg/m2 or higher and individuals who had previous experience with PBU tests. The subjects were also requested not to take stimulants or medications or to consume alcohol that could alter their conscious nervous system perception before the tests.
The study was approved by the institutional ethics committee according to the latest version of the Declaration of Helsinki. Participants signed the inform consent prior to start of the study.
2.3. Instrumentation
The PBU (Stabilizer®, Chattanooga Group, Inc., Hixson, TN, USA) was used to monitor LP movement in four MC tests. The same PBU unit was used throughout the study to avoid between-device differences.27.
A manual chronometer (Namaste© model 898, Spain) was used to identify the duration (in seconds) that the subjects maintained each position in the ASLR and PRONE tests.
A simple long-arm goniometer (Orthopedic Equipment Co., Bourbon, KY, USA) with a 360°, marked in 1-degree increments, was used to control the starting positions of the hips and knees during the KLAT and BKFO tests.
2.4. Procedure
Before the testing session, the order of the visual feedback, the order of the subjects and the order of the applied tests were randomly determined using a true random number generator to avoid order effects.
Four commonly used clinical tests were applied to evaluate LPMC (KLAT, BKFO, ASLR and PRONE tests). The pressure was inflated to 40 mmHg for the supine tests and 70 mmHg for the PRONE test (baseline pressure).22,30 Before the tests, the subjects performed two inspirations and expirations, and the pressure was then readjusted. The participants were instructed to maintain a neutral lumbar spine position while moving their lower limbs and while lying on a hard surface to ensure that foam did not interfere with the PBU-measured pressures.35 The subjects received no verbal feedback to encourage them for any of the measurements.
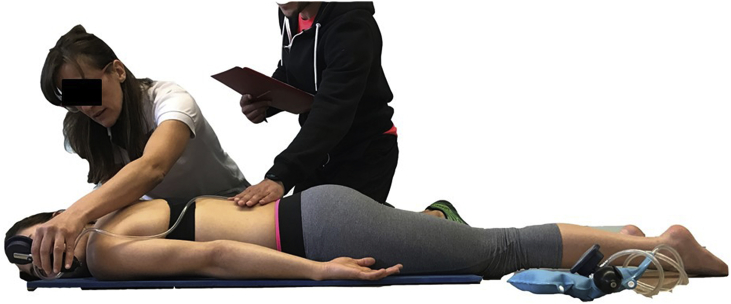
The ASLR (Fig. 1) was performed in a supine position. The participant lifted one extended lower limb 20 cm above the mat marked by a small bottle of water. Next, the subject held this position for20 sec based on input from the researchers. The PBU was placed horizontally under the lumbar spine of the participant, with the lower edge at the level of the posterior superior iliac spines.
Fig. 1.
ASLR
For the BKFO (Fig. 2), the subjects were positioned in a supine position while lying on a mat with a partial crook, with one knee flexed by 120° and the other lower limb in a neutral position.16 The participants were asked to slowly bend their hip to approximately 45° of abduction/lateral rotation while keeping their foot supported beside their straight knee and then return to the starting position. Two joined biofeedback units were placed under the centre of the back at the L3 level and connected along the spine to avoid differences in the lumbar tactile cue, although only the data of the PBU from the moving limb were considered.
Fig. 2.
BKFO
During the KLAT (Fig. 3), the subjects were positioned in a crook position and were asked to lift one foot off the mat until they attained a hip and knee flexion of 90°. At the same time, they were challenged to keep their lumbar spine in a neutral position. The PBU was placed horizontally under the spine of the participant, with the lower edge at the level of the posterior superior iliac spines.
Fig. 3.
KLAT
In the PRONE test (Fig. 4), the participants were instructed to lay down in a prone position on the mat with their arms beside the trunks. The inflatable bag was placed between the anterior superior iliac spine and the navel. Before starting the contraction, the bag was inflated to a pressure of 70 mmHg, and the participants were instructed to breathe deeply using mainly their abdominal wall. After the participants completed two normal breaths, the inflatable bag was readjusted to 70 mmHg. The subjects were requested to perform three contractions with the following verbal command: “Draw in your abdomen without moving your lumbar spine or pelvis and hold that position until I tell you otherwise”. Using palpation, the examiner checked whether the participants were moving their spine or pelvis over the course of 10 s.
Fig. 4.
PRONE test
2.5. Outcome measures
The maximum absolute mmHg deviation from the preset baseline pressure (40 or 70 mmHg) was recorded during each test. Excessive pressure changes were interpreted as uncontrolled movement of the lumbar spine for the asymmetric test, and higher deviations in prone test were related to an increased ability to voluntarily contract the transversus abdominis (TrA).
2.6. Data analysis
The data are presented as mean values ± standard deviations (SDs). The Kolmogorov-Smirnov test was used to test for a normal distribution of the data. A two-way, mixed-model ANOVA (visual feedback [with and without] × type of test [KLAT-right, KLAT-left, ASLR-right, ASLR-left, BKFO-right, BKFO-left and PRONE]) was applied to assess the effects of visual feedback and the type of test on the dependent variable (the absolute value of the maximum mmHg [ABSmmHgmax]). Where appropriate, univariate contrast and Bonferroni-adjusted post-hoc analyses were performed. Additional information about the percentages of the increment of the ABSmmHg (%ΔABSmmHg) between conditions (with and without visual feedback) was added to the descriptive analyses.
Descriptive analyses of frequencies were used to determine the percentage of responders or nonresponders to the visual feedback factor. The responders included subjects who reduced their ABSmmHgmax score between their results with and without visual feedback. The nonresponders were individuals whose ABSmmHgmax scores were the same or higher for the visual feedback and nonvisual feedback conditions. Statistical analyses were performed with SPSS for Windows (V.22, IBM, Armonk, NY, USA), and p ≤ 0.05 was used to established statistical significance.
3. Results
The descriptive data are listed in Table 1.
Table 1.
Maximal ABSmmHg scores for each test with and without visual feedback and percentage of the difference between mean values.
| Test | Right Side | Right Side Visual Input | P | %Δ | Left Side | Left Side Visual Input | P | % Change |
|---|---|---|---|---|---|---|---|---|
| KLAT | 18.3 (13.4) | 10.4 (6.4) | 0.00 | 43.1 | 18.3 (13.1) | 10.7 (7.8) | 0.00 | 41.4 |
| ASLR | 8.0 (7.0) | 4.5 (4.0) | 0.00 | 43.9 | 7.8 (7.3) | 4.3 (4.0) | 0.00 | 44.3 |
| BKFO | 11.5 (9.2) | 8.2 (5.4) | 0.61 | 28.3 | 12.6 (10.9) | 9.2 (6.7) | 0.10 | 27.0 |
| PRONE | 17.3 (7.8) | 19.3 (8.2) | 0.14 | 10.3 | – | – | – | - |
KLAT: Knee Lift Abdominal Test, ASLR: Active Straight Leg Raise Test, BKFO: Bent Knee Fall Out Test, PRONE: PRONE Test.
There was a significant effect for visual feedback F(1, 76) = 10.07, p = 0.002, η2p = 0.117 and a significant effect for the type of test F(3.4, 260.19) = 32.53, p = 0.000, η2p = 0.300 on the LPMC tests scores. The sphericity assumption was violated, and the number of degrees of freedom was adjusted using the Huynh-Feldt method.
There was a nonsignificant interaction effect for visual feedback × type of test (p = 0.073).
Pair-wise comparisons revealed no significant differences between the scores for both sides for asymmetrical tests (p = 1.000). Moreover, there were nonsignificant differences between scores obtained with or without visual feedback for the BKFO developed with right and left side (p = 0.61 and p = 0.10, respectively) and for the PRONE (p = 0.278).
The descriptive data revealed %Δ between mean values from 27.0% to 44.3%; scores were better for visual feedback conditions (Table 1).
Mean Scores are presented by ABSmmHgmax mean values (SD). %Δ: The percentage of the difference between mean values is expressed by %.
The frequency analyses revealed there were higher percentages of responders for visual feedback than no visual feedback for all LPMC tests (Table 2).
Table 2.
Percentages of responders with visual feedback.
| Test | Improve (%) | Not improve (%) |
|---|---|---|
| KLAT moving the right lower limb | 71.8 | 28.2 |
| KLAT moving the left lower limb | 69.2 | 30.8 |
| ASLR moving the right lower limb | 64.1 | 35.8 |
| ASLR moving the left lower limb | 66.7 | 33.3 |
| BKFO moving the right lower limb | 66.7 | 33.3 |
| BKFO moving the left lower limb | 53.8 | 46.2 |
| PRONE test | 64.1 | 35.9 |
KLAT: Knee Lift Abdominal Test, ASLR: Active Straight Leg Raise Test, BKFO: Bent Knee Fall Out Test, PRONE: PRONE Test.
4. Discussion
This study revealed that the presence of visual feedback can significantly improve performance in asymmetrical motor control tests (e.g., KLAT, ASLR). However, visual feedback does not have a significant effect on BKFO or PRONE test scores. Additionally, there were no significant differences in the scores obtained with the right or left lower limbs, although there were significant differences among the scores for the different tests.
Receiving manometer visual feedback has been shown to increase subjects’ capacity for recognising specific lumbar pressure feedback and processing this signal to maintain the LP area in a stable position in which the lower limbs are moved in the sagittal plane. This result is in agreement with the findings of previous studies that reported that visual feedback increased multifidus isometric activation while subjects received ultrasound image feedback.36 The results support the idea of the convergence between visual and sensory afferent information when inputs arrive to the primary sensitive parietal area in the brain. Furthermore, other imaging studies have also shown substantial activation in parietal regions during visually guided limb movements.37,38 All afferent information is processed in the secondary sensitive parietal area to yield a better motor response to that kind of stimulus. This neurophysiologic mechanism could interfere with the modulation of LPMC muscles to enable processing of the relations between the manometer nail movements and PBU tactile cues.
On the other hand, visual feedback did not interfere significantly with PRONE or BKFO test scores. The PRONE test is one of the LPMC tests that checks an individual's ability to activate his/her TrA muscle rather than testing subjects' LP ability to remain stable while their limbs are moving.39 Moreover, during the PRONE test, there is no limb and lumbar movement, and there is no challenge in terms of LP stability, which reduces proprioceptive afferent implications and therefore visuo-motor pathway intervention.
Lumbopelvic motor control is likely to affect the race times of elite swimmers.40 Additionally, the BKFO is an LMPC test that challenges subjects’ ability to control their core while their limbs are moving in transversal and coronal planes and they are performing hip external rotation and abduction, movements that are rare for swimmers (with the exception of those who perform the breaststroke). It is known that familiar movements are better controlled than unfamiliar movements. Therefore, the type of movements developed during the tests will be better controlled if these movements are performed during the technical movements of each sport.
Applying the LPMC tests using the PBU in swimmers could be an easy, nonpainful and rapid way to assess the core control status of elite swimmers, and they may be used to improve and follow up swimmers’ core control exercise programs. Therefore, MC tests with the PBU in swimmers could also be used to predict LP fatigue or injury risk.8,12
To the best of our knowledge, there is a lack of athlete-based studies objectively assessing the effect of visual feedback on LPMC using PBU tests. This situation makes it difficult to compare the swimmers' PBU performance in the present study with previous athlete-based studies.25,41,42 Nevertheless, it is advisable that specific lower-limb technical requirements of different sports can interfere with LPMC during PBU tests. For instance, dancers with hypermobility exhibited lower scores on the KLAT, ASLR and BKFO tests than our swimmers.25 Swimmers exhibited lower scores on the KLAT and BKFO than soccer players but better scores on the ASLR test.41 It seems that LPMC is better when the range of movement of the lower limbs during the tests is closer to that developed in each sport. For example, swimmers exhibit better control on the ASLR than the KLAT because swimmers’ kicking ranges from 10 to 20° of hip extension to 20–30° of flexion.36
We observed that each PBU test yielded different information about the subjects' LPMC. Indeed, large manometer displacements in the KLAT were revealed that subjects were not able to stabilise their lumbar spine while their limbs are moving from 45 to 90° of hip flexion.22,23 On the other hand, the ASLR test produced a larger torque to stabilise the LP area while the lower limbs were moved to 10–20° of hip flexion with the knee in an extended position.43 During the KLAT movements, it was more difficult to avoid posterior pelvic tilt during hip flexion than starting from a neutral position, which is the case in the ASLR test. It is possible that the relaxed starting position of the abdominal muscles in the KLAT test reduced the tension of muscle spindles and therefore their ability to stabilise the LP region when it was perturbed. Because the KLAT test requires more LPMC from passive soft-tissue proprioceptors, the ASLR requires more strength and assesses the participant's ability to transfer a load between his/her spine and lower limbs via the pelvis.43.
The BKFO contributed information about the subjects' ability to control lumbar rotations during abduction/lateral rotation of the hip.16 This is the only test that yielded information about the subjects' LPMC during transversal and coronal hip movements, in contrast with the sagittal plane of the other tests. Finally, the PRONE test is the only one that assesses subjects’ ability to voluntarily recruit the TrA in a prone position, a geometry that has been reported to be challenging for these muscles.19,26
The results of the present study could help practitioners consider different practical applications. First, the LPMC tests using the PBU must to be applied without visual feedback because this behaviour is closer to the function that the LP area develops during athletes' training and competitions. In this situation, the nervous system will be able to reproduce trained LPMC behaviours without the aid of PBU visual feedback. Nevertheless, if we ask patients or athletes to train LPMC using this PBU visual feedback, then the best choice to assess subjects’ progress will be in the same conditions in which they trained. Secondly, our results revealed non-significant differences between body sides in the tests, which is consistent with the findings of previous studies of athletes.25,42 This finding suggests it is not necessary to perform asymmetrical tests with both sides—practitioners could save time in terms of assessing LPMC by taking measurements only with one side. Finally, depending on the requirements of the lower limbs in each sport, practitioners should choose the LPMC test that is better adapted to the functional technical abilities of their athletes. For example, swim coaches and physiotherapists should use the BKFO only on breaststroke swimmers because they need LPMC while their lower limbs move on abduction and external rotation during the breaststroke kick.
Certain issues and limitations regarding the design, methodology and overall validity of this study need to be considered. The coaches imposed restrictions in terms of testing so as not to disturb the athletes and their preparations for competition. Further investigations are necessary in larger samples of healthy athletes and asymmetrical sports to obtain baseline reference data of the levels of performance of LPMC using the PBU. Although the reliability of PBU tests has been studied, there is still a lack of studies pertaining to the validity of these tests, which could help practitioners assess the LPMC of patients or athletes outside of laboratories without using expensive and difficult-to-manage devices.
5. Conclusions
Watching the PBU's sphygmomanometer decreases the nail deviations in KLAT and ASLR tests but does not affect the results in BKFO and PRONE tests. Swimmers did not exhibit differences between sides in asymmetrical tests. Future studies using the PBU must consider not allowing subjects to watch the sphygmomanometer during the tests to ensure that the central nervous system determines the correct LP position based on proprioceptors and tactile cues, a situation consistent with most daily activities and sports.
Conflicts of interest
The authors stated no conflict of interest.
Ethical approval
The study was approved by the institutional ethics committee according the latest version of the Declaration of Helsinki.
Funding
Not applicable.
Acknowledgements
The authors thanks the Spanish Royal Federation of Swimming (RFEN), swimming coaches and swimmers for their assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jor.2019.06.002.
Contributor Information
Mònica Solana-Tramunt, Email: monicast2@blanquerna.url.edu.
Alberto Ortegón, Email: albertoop@blanquerna.url.edu.
José Morales, Email: josema@blanquerna.url.edu.
Ainhoa Nieto, Email: ainhoang@blanquerna.url.edu.
María Betina Nishishinya, Email: betinanishishinya@yahoo.es.
Jorge Hugo Villafañe, Email: mail@villafane.it.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cynn H.S., Oh J.S., Kwon O.Y., Yi C.H. Effects of lumbar stabilization using a pressure biofeedback unit on muscle activity and lateral pelvic tilt during hip abduction in sidelying. Arch Phys Med Rehabil. 2006;87(11):1454–1458. doi: 10.1016/j.apmr.2006.08.327. [DOI] [PubMed] [Google Scholar]
- 2.Negrini S., Imperio G., Villafane J.H., Negrini F., Zaina F. Systematic reviews of physical and rehabilitation medicine Cochrane contents. Part 1. Disabilities due to spinal disorders and pain syndromes in adults. Eur J Phys Rehabil Med. 2013;49(4):597–609. [PubMed] [Google Scholar]
- 3.Vera-García F.J., Barbado D., Moreno-Pérez V., Hernández-Sánchez S., Juan-Recio C J.L.L.E. Core stability. Concepto y aportaciones al entrenamiento y la prevención de lesiones. Rev Andaluza Med del Deport. 2015;8(2):79–85. [Google Scholar]
- 4.Langella F., Villafane J.H., Ismael M. Reliability of the xipho-pubic angle in patients with sagittal imbalance of the spine. J Neurosurg Sci. 2018;62(2):121–127. doi: 10.23736/S0390-5616.16.03556-6. [DOI] [PubMed] [Google Scholar]
- 5.Langella F., Villafane J.H., Lafage V. Xipho-pubic angle (XPA) correlates with patient's reported outcomes in a population of adult spinal deformity: results from a multi-center cohort study. Eur Spine J : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2018;27(3):670–677. doi: 10.1007/s00586-017-5460-5. [DOI] [PubMed] [Google Scholar]
- 6.Borghuis J., Hof A.L., Lemmink K.A. The importance of sensory-motor control in providing core stability: implications for measurement and training. Sports Med. 2008;38(11):893–916. doi: 10.2165/00007256-200838110-00002. [DOI] [PubMed] [Google Scholar]
- 7.Leonard J.H., Paungmali A., Sitilertpisan P., Pirunsan U., Uthaikhup S. Changes in transversus abdominis muscle thickness after lumbo-pelvic core stabilization training among chronic low back pain individuals. La Clinica terapeutica. 2015;166(5):e312–e316. doi: 10.7417/T.2015.1884. [DOI] [PubMed] [Google Scholar]
- 8.Zazulak B.T., Hewett T.E., Reeves N.P., Goldberg B., Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am J Sports Med. 2007;35(7):1123–1130. doi: 10.1177/0363546507301585. [DOI] [PubMed] [Google Scholar]
- 9.Panjabi M.M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5(4):390–396. doi: 10.1097/00002517-199212000-00002. discussion 7. [DOI] [PubMed] [Google Scholar]
- 10.Hodges P., van den Hoorn W., Dawson A., Cholewicki J. Changes in the mechanical properties of the trunk in low back pain may be associated with recurrence. J Biomech. 2009;42(1):61–66. doi: 10.1016/j.jbiomech.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Radebold A., Cholewicki J., Polzhofer G.K., Greene H.S. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26(7):724–730. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Cholewicki J., McGill S.M. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech. 1996;11(1):1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari S., Manni T., Bonetti F., Villafane J.H., Vanti C. A literature review of clinical tests for lumbar instability in low back pain: validity and applicability in clinical practice. Chiropr Man Ther. 2015;23:14. doi: 10.1186/s12998-015-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillastrini P., Ferrari S., Rattin S., Cupello A., Villafane J.H., Vanti C. Exercise and tropism of the multifidus muscle in low back pain: a short review. J Phys Ther Sci. 2015;27(3):943–945. doi: 10.1589/jpts.27.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comerford M.J., Mottram S.L. Movement and stability dysfunction--contemporary developments. Man Ther. 2001;6(1):15–26. doi: 10.1054/math.2000.0388. [DOI] [PubMed] [Google Scholar]
- 16.Comerford M.J., Mottram S.L. Functional stability re-training: principles and strategies for managing mechanical dysfunction. Man Ther. 2001;6(1):3–14. doi: 10.1054/math.2000.0389. [DOI] [PubMed] [Google Scholar]
- 17.Hides J.A., Jull G.A., Richardson C.A. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine. 2001;26(11):E243–E248. doi: 10.1097/00007632-200106010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Dankaerts W., O'Sullivan P.B., Straker L.M., Burnett A.F., Skouen J.S. The inter-examiner reliability of a classification method for non-specific chronic low back pain patients with motor control impairment. Man Ther. 2006;11(1):28–39. doi: 10.1016/j.math.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Lima P.O., Oliveira R.R., Moura Filho A.G., Raposo M.C., Costa L.O., Laurentino G.E. Concurrent validity of the pressure biofeedback unit and surface electromyography in measuring transversus abdominis muscle activity in patients with chronic nonspecific low back pain. Rev Brasileira Fisioterapia. 2012;16(5):389–395. doi: 10.1590/s1413-35552012005000038. [DOI] [PubMed] [Google Scholar]
- 20.Richardson C., Jull G., Toppenberg R., Comerford M. Techniques for active lumbar stabilisation for spinal protection: a pilot study. Aust J Physiother. 1992;38(2):105–112. doi: 10.1016/S0004-9514(14)60555-9. [DOI] [PubMed] [Google Scholar]
- 21.Jull G., Richardson C., Toppenberg R., Comerford M., Bui B. Towards a measurement of active muscle control for lumbar stabilisation. Aust J Physiother. 1993;39(3):187–193. doi: 10.1016/S0004-9514(14)60481-5. [DOI] [PubMed] [Google Scholar]
- 22.Wohlfahrt D., Jull G., Richardson C. The relationship between the dynamic and static function of abdominal muscles. Aust J Physiother. 1993;39(1):9–13. doi: 10.1016/S0004-9514(14)60464-5. [DOI] [PubMed] [Google Scholar]
- 23.Roussel N., Nijs J., Truijen S., Vervecken L., Mottram S., Stassijns G. Altered breathing patterns during lumbopelvic motor control tests in chronic low back pain: a case-control study. Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2009;18(7):1066–1073. doi: 10.1007/s00586-009-1020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azevedo D.C., Lauria A.C., Pereira A.R. Intraexaminer and interexaminer reliability of pressure biofeedback unit for assessing lumbopelvic stability during 6 lower limb movement tests. J Manip Physiol Therapeut. 2013;36(1):33–43. doi: 10.1016/j.jmpt.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Roussel N.A., Nijs J., Mottram S., Van Moorsel A., Truijen S., Stassijns G. Altered lumbopelvic movement control but not generalized joint hypermobility is associated with increased injury in dancers. A prospective study. Man Ther. 2009;14(6):630–635. doi: 10.1016/j.math.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Lima P.O., de Oliveira R.R., de Moura Filho A.G., Raposo M.C., Costa L.O., Laurentino G.E. Reproducibility of the pressure biofeedback unit in measuring transversus abdominis muscle activity in patients with chronic nonspecific low back pain. J Bodyw Mov Ther. 2012;16(2):251–257. doi: 10.1016/j.jbmt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 27.von Garnier K., Koveker K., Rackwitz B. Reliability of a test measuring transversus abdominis muscle recruitment with a pressure biofeedback unit. Physiotherapy. 2009;95(1):8–14. doi: 10.1016/j.physio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Noh K.H., Kim J.W., Kim G.M., Ha S.M., Oh J.S. The influence of dual pressure biofeedback units on pelvic rotation and abdominal muscle activity during the active straight leg raise in women with chronic lower back pain. J Phys Ther Sci. 2014;26(5):717–719. doi: 10.1589/jpts.26.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roussel N.A., Nijs J., Truijen S., Smeuninx L., Stassijns G. Low back pain: clinimetric properties of the Trendelenburg test, active straight leg raise test, and breathing pattern during active straight leg raising. J Manip Physiol Therapeut. 2007;30(4):270–278. doi: 10.1016/j.jmpt.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Hides J., Stanton W., Mendis M.D., Sexton M. The relationship of transversus abdominis and lumbar multifidus clinical muscle tests in patients with chronic low back pain. Man Ther. 2011;16(6):573–577. doi: 10.1016/j.math.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Graziano M.S. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc Natl Acad Sci U S A. 1999;96(18):10418–10421. doi: 10.1073/pnas.96.18.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touzalin-Chretien P., Ehrler S., Dufour A. Dominance of vision over proprioception on motor programming: evidence from ERP. Cerebr Cortex. 2010;20(8):2007–2016. doi: 10.1093/cercor/bhp271. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard C., Roll R., Roll J.P., Kavounoudias A. Differential contributions of vision, touch and muscle proprioception to the coding of hand movements. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simoneau G., Ulbrecht J., Derr J., Cavanagh P. Role of somatosensory input in the control of human posture. Gait Posture. 1995;3(3):115–122. [Google Scholar]
- 35.Storheim K., Bo K., Pederstad O., Jahnsen R. Intra-tester reproducibility of pressure biofeedback in measurement of transversus abdominis function. Physiother Res Int : the journal for researchers and clinicians in physical therapy. 2002;7(4):239–249. doi: 10.1002/pri.263. [DOI] [PubMed] [Google Scholar]
- 36.Van K., Hides J.A., Richardson C.A. The use of real-time ultrasound imaging for biofeedback of lumbar multifidus muscle contraction in healthy subjects. J Orthop Sport Phys Ther. 2006;36(12):920–925. doi: 10.2519/jospt.2006.2304. [DOI] [PubMed] [Google Scholar]
- 37.Ellermann J.M., Siegal J.D., Strupp J.P., Ebner T.J., Ugurbil K. Activation of visuomotor systems during visually guided movements: a functional MRI study. J Magn Reson. 1998;131(2):272–285. doi: 10.1006/jmre.1998.1379. [DOI] [PubMed] [Google Scholar]
- 38.Inoue K., Kawashima R., Satoh K. PET study of pointing with visual feedback of moving hands. J Neurophysiol. 1998;79(1):117–125. doi: 10.1152/jn.1998.79.1.117. [DOI] [PubMed] [Google Scholar]
- 39.de Paula Lima P.O., de Oliveira R.R., Costa L.O., Laurentino G.E. Measurement properties of the pressure biofeedback unit in the evaluation of transversus abdominis muscle activity: a systematic review. Physiotherapy. 2011;97(2):100–106. doi: 10.1016/j.physio.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Uematsu A., Kurita Y., Inoue K., Okuno K., Hortobagyi T., Suzuki S. A 200-m all-out front-crawl swim modifies competitive swimmers' shoulder joint position sense. Int J Sports Med. 2015;36(13):1081–1086. doi: 10.1055/s-0035-1554698. [DOI] [PubMed] [Google Scholar]
- 41.Grosdent S., Demoulin C., Rodriguez de La Cruz C. Lumbopelvic motor control and low back pain in elite soccer players: a cross-sectional study. J Sport Sci. 2016;34(11):1021–1029. doi: 10.1080/02640414.2015.1085077. [DOI] [PubMed] [Google Scholar]
- 42.Olivier B., Stewart A.V., Olorunju S.A., McKinon W. Static and dynamic balance ability, lumbo-pelvic movement control and injury incidence in cricket pace bowlers. J Sci Med Sport. 2015;18(1):19–25. doi: 10.1016/j.jsams.2013.10.245. [DOI] [PubMed] [Google Scholar]
- 43.Hu H., Meijer O.G., van Dieen J.H. Muscle activity during the active straight leg raise (ASLR), and the effects of a pelvic belt on the ASLR and on treadmill walking. J Biomech. 2010;43(3):532–539. doi: 10.1016/j.jbiomech.2009.09.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.