Introduction
Linear IgA bullous dermatosis (LABD) is a rare autoimmune bullous disease characterized by linear deposition of IgA along the cutaneous basement membrane. Clinically, LABD has a variable presentation characterized by tense vesiculobullous lesions on the trunk and extremities that often appear in a herpetiform arrangement or at the center of annular erythematous plaques. Omalizumab (Xolair; Genentech, South San Francisco, CA) is a monoclonal anti–immunoglobulin E (IgE) antibody that has been approved for chronic idiopathic urticaria (CIU). To date, omalizumab has not been reported to be of benefit in the treatment of LABD. However, omalizumab has been reported to improve control of other bullous dermatoses, particularly bullous pemphigoid.1
Case report
We report a case of a 55-year-old woman with no pertinent past medical history who received a diagnosis of chronic LABD more 10 years earlier. She had initially presented with pruritic vesiculobullous lesions, classically described as “cluster of jewels” and “string of pearls,” located mainly on the trunk, neck, and arms (Fig 1). She did not have any systemic symptoms, mucosal involvement, or lymphadenopathy on examination. Her medications included progesterone, estradiol, vitamin D, escitalopram, diphenhydramine, and cetirizine, as needed.
Fig 1.
A, Clinical presentation on the patient's back. B, Erythematous scaly and crusted papules and plaques with grouped vesicles and bullae.
Laboratory testing showed mild leukocytosis with eosinophilia. Liver function test results, renal function, thyroid hormones, and antinuclear antibodies were all within normal ranges.
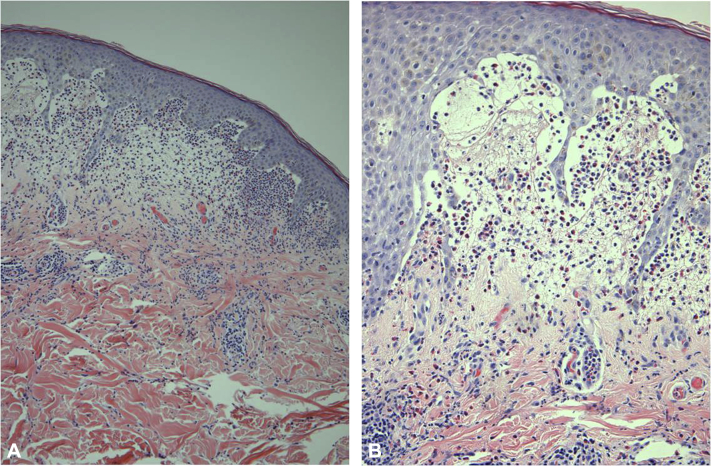
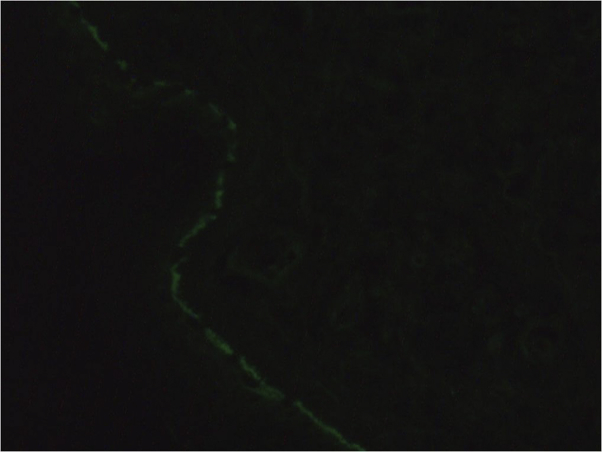
Cutaneous biopsies were performed for both histopathology and direct immunofluorescence. Histology showed subepidermal bullae, epidermal acanthosis and papillomatosis, perivascular inflammation with predominant neutrophils, and occasional eosinophils in the superficial dermis (Fig 2). Direct immunofluorescence showed linear IgA deposition along the basement membrane (Fig 3), which was consistent with the diagnosis of LABD.
Fig 2.
Histology consistent with linear IgA bullous dermatosis. Hematoxylin-eosin stain. Original magnification, A, ×40; B, ×100. Subepidermal bullae, epidermal acanthosis and papillomatosis, perivascular inflammation with predominant neutrophils, and occasional eosinophils in the superficial dermis.
Fig 3.
Direct immunofluorescence of perilesional biopsy showing linear IgA deposition along the cutaneous basement membrane. Original magnification, ×100.
Although the patient responded appropriately to dapsone for the first 3 years of treatment, the response eventually became suboptimal despite dose optimization (300 mg daily). The patient experienced multiple adverse effects secondary to the high-dose dapsone therapy. Complications included methemoglobinemia, which resulted in functional anemia and subsequent shortness of breath and fatigue. The patient was then treated with a 2-year course of sulfapyridine (up to 6 g daily divided into 3 doses), during which time she showed little improvement. She did not respond to a subsequent trial of gluten-free diet. Cutaneous biopsy specimens with direct immunofluorescence repeated 6 years after the initial diagnosis remained consistent with the diagnosis of LABD. However, the second biopsy specimen contained fewer eosinophils than the first one. Direct immunofluorescence again showed linear IgA deposition along the basement membrane (IgG, IgM, C3, and fibrinogen were again negative).
Dapsone at a lower dosage (150 mg daily) was reinitiated along with tetracycline (at a dosage of 500 mg twice daily) to optimize management of the disease while minimizing adverse effects. The patient showed mild improvement of skin lesions with this combination therapy. Throughout the course of the disease, she was also treated with a strong topical corticosteroid as needed (clobetasol propionate 0.05%).
Ten years after the initial diagnosis of LABD, the patient developed chronic spontaneous urticaria that became incapacitating despite up to 4 times the standard dose of second-generation antihistamines (cetirizine 20 mg twice daily). The patient presented mild peripheral blood eosinophilia throughout her 10-year LABD history, which did not worsen with the CIU diagnosis (Table I). She was started on omalizumab 300 mg subcutaneously every 4 weeks.
Table I.
Patient's peripheral eosinophil count throughout the course of the disease
| Year | Absolute eosinophil value (10e9/L)∗ |
|---|---|
| 2001 | 0.5 |
| 2013 | 0.3-0.4 |
| 2014 | 0.4 |
| 2015 | 0.3 |
| 2016 | 0.4 |
| 2017 | 0.2 |
| 2018 (CIU diagnosis) | 0.3 |
CIU, chronic idiopathic urticaria.
Normal range, 0-0.2.
Within 3 weeks of starting treatment with omalizumab, the patient had complete resolution of both her chronic urticaria and her LABD. Dapsone and tetracycline were tapered over the course of the following 3 months, and the patient did not present any signs of relapse during the 6-month treatment with omalizumab. However, LABD lesions recurred within a month of omalizumab cessation and completely disappeared when omalizumab was reintroduced 2 months later.
Discussion
LABD is a rare, autoimmune blistering disorder characterized by a diffuse vesiculobullous eruption located mainly on the trunk, thighs, and face. LABD is usually an idiopathic disease, but it can be associated with medications,2, 3 lymphoproliferative disorders, carcinomas4 and systemic diseases.5 Adult-onset LABD typically occurs in patients older than 60 years and has a spontaneous remission rate of 30%.6 LABD generally responds well to dapsone, corticotherapy, and systemic antibiotics such as tetracyclines and erythromycin. Other treatments, such as mycophenolate mofetil and azathioprine, may be necessary in refractory cases. However, long-term evolution of LABD is poorly understood. In a retrospective study in 2017, Gottlieb and Ingen-Housz-Oro7 found that patients with LABD who were younger than 70 years and have mucosal involvement were at increased risk of chronic evolution.
We describe a case of a patient with chronic idiopathic LABD diagnosed more than 10 years earlier that was refractory to optimal usual therapy. No associated systemic disease or iatrogenic etiologies were found. Because of the reported incidence of celiac disease of up to 24% in LABD, a gluten-free diet was attempted. However, the diet was unsuccessful in reducing skin lesions, and results of a small-bowel biopsy were negative for gluten-sensitive enteropathy. Omalizumab, although initiated to treat the patient's chronic spontaneous urticaria, showed complete resolution of the patient's LABD-related skin lesions and improved her quality of life. Furthermore, upon discontinuation of omalizumab, LABD lesions recurred within a month and completely disappeared with reintroduction of omalizumab.
Because the urticarial lesions of the patient were typical and lasted less than 4 to 5 hours, they were not biopsied. It is, however, not impossible that the urticaria in this patient could actually represent urticarial bullous pemphigoid and that the patient could have progressed from LABD to the overlap condition of LABD and bullous pemphigoid, commonly named linear IgA and IgG bullous dermatosis.8, 9, 10
Omalizumab is a monoclonal IgE antibody approved for the treatment of CIU. It is typically administered subcutaneously once a month for a period of 6 months. The role of omalizumab in linear IgA bullous dermatosis is not fully understood, but we hypothesize that IgE immunoglobulins are either directly or indirectly implicated in the pathogenesis of this disease. Omalizumab prevents free circulating IgE from interacting with receptors on mast cells and basophils, thus preventing their activation. These cells produce cytokines such as interleukin 3, interleukin 5, and granulocyte-macrophage colony-stimulating-factor, which are essential for eosinophil development. In LABD biopsy specimens, neutrophils predominate but have variable amounts of eosinophils. The presence of peripheral blood eosinophilia and significant eosinophils in histology might be a sign of a patient who may respond to omalizumab. Considering its safety profile and tolerability, omalizumab could become a potential therapeutic alternative in refractory cases of LABD.
In our case, both clinical and temporal correlations exist between initiation of omalizumab therapy and remission of LABD lesions. The lack of iatrogenic or systemic etiologies, LABD recurrence after treatment cessation, and resolution with reintroduction of treatment support our hypothesis of a causal association between the LABD clinical improvement and the use of omalizumab. Further studies are needed to corroborate these finding.
Acknowledgments
We would like to show our gratitude to our colleagues Dr Edmond Rizcallah and Dr Myrna Chababi-Atallah, both dermatopathologists who assisted us by providing insight and expertise on the pathology reports.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Yu K.K., Crew A.B., Messingham K.A., Fairley J.A., Woodley D.T. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71(3):468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shpadaruk V., Miller J., Da Forno P., Johnson G.A. Vancomycin-induced linear IgA bullous dermatosis. J Am Acad Dermatol. 2016;74(5, Supplement 1):AB77. [Google Scholar]
- 3.Fortuna G., Salas-Alanis J.C., Guidetti E., Marinkovich M.P. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66(6):988–994. doi: 10.1016/j.jaad.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Yang C.S., Robinson-Bostom L., Landow S. Linear IgA bullous dermatosis associated with metastatic renal cell carcinoma. JAAD Case Rep. 2015;1(2):91–92. doi: 10.1016/j.jdcr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayakawa K., Shiohara T., Yagita A., Nagashima M. Linear IgA bullous dermatosis associated with rheumatoid arthritis. J Am Acad Dermatol. 1992;26(1):110–113. doi: 10.1016/0190-9622(92)70017-a. [DOI] [PubMed] [Google Scholar]
- 6.Bolognia J., Schaffer J., Cerroni L. Dermatology. 4th ed. 2018. Chapter 31. [Google Scholar]
- 7.Gottlieb J., Ingen-Housz-Oro S. Idiopathic linear IgA bullous dermatosis: prognostic factors based on a case series of 72 adults. Br J Dermatol. 2017;177(1):212–222. doi: 10.1111/bjd.15244. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes K., Galvis K.H., Gomes A.C. Linear IgA and IgG bullous dermatosis. An Bras Dermatol. 2016;91(5 Suppl 1):32–34. doi: 10.1590/abd1806-4841.20164630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi M., Bito T., Oda Y. Three cases of linear IgA/IgG bullous dermatosis showing IgA and IgG reactivity with multiple antigens, particularly laminin-332. JAMA Dermatol. 2013;149:1308–1313. doi: 10.1001/jamadermatol.2013.5691. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu S., Natsuga K., Shinkuma S., Yasui C., Tsuchiya K., Shimizu H. Localized linear IgA/IgG bullous dermatosis. Acta Derm Venereol. 2010;90(6):621–624. doi: 10.2340/00015555-0985. [DOI] [PubMed] [Google Scholar]