Abstract
Aims
Aquaporins (AQPs) are channel proteins that facilitate the rapid passive movement of water. In our studies it was proved that the decreased AQP5 expression is followed by the increase of uterine contractility. The transient receptor potential vanilloid 4 (TRPV4) is a calcium channel, which is activated in response to osmotic changes. Our aim was to determine the possible role of AQP5 in this osmotic regulation of TRPV4, thus in pregnant uterine contraction.
Main methods
We used RT-PCR and Western blot techniques for the detection of the TRPV4 expression during pregnancy in rat uterus. The localization of AQP5 and TRPV4 was determined by immunohistochemical studies. The role of TRPV4 in uterus contraction was investigated in an isolated organ bath system. In vitro uterus contractions were stimulated with KCl and its effect was investigated with the selective TRPV4 agonist (RN1747) and antagonist (RN1734).
Key findings
The TRPV4 expression continuously increased from day 18 to the last day of pregnancy. The co-expression of TRPV4 and AQP5 in the myometrium and endometrium was determined in the late pregnant uterus. The TRPV4 antagonist and agonist significantly decreased and increased uterine contraction, respectively, especially on the last day of pregnancy.
Significance
We presume the decreased AQP5 expression triggers hypertonic stress, which activates TRPV4 and increases uterus contraction on the day of labor. Based on these findings, we suppose the TRPV4 effect on uterus contraction is AQP5 control, which could be a new target in preterm birth therapy.
Keywords: Obstetrics, Pharmacology, Physiology, Pregnancy, Molecular biology, Reproductive system, AQP5, Pregnancy, Preterm birth, TRPV4
Obstetrics; Pharmacology; Physiology; Pregnancy; Molecular biology; Reproductive system; AQP5; Pregnancy; Preterm birth; TRPV4
1. Introduction
The signals and mechanisms that synchronize the timing of parturition are not fully understood and a better understanding of these processes would be essential to avert adverse pregnancy outcomes [1]. The understanding of myometrial contraction at the time of labor is important for the development of novel therapeutic strategies against preterm labor, which is the main cause of prenatal mortality and morbidity.
Evidence suggests that myometrial smooth muscle Ca2+ homeostasis is modulated near the term to promote uterine contractility. In the myometrium, the entry of extracellular Ca2+ is essential for the maintenance of spontaneous rhythmic contractions [2]. The transient receptor potential vanilloid (TRPV) channels belong to the vanilloid subfamily of the TRP channel family, a group of nonselective cation channels permeable to sodium, Ca2+ and magnesium. The TRPV4 channel is a Ca2+ channel activated by a variety of stimuli, including warm temperature (27–42 °C), osmotic changes and endogenous lipids. The possible influence of TRPV4 has been reported in the smooth muscle contraction of pregnant and non-pregnant uterus [3, 4] but its exact role is still unknown.
Normal water homeostasis in the female reproductive system is indispensable for healthy pregnancy and successful delivery. Aquaporins (AQPs) are present in the uterus during parturition, participating in the control of pregnant myometrial contractions and cervical ripening [5]. Earlier we identified AQP1, 2, 3, 5, 8 and 9 in rat uteri on gestational days 18–22. At the end of pregnancy, the expression of AQP5 was much higher as compared with other AQPs, however, its expression dropped dramatically on the last day of gestation. It was also proved that this type of water channel is selectively down-regulated by oxytocin [6]. Progesterone and progesterone derivatives up-regulated the AQP5 expression, which was more predominant as compared with estrogen pretreatment. This finding was confirmed by hormonally-induced (antigestagen with prostaglandin E2) preterm labor, resulting in a significant drop in the expression of AQP5 in the rat uterus. The decrease in uterine AQP5 expression during preterm delivery was similar to the normal term uteri, suggesting that reduced AQP5 expression after progesterone deprivation may contribute to the initiation of labor [7].
Although this inverse correlation between the AQP5 level and myometrial contraction was found, the mechanism of this phenomenon is still unknown. We hypothesize an osmotic pathway – through AQP5 – might have influence on the changes in TRPV4 function. These osmotic stimuli for TRPV4 may modify the intracellular Ca2+ level, which results in myometrial contraction. The aim of our study was to investigate the co-expression and cooperation of AQP5 and TRPV4 in the pregnant uterus and their mutual regulatory effect on myometrial contraction.
2. Materials and methods
2.1. Housing and handling of the animals
The animals were treated in accordance with the European Communities Council Directives (2010/63/EU) and the Hungarian Act for the Protection of Animals in Research (Article 32 of Act XXVIII). All experiments involving animal subjects were carried out with the approval of the National Scientific Ethical Committee on Animal Experimentation (registration number: IV/198/2013). Sprague–Dawley rats (INNOVO Ltd., Gödöllő, Hungary) were kept at 22 ± 3 °C; the relative humidity was 30–70% and the light/dark cycle was 12/12 h. The animals were maintained on a standard rodent pellet diet (INNOVO Ltd., Gödöllő, Hungary) with tap water available ad libitum. They were sacrificed by CO2 inhalation.
2.2. Mating of the animals
Mature female (180–200 g) and male (240–260 g) Sprague-Dawley rats were mated in a special mating cage before dawn. Within 4 h after mating, vaginal smears were taken and a sperm search was performed under a microscope. If the search proved positive, the female rats were separated and were regarded as first-day pregnant animals.
2.3. RT-PCR studies
2.3.1. Tissue isolation
Rats were terminated by CO2 inhalation, while the fetuses were terminated by cervical dislocation. The non-pregnant uteri and the pregnant uterine tissues (from between two implantation places) were rapidly removed and placed in RNAlater Solution (Sigma-Aldrich, Hungary). The tissues were frozen in liquid nitrogen and then stored at -70 °C until the extraction of total RNA.
2.3.2. Total RNA preparation from tissue
Total cellular RNA was isolated by extraction with guanidinium thiocyanate-acid-phenol-chloroform according to the procedure of Chomczynski and Sacchi [8]. After precipitation with isopropanol, the RNA was washed with 75% ethanol and then resuspended in diethyl pyrocarbonate-treated water. RNA purity was controlled at an optical density of 260/280 nm with BioSpec Nano (Shimadzu, Japan); all samples exhibited an absorbance ratio in the range of 1.6–2.0. RNA quality and integrity were assessed by agarose gel electrophoresis.
2.3.3. Real-time quantitative reverse transcription-PCR (RT-PCR)
Reverse transcription and amplification of the PCR products were performed by using the TaqMan RNA-to-CT-Step One Kit (Life Technologies, Hungary) and an ABI StepOne Real-Time cycler. Reverse-transcriptase PCR amplifications were performed as follows: at 48 °C for 15 min and at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 1 min. The generation of specific PCR products was confirmed by melting curve analysis. The following primers were used: assay ID Rn00562837_m1 for the Aqp5 water channel, Rn00576745_m1 for Trpv4 and Rn00667869_m1 for β-actin as endogenous control. All samples were run in triplicate. The fluorescence intensities of the probes were plotted against PCR cycle number. The amplification cycle displaying the first significant increase of the fluorescence signal was defined as the threshold cycle (CT).
2.4. Western blot analysis
25 μg of protein per well was subjected to electrophoresis on 4–12% NuPAGE Bis-Tris Gel in XCell SureLock Mini-Cell Units (Thermo Fisher Scientific, Hungary). Proteins were transferred from gels to nitrocellulose membranes, using the iBlot Gel Transfer System (Thermo Fisher Scientific, Hungary). The antibody binding was detected with the WesternBreeze Chromogenic Western blot immunodetection kit (Thermo Fisher Scientific, Hungary). The blots were incubated on a shaker with AQP5 (cat. no sc-514022), β-actin (cat. no sc-8432) monoclonal antibody (Santa Cruz Biotechnology, California, 1:200) and TRPV4 (Thermo Fisher Scientific, Hungary, cat. no OSR00136W, 1:200) in the blocking buffer. Images were captured with the EDAS290 imaging system (Csertex Ltd., Hungary), and the optical density of each immunoreactive band was determined with Kodak 1D Images analysis software. Optical densities were calculated as arbitrary units after local area background subtraction.
2.5. Immunohistochemistry
The localization of TRPV4 and AQP5 in the rat uterus was examined by immunohistochemistry. Late pregnant (pregnancy days 18 and 22) uteri were fixed in paraformaldehyde and then embedded in paraffin, sectioned (5-μm-thick tissue sections) deparaffinized, rehydrated and incubated in acidic citrate buffer (pH6) in microwave for antigen recovery, then treated with 3% hydrogen peroxide to quench endogenous peroxidase activity. After washing, sections were placed on normal blocking solution, treated with rabbit polyclonal anti-TRPV4 (cat. no. 20987-1-AP, Proteintech, UK) and AQP5 (cat. no PA5-36529, ThermoFischer Scientific, Hungary) primary antibodies in a dilution of 1:200 for 1 h at room temperature. Incubation was performed with the Histo-Labeling system anti-rabbit secondary antibody conjugated with peroxidase (Histols Reagent, Hungary) and the reaction was visualized using 3,3-diaminobenzidine tetrachloride (Histols DAB, Histols Reagent, Hungary). Histological counterstaining was performed with haematoxylin. For double immunofluorescence analysis, the Tyramide Signal Amplification Kit (Molecular Probes/ThermoFischer Scientific, Hungary) was used with fluorescent-labeled tyramide (Alexa Fluor 594-labeled, cat. no. T20925, Invitrogen, 1:100) to detect color red and directly labeled secondary antibody (Alexa Fluor 488 goat anti-rabbit, Invitrogen, 1:200) to detect color green. Micrographs were generated using an Olympus Fluoview-1000 system on an Olympus IX81 microscope stage equipped with an Olympus DP70 digital camera and through an Olympus UPlan FL N, Phase2 objective. The scale bar represents 50 μm. The counting of TRPV4 and aquaporin positive myometrial cells was performed in 3 different standardized areas from each slides, using ImageJ software.
2.5.1. Statistical analysis
D'Agostino-Pearson omnibus test was performed to determine the normal distribution of the data. One-way ANOVA followed by Bonferroni's post hoc test was used for statistical analysis of the immunochemistry. A value of p < 0.05 was considered statistically significant.
2.6. Isolated organ bath study
Uteri were removed from rats on day 18 or 22 of pregnancy. 5-mm-long muscle rings were sliced from the uterine horns and mounted vertically in an organ bath containing 10 ml de Jongh solution (composition: 137 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 12 mM NaHCO3, 4 mM NaH2PO4, 6 mM glucose, pH = 7.4). The organ bath was maintained at 37 C and carbogen (95% O2 + 5% CO2) was bubbled through it. After mounting, the rings were equilibrated for about 1 h before experiments were undertaken; with a solution change every 15 min.
2.6.1. Contractility studies
In the isolated uterine tissue rings, rhythmic contractions were induced with 25mM KCl solution. Without washing out the contractile agent, the effects of a TRPV4 antagonist (RN1734, Sigma-Aldrich, Hungary) and a TRPV4 agonist (RM1747, Sigma-Aldrich, Hungary) were tested on the uterine contractions in the concentration range of 3 × 10−8-10−5M in a cumulative manner. Control uteri were treated with the solvent of the compounds. Recording was performed at each concentration of the examined agents for 5 min. The tension of the myometrial rings was measured with a strain gauge transducer (SG-02; MDE Ltd., Budapest, Hungary) and contractions were recorded and later analyzed with the SPEL Advanced ISOSYS Data Acquisition System (MDE Ltd., Budapest, Hungary). The effects of RN1734 and RN1747 were expressed as the percentage of the area under curve (AUC) of KCl induced contractions. The dose-response curves were fitted and the statistical analysis of EC50 and Emax values was performed.
2.7. Statistical analyses
All experiments were carried out on at least 6 animals, repeated 3 times, and each value is given as a mean ± S.E.M. All curve fittings, data calculations and statistical analyses were performed with Prism 5.0 computer software (Graph Pad Software Inc, San Diego, CA, USA). The curves were generated by fitting the data with an equation using the Graphpad Prism software (Y=Bottom + (Top-Bottom)/(1 + 10ˆ((LogEC50-X))), where x = log (concentration). Comparisons were made by one-way ANOVA tests with the Tukey posttest or two-tailed unpaired t-test.
3. Results
TRPV4 mRNA and protein expression were determined in the non-pregnant and pregnant rat uteri (Fig. 1A and B). The changes in the mRNA and protein level showed correlation on the investigated day. The lowest expression was measured on pregnancy day 18 and it continuously increased towards the day of labor. Strong correlation was found between the AQP5 [6] and the TRPV4 mRNA expression from pregnancy day 18–22 (r2 = 0.9577) (Fig. 1C) and moderate correlation in the protein expression between AQP5 and TRPV4 (r2 = 0.6452) (Fig. 1D).
Fig. 1.
The changes in mRNA (A) and protein expression (B) of TRPV4 in the non-pregnant uterus (estrus phase) and on different gestational days in pregnant rat uteri (n = 6 on investigated days of pregnancy). Correlation between TRPV4 and AQP5 mRNA (C) and protein (D) expression from pregnancy day 18 to day 22 in uterus. Original uncropped gel images can be viewed in Supplementary Fig. 1. NP: non-pregnant, ns > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001; compared to the previous day.
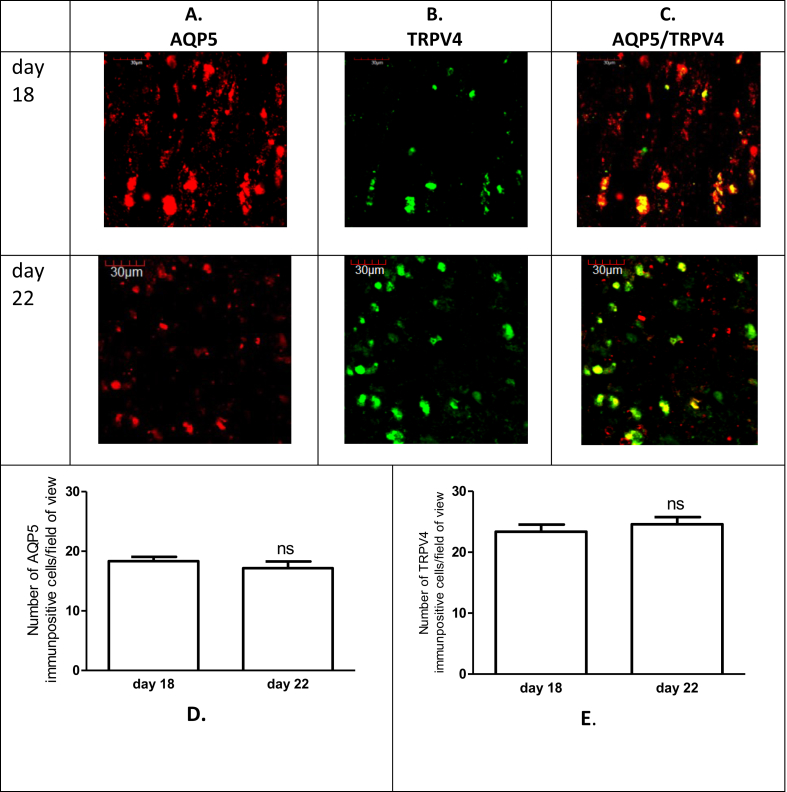
Based on the summarized immunohistochemical studies, we determined an inverse expression of the AQP5 and TRPV4 expression in the uterus tissue. The changes of the myometrial expression of AQP5 and TRPV4 were not significant on pregnancy day 18 and day 22 (Fig. 2). The endometrial expressions of both of investigated proteins were significantly higher than in myometrium. The number of AQP5 immunpositive cells were significantly lower on the last day of pregnancy (Fig. 3D) in contrast the number of TRPV4 immunopositive cells were higher on day 22 (Fig. 3E). The endometrial and myometrial co-expression of AQP5 and TRPV4 was proved on both investigated days of late pregnancy (Figs. 2C and 3C).
Fig. 2.
Representative pictures showing expressions of the AQP5 (A) and TRPV4 (B) and these co-expressions (C) in the myometrium on days 18 and 22 of pregnancy. n = 6, ns > 0.05, compared to the previous day.
Fig. 3.
Representative pictures showing expressions of the AQP5 (A) and TRPV4 (B) and these co-expressions (C) in the endometrium on days 18 and 22 of pregnancy. n = 6,*p < 0.05, ***p < 0.001 compared to the previous day.
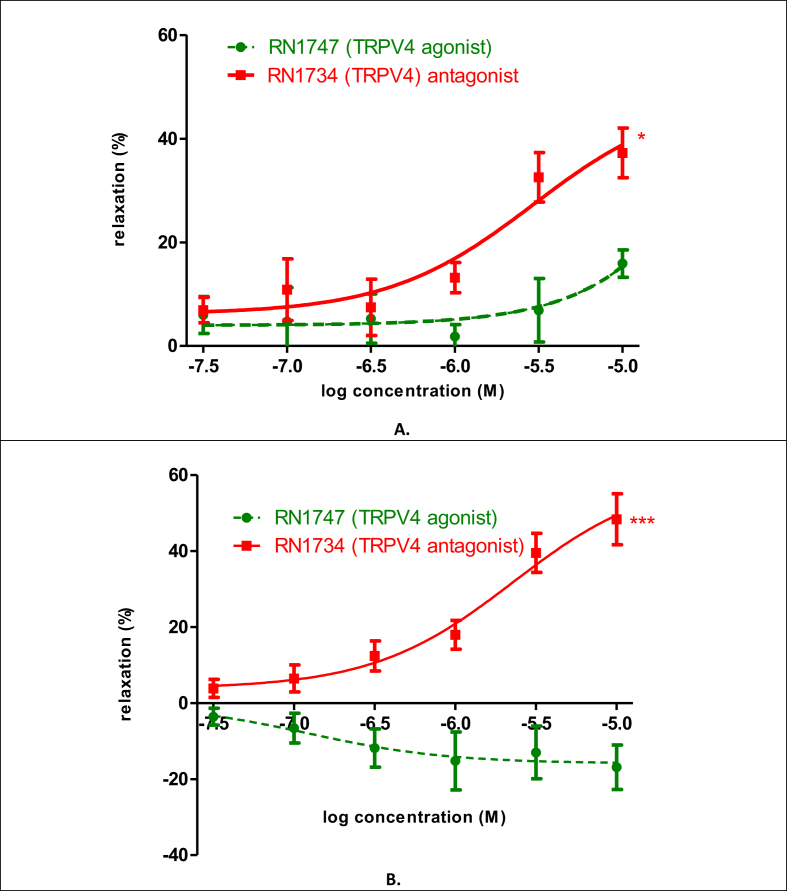
The uterine contraction influencing effects of TRPV4 agonist and antagonist were investigated in an isolated organ bath system (Fig. 4.). The agonist (RN1747) had a slight relaxing effect on day 18 (9.93%) in 10−5 M concentration. The antagonist (RN1734) had a significant relaxing effect (30.33 %) on the same day and in the same concentration (Fig. 4A).
Fig. 4.
Effect of selective TRPV4 agonist (RN1747) and antagonist (RN1734) on KCl-evoked control contraction of rat uteri on days 18 (A) and 22 (B) of gestation. The statistical analyses were carried out with the two-tailed unpaired t-test. Each value denotes the mean ± S.E.M, n = 6. *p < 0.05; ***p < 0.001.
The agonist had no effect on uterus relaxation (-16.87%), whereas it induced uterus contraction on pregnancy day 22. The antagonist had a pronounced relaxing effect (44.52%) on the last of pregnancy (Fig. 4B).
4. Discussion
The exact mechanisms of preterm delivery, which can be associated with immediate and long-term neonatal complications, are largely unknown [9]. The management of preterm birth involves the identification of the factors which induced the untimely uterine contraction [10]. In our study we looked for new mechanisms through which uterine contraction is adjustable.
The normal homeostasis of water in the female reproductive system is indispensable for healthy pregnancy and successful birth. AQPs are present during parturition, participating in the control of pregnant myometrial contractions and cervical ripening [5]. Based on earlier studies, we know that the expression of AQP5 is dominant during pregnancy and this expression dramatically decreases on the last day of gestation in rat. It was also proved that this type of water channel is selectively down-regulated by oxytocin, which stimulated uterus contractions [6], and the uterus relaxing progesterone and progesterone derivatives up-regulated the AQP5 expression [7]. Based on these findings, we confirmed an inverse correlation between the AQP5 level and myometrial contractions. We hypothesize that a low AQP5 level may induce or increase the late pregnant myometrium contractility. The key question is the clarification of the mechanism of this phenomenon, especially at the time of delivery. We suppose an osmotic pathway in this regulatory mechanism in the pregnant uterus. We investigated pregnancy days 18 and 22 with special attention because the changes of AQP5 and TRPV4 expression were the most significant in rat.
TRPV channels are expressed in the male and female reproductive tissues and play a crucial regulatory role in various physiological actions. The expression of TRPV4 mRNA was observed in rat prostatic tissue [11]. They are responsible for oocyte maturation and activation and for fertilization [12]. TRPV1 is present in the human placenta and the deregulation in TRPV1 expression was found in preeclampsia [13]. TRPV6 expression was proved during pregnancy in the bovine uterine endometrium and placenta [14].
The TRPV4 channel is a Ca2+ channel, which is activated by osmotic changes. This channel is widely expressed in the smooth muscle of the cardiovascular [15], digestive [16, 17], respiratory [18] and reproductive system [11]. Current evidence suggests that myometrial smooth muscle Ca2+ homeostasis is modulated near term to promote uterine contractility. In the human myometrium, the entry of extracellular Ca2+ is essential for the maintenance of spontaneous rhythmic contractions [19].
It is supported by much evidence that the AQP5 water channel is an interacting partner of the TRPV4 channel in the smooth muscles of the airway system [20] and the gastrointestinal tract [21]. Aure et al. [21] recognized the functional connection between AQP5 and TRPV4 in salivary glands, other studies proved that the hypotonic reduction of AQP5 expression requires the TRPV4 function, furthermore it is essential for the mechanism of regulatory volume decrease in salivary gland cells [22, 23].
We determined the dynamic changes of TRPV4 expression during pregnancy in rat uterus. Based on literature data, this alteration of TRPV4 expression in the uterus seems to be sex hormone-dependent, mostly depending on progesterone (P4) effect. P4 analogue, levonorgestrel could down regulate the expression of TRPV4 to reduce the ciliary beat frequency in both humans and mice, suggesting the possible mechanism of tubal pregnancy [24]. P4 reduces TRPV4 expression in human tracheal and mammary gland ductal epithelial cell lines [25]. Decreased TRPV4 expression and promoter activity were also observed in the presence of P4 in human aortic vascular smooth muscle cells [25]. This is consistent with our results because the level of P4 decreases at the end of pregnancy [26], which increases TRPV4 expression. It is known from our previous results that AQP5 expression is up-regulated by P4 [7]. We suppose a potential hormone regulated cooperation between the AQP5 and TRPV4 expression.
We found an inverse correlation between the AQP5 and TRPV4 mRNA and protein expression. The putative cooperation between AQP5 and TRPV4 was revealed by our immunohistochemical findings. We have confirmed the changes in the AQP5 and TRPV4 expressions in the uterus and the co-expression of these proteins at the end of pregnancy. Similar co-expression and functional connection were found in the central nervous system. Astrocytes possess a TRPV4/AQP4 complex, which is an essential component in the brain's volume homeostasis [27].
Strategies designed to modulate the entry of extracellular Ca2+ into smooth muscle cells, and thereby uterus contractility, have been less than successful in preventing or halting the progression of preterm labor thus far [28]. The changes in the intracellular concentration of free Ca2+ influence muscle contraction, hormone secretion, gene expression, cell proliferation, and many other critical processes [29]. Current evidence suggests that myometrial smooth muscle cell Ca2+ homeostasis is modulated near term to promote uterine contractility. TRPV4, which is the osmotic calcium channel, might have a role in myometrial contractility and Ca2+ signaling. Earlier the functional role of TRPV4 in modulating myometrial contractility was investigated in murine model by HC067074 (TRPV4 antagonist), which prolonged pregnancy [4]. We determined the effect of the TRPV4 channel in the regulation of late pregnant rat uterus contraction with TRPV4 agonist and antagonist. The TRPV4 agonist had a negligible relaxing effect on uterine contraction on day 18 at the highest concentration. In contrast, a contraction-inducing effect was measured on the last day of pregnancy.
RN-1734, the antagonist which was used in our studies, completely inhibits both ligand-induced and hypotonicity-induced activation of TRPV4, without affecting the activity of other TRP channels [30]. The agonist RN-1747 is mostly selective for TRPV4 although low level activation of TRPV1 became apparent at high (100 μM) concentration and antagonism of TRPM8 was observed, too [31]. We proved the relaxing effect of RN-1734 on both of the investigated pregnancy days, with the major effect on the last day of rat pregnancy. We presume it can be explained by the increased expression of TRPV4 and the decreased expression of AQP5. In non-pregnant and pregnant mouse uterus another TRPV4 antagonist (HC067047) also inhibited contraction induced by PGF2α (3). There is no way to investigate the physiological role of AQP5 in the myometrial contraction because of the lack of non-toxic tissue- and subtype-selective agonists or antagonists.
Based on our findings we presume the decreased AQP5 expression triggers an osmotic stress, which activates TRPV4 and increases uterus contraction on the day of labor. This phenomenon can confirm the future role of TRPV4 antagonist in the tocolytic therapy.
5. Conclusion
Nowadays, we do not have exact information on the initiation step of labor. The therapy of preterm birth is an unresolved problem; therefore we were looking for new targets for the tocolytic therapy. The dynamic change of AQP5 expression was proved during pregnancy with the negative correlation between the AQP5 expression and myometrial contraction. First, we determined the co-expression of the osmotic active Ca2+-channel, TRPV4 and AQP5 in pregnant myometrial and endometrial tissues. The demonstration of TRPV4 antagonists in the uterine relaxation effect, on the day of birth, can be the new pathway in influencing myometrial contractility.
Declarations
Author contribution statement
Eszter Ducza, Robert Gaspar: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Adrienn Csanyi, Éva Szoke, Zita Tiszai: Performed the experiments; Analyzed and interpreted the data.
Krisztina Pohóczky, Judit Hajagos-Tóth, Anna Kothencz: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Ministry of Human Capacities, Hungary grant 20391-3/2018/FEKUSTRAT; and by GINOP-2.3.2-15-2016-00050. É. Szőke was supported by János Bolyai fellowship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Eszter Ducza, Email: ducza@pharm.u-szeged.hu.
Róbert Gáspár, Email: gaspar.robert@med.u-szeged.hu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Menon R., Bonney E.A., Condon J., Mesiano S., Taylor R.N. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum. Reprod. Update. 2016;22(5):535–560. doi: 10.1093/humupd/dmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bru-Mercier G., Gullam J.E., Thornton S., Blanks A.M., Shmygol A. Characterization of the tissue-level Ca2+ signals in spontaneously contracting human myometrium. J. Cell Mol. Med. 2012;16(12):2990–3000. doi: 10.1111/j.1582-4934.2012.01626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh V., Ram M., Kandasamy K., Thangamalai R., Choudhary S., Dash J.R., Kumar D., Parida S., Singh T.U., Mishra S.K. Molecular and functional characterization of TRPV4 channels in pregnant and nonpregnant mouse uterus. Life Sci. 2015;122:51–58. doi: 10.1016/j.lfs.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Ying L., Becard M., Lyell D., Han X., Shortliffe L., Husted C.I., Alvira C.M., Cornfield D.N. The transient receptor potential vanilloid 4 channel modulates uterine tone during pregnancy. Sci. Transl. Med. 2015;7(319) doi: 10.1126/scitranslmed.aad0376. 319ra204. [DOI] [PubMed] [Google Scholar]
- 5.Ducza E., Csányi A., Gáspár R. Aquaporins during pregnancy: their function and significance. Int. J. Mol. Sci. 2017;18(12) doi: 10.3390/ijms18122593. pii: E2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ducza E., Seres A.B., Hajagos-Tóth J., Falkay G., Gáspár R. Oxytocin regulates the expression of aquaporin 5 in the late-pregnant rat uterus. Mol. Reprod. Dev. 2014;81(6):524–530. doi: 10.1002/mrd.22320. [DOI] [PubMed] [Google Scholar]
- 7.Csányi A., Bóta J., Falkay G., Gáspár R., Ducza E. The effects of female sexual hormones on the expression of aquaporin 5 in the late-pregnant rat uterus. Int. J. Mol. Sci. 2016;17(8) doi: 10.3390/ijms17081300. pii: E1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Georgiou H.M., Di Quinzio M.K., Permezel M., Brennecke S.P. Vol. 2015. Dis Markers; 2015. p. 435014. (Predicting Preterm). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzano S., Padula F., Meloni P., Anceschi M.M. Preterm delivery at low gestational age: risk factors for short latency. A multivariated analysis. J. Prenat. Med. 2008;2(2):15–18. [PMC free article] [PubMed] [Google Scholar]
- 11.Guibert C., Ducret T., Savineau J.P. Expression and physiological roles of TRP channels in smooth muscle cells. Adv. Exp. Med. Biol. 2011;704:687–706. doi: 10.1007/978-94-007-0265-3_36. [DOI] [PubMed] [Google Scholar]
- 12.Björkgren I., Lishko P.V. Fertility and TRP channels. In: Emir T.L.R., editor. Neurobiology of TRP Channels, Frontiers in Neuroscience. CRC Press/Taylor & Francis; Boca Raton (FL: 2017. [PubMed] [Google Scholar]
- 13.Martínez N., Abán C.E., Leguizamón G.F., Damiano A.E., Farina M.G. TPRV-1 expression in human preeclamptic placenta. Placenta. 2016;40:25–28. doi: 10.1016/j.placenta.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Sprekeler N., Kowalewski M.P., Boos A. TRPV6 and Calbindin-D9k-expression and localization in the bovine uterus and placenta during pregnancy. Reprod. Biol. Endocrinol. 2012;10:66. doi: 10.1186/1477-7827-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randhawa P.K., Jaggi A.S. TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 2015;110(6):54. doi: 10.1007/s00395-015-0512-7. [DOI] [PubMed] [Google Scholar]
- 16.Holzer P. TRP channels in the digestive system. Curr. Pharmaceut. Biotechnol. 2011;12(1):24–34. doi: 10.2174/138920111793937862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihara H., Uchida K., Koizumi S., Moriyama Y. Involvement of VNUT-exocytosis in transient receptor potential vanilloid 4-dependent ATP release from gastrointestinal epithelium. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0206276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonsen U., Wandall-Frostholm C., Oliván-Viguera A., Köhler R. Emerging roles of calcium-activated K channels and TRPV4 channels in lung oedema and pulmonary circulatory collapse. Acta Physiol. 2017;219(1):176–187. doi: 10.1111/apha.12768. [DOI] [PubMed] [Google Scholar]
- 19.Parkington H.C., Tonta M.A., Brennecke S.P., Coleman H.A. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am. J. Obstet. Gynecol. 1999;181(6):1445–1451. doi: 10.1016/s0002-9378(99)70390-x. [DOI] [PubMed] [Google Scholar]
- 20.Sidhaye V.K., Güler A.D., Schweitzer K.S., D'Alessio F., Caterina M.J., King L.S. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc. Natl. Acad. Sci. U. S. A. 2006;103(12):4747–4752. doi: 10.1073/pnas.0511211103. Epub 2006 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aure M.H., Røed A., Galtung H.K. Intracellular Ca2+ responses and cell volume Regulation upon cholinergic and purinergic stimulation in an immortalized salivary cell line: agonist stimulation, calcium, and cell volume in salivary gland cells. Eur. J. Oral Sci. 2010;118:237–244. doi: 10.1111/j.1600-0722.2010.00738.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Bandyopadhyay B., Nakamoto T., Singh B., Liedtke W., Melvin J.E., Ambudkar I. A Role for AQP5 in Activation of TRPV4 by Hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 2006;281:15485–15495. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 23.Hosoi K. Physiological role of aquaporin 5 in salivary glands. Pflüg. Arch. 2016;468(4):519–539. doi: 10.1007/s00424-015-1749-6. [DOI] [PubMed] [Google Scholar]
- 24.Li C., Wu Y.T., Zhu Q., Zhang H.Y., Huang Z., Zhang D., Qi H., Liang G.L., He X.Q., Wang X.F., Tang X., Huang H.F., Zhang J. TRPV4 is involved in levonorgestrel-induced reduction in oviduct ciliary beating. J. Pathol. 2019;248(1):77–87. doi: 10.1002/path.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung C., Fandos C., Lorenzo I.M., Plata C., Fernandes J., Gené G.G., Vázquez E. Valverde MA the progesterone receptor regulates the expression of TRPV4 channel. Pflüg. Arch. 2009;459(1):105–113. doi: 10.1007/s00424-009-0706-7. [DOI] [PubMed] [Google Scholar]
- 26.Boroditsky R.S., Reyes F.I., Winter J.S., Faiman C. Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet. Gynecol. 1978;51:686–691. [PubMed] [Google Scholar]
- 27.Benfenati V., Caprini M., Dovizio M., Mylonakou M.N., Ferroni S., Ottersen O.P., Amiry-Moghaddam M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. U. S. A. 2011;108(6):2563–2568. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muglia L.J., Katz M. The enigma of spontaneous preterm birth. N. Engl. J. Med. 2010;362(6):529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 29.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 30.White J.P., Cibelli M., Urban L., Nilius B., McGeown J.G., Nagy I. TRPV4: molecular conductor of a diverse orchestra. Physiol. Rev. 2016;96(3):911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 31.Vincent F., Acevedo A., Nguyen M.T., Dourado M., DeFalco J., Gustafson A., Spiro P., Emerling D.E., Kelly M.G., Duncton M.A. Identification and characterization of novel TRPV4 modulators. Biochem. Biophys. Res. Commun. 2009;389(3):490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.