Abstract
Backgrounds
Acute lung injury (ALI) often occurs early and seriously in the progress of sepsis. Netrin-1 is demonstrated to be an effective anti-inflammatory agent. However, whether netrin-1 can relieve sepsis-induced ALI remains unknown.
Material/Methods
The sepsis rat model was built with the method of cecal ligation and puncture (CLP). The lung tissue changes were represented as the results of hematoxylin-eosin (HE) staining, wet-to-dry (W/D) ratio, Western blot analysis, and immunohistochemistry. An in vitro lung injury model was simulated with LPS-induced BEAS-2B cells. The cell transfection effects were evaluated by Western blot analysis and RT-qPCR analysis. TNF-α, IL-1β, and IL-6 levels were detected by Western blot analysis in LPS-induced BEAS-2B cells.
Results
Obvious inflammation caused by sepsis appeared in lung tissues with the increase of the W/D ratio and expression of inflammatory cytokines. Netrin-1 and its receptor UNC5B were reduced in sepsis. However, upregulation of netrin-1 alleviated the levels of inflammation and increased the UNC5B levels in BEAS-2B cells.
Conclusions
Netrin-1 protects against ALI in sepsis rats through its anti-inflammation effect and may provide a novel treatment to prevent lung injury caused by sepsis.
MeSH Keywords: Acute Lung Injury, Exosomes, Sepsis
Background
Sepsis is a systemic inflammatory response syndrome (SIRS) that is often secondary to severe infection, burn injury, wounds, and major surgery. It is a refractory disease with high morbidity and mortality rates. The case fatality rate of sepsis is about 30–50% [1]. In sepsis, acute lung injury (ALI) appears earliest and is a main cause of death. Sepsis-related mortality can reach 70% [2,3]. ALI is a difficult problem encountered in clinical practice.
Excessive inflammatory response is a main mechanism of sepsis-induced ALI [4]. During sepsis, many inflammatory mediators and lipid metabolites enter into the blood circulation, which stimulate inflammation recruitment and activation of cells in lung tissue to produce cytokines, chemokines, and oxygenation. Based on the above changes, the inflammatory response is expanded to form a cascading chain reaction. The imbalance of pro-inflammatory/anti-inflammatory mediators causes damage to lung capillary endothelial cells and alveolar epithelial cells and increases permeability of alveolar capillary membranes for water and protein, which form permeable pulmonary edema [5,6]. Many studies indicate that the inflammatory response during sepsis is successfully relieved by many different agents with antioxidant properties [7–9]. Therefore, to effectively treat sepsis-induced ALI, attenuating inflammation is crucial.
Increasing evidence shows that exosomes can transfer protein, cytokines, mRNA, and miRNAs from donor cells to recipient tissues. Exosomes released by lung epithelial cells can effectively improve lung injury in mice by restoring integrity of pulmonary capillaries to reduce lung inflammation and edema formation [10]. Netrin-1 is initially an anti-inflammatory factor that can control axial growth and cone shift in the central nervous system during neuro-development. Netrin-1 can promote leukocyte movement into the acute inflammatory region [11]. Studies have shown that netrin-1 participates in the anti-inflammatory effect by activating the A2BAR receptor protein [12,13]. However, the role of netrin-1 in sepsis has been rarely studied. Netrin-1 was shown to be a biomarker of acute kidney injury in sepsis [14,15]. In addition, studies have shown that netrin-1 can reduce the kidney injury induced by inflammation by binding to its receptor UNC5B, as well as reducing neuroinflammation and brain injury [16]. However, whether netrin-1 can relieve the inflammation of ALI in sepsis has been rarely studied.
Therefore, this study assessed the expression and mechanism of netrin-1 in ALI caused by sepsis.
Material and Methods
Sepsis rat model
Twenty male Sprague-Dawley (SD) rats (6 weeks old, weighing 180–220 g) were supplied by Shanghai Jiesijie Experimental Animal Co. Twenty rats were fed with standard food and water in a room at 22±2°C with 12-h light/12-h dark cycle for 1 week. After fasting for 12 h, male SD rats were anesthetized by intraperitoneal injection of thiopental sodium and then were fixed with skin preparation and routine disinfection. Rats in the model group were treated with cecal ligation and perforation to form the sepsis model. The standard steps of the sepsis model follow the method described by Rittirsch et al. [17]. The abdominal skin was cut for about 2 cm, and the cecum was probed with sterile tweezers. The cecum was gently pulled out and the root of the cecum was ligated. The ileocolonic-colon was kept unobstructed. Then, the cecum was perforated at the head and tail of the cecum with 18-G sterile needles. The 2 holes were about 1 cm apart. The cecum was returned to the abdominal cavity and the muscles and skin were sutured layer by layer. Rats in the control group only had the abdominal cavity opened and the cecum exposed, without ligation.
Hematoxylin-eosin (HE) staining
The right lung tissues taken from each rat were stained with HE (Sigma-Aldrich). The right lung tissues were fixed in 4% paraformaldehyde for 12 h and then embedded in paraffin wax blocks. We stained 5-mm paraffin sections with HE. The stained images were obtained with a digital camera (Olympus BX 53 microscope, Tokyo, Japan).
Detection of wet-to-dry (W/D) ratio of lung tissue
The right lung middle lobes were obtained from each rat. Filter paper was used to drain blood from the surface of the right lung middle lobes, and the wet weight of the right lung middle lobe was immediately weighed. Then, the lung tissue was dried in an oven at 75°C for 72 h and the dry weight was immediately measured. The W/D ratio was calculated from the ratio of wet to dry lung mass.
Western blot analysis
In brief, total protein was isolated with RIPA lysis buffer at 14 000 g for 15 min at 4°C. The proteins were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% dry nonfat milk in PBST for 1 h and incubated with primary antibodies against TNF-α (cat no. 3707; Cell Signaling Technology, Inc.; dilution, 1: 1000), IL-1β (cat no. 12703; Cell Signaling Technology, Inc.; dilution, 1: 1,000), IL-6 (ab6672; Abcam, USA; dilution, 1: 1000), netrin-1 (ab126729; Abcam, USA; dilution, 1: 1000), UNC5B (ab104871; Abcam, USA; dilution, 1: 500), and GAPDH (cat no. 5174; Cell Signaling Technology, Inc.; dilution, 1: 1000) overnight at 4°C and then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at 37°C. Finally, membranes were washed with PBST and the protein bands were detected using an ECL Western blot kit (Amersham Biosciences, UK).
Immunohistochemistry
The 5-μm lung slices were deparaffinized, treated with 3% hydrogen peroxide, blocked with 5% normal rabbit serum for 1 h, and incubated with primary antibodies against netrin-1 (ab126729; Abcam, USA; dilution, 1: 500) and UNC5B (ab104871; Abcam, USA; dilution, 1: 500) at 4°C overnight. Then, lung slides were incubated with a biotinylated secondary IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 90 min. The sections were counterstained with HE, dehydrated, and fixed on glass slides with neutral resin. The sections were observed using an LSM 5 PASCAL confocal microscope (Carl Zeiss AG, Oberkochen, Germany).
Cell culture
The human normal lung epithelial cells BEAS-2B cell lines (ATCC, CRL-9609) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA), with 10% fetal bovine serum (FBS) (HyClone, Australia) and 1% antibiotic-antimycotic solution in a humidified atmosphere at 37°C with 5% CO2.
Cell transfection
The BEAS-2B cells, reseeded into a 12-well-plate, were cultured to reach 80% confluence before transfection. The pcDNA and pcDNA-netrin-1 were transfected into BEAS-2B cells using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s protocol. BEAS-2B cells, receiving no treatment, were identified as the control group. Subsequent experiments were performed after 48 h of cell transfection. Western blot analysis and RT-qPCR analysis were used for the assessment of transfection effects by detecting netrin-1 expression.
RT-qPCR analysis
Total RNA was extracted from BEAS-2B cells with an RNA extraction kit (Beijing Tianenze Gene Technology Co., Beijing, China), which was used for the production of cDNA using a RT-qPCR kit (Hangzhou Bioer Technology Co., Zhejiang, China). The thermal cycling conditions were including: initial denaturation: 95°C for 2 min, denaturation at 95°C for 30 s, annealing at 59°C for 45 s, and elongation at 72°C for 60 s for 30 cycles, followed by elongation at 72°C for 7 min. GAPDH was used as the endogenous control for the expression levels of mRNA. The primer sequences for qPCR were as follows:
GAPDH forward, 5′-TATGTCGTGGAGTCTACTGG-3′, and
reverse, 5′-AGTGATGGCATGGACTGTGG-3′;
netrin-1 forward, 5′-CCCTGGTTACTGCCTCTTGA-3′, and
reverse, 5′-ACTTTGCTGCCTCCTCTGAA-3′.
The results were presented as fold changes relative to GAPDH and calculated using the 2−ΔΔCq method.
Lung injury cell model
BEAS-2B cells (106 cells per well) were inoculated into 6-well plates at 37°C with 5% CO2 before treatment. The BEAS-2B cells in LPS group were treated with 1 μg/ml LPS for 48 h. pcDNA-netrin-1 was transfected into LPS-induced BEAS-2B cells using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Statistical analysis
The statistical analysis of data was performed with SPSS 25.0 software. The t test was used to analyze differences between 2 groups, and one-way analysis of variance (ANOVA) was used to analyze differences between multiple groups (p<0.05). Data are shown as mean ± standard error.
Results
Effect of sepsis on lung tissue
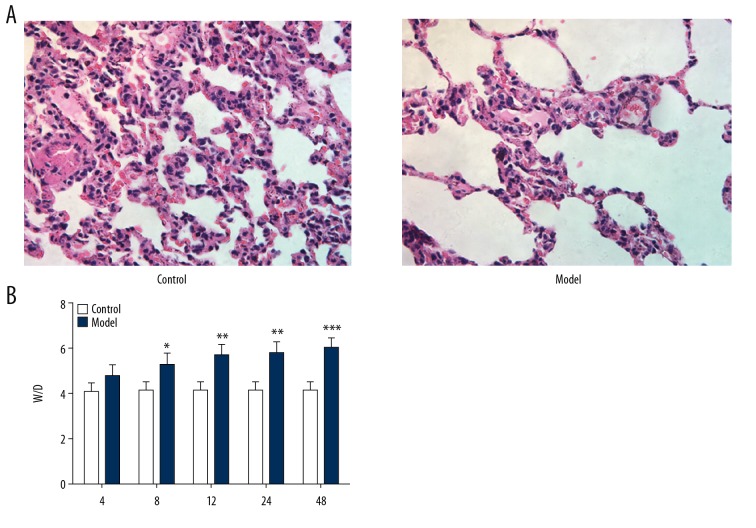
The pathologic changes of lung tissue were analyzed with HE staining. In the control group, there was no obvious lung pathological change in rats. There were obvious pathological changes of lung in the model group, including alveolar congestion, hemorrhage, edema, infiltration of inflammatory cells in the airspace, atelectasis, and hyaline membrane formation (Figure 1A). The lung W/D ratios in the model group were higher than that in the control group at all time points (Figure 1B). These results indicate that sepsis changes the normal lung tissue and causes severe edema in the inflamed lung tissues.
Figure 1.
Effect of sepsis on lung tissue. (A) Lung pathological changes. (B) Wet-to-dry (W/D) ratio of lung tissue. * P<0.05, ** P<0.01 and *** P<0.001 vs. control group.
Effect of sepsis on the expression of inflammatory factors, netrin-1, and its receptor UNC5B in lung tissues
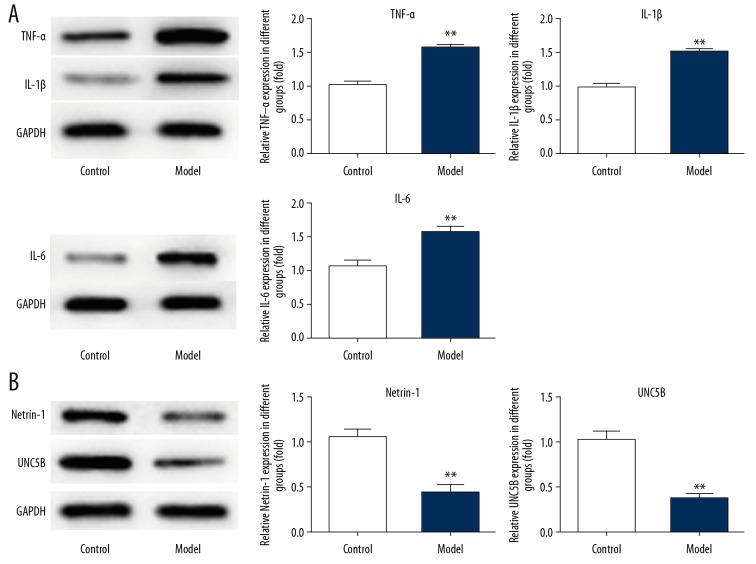
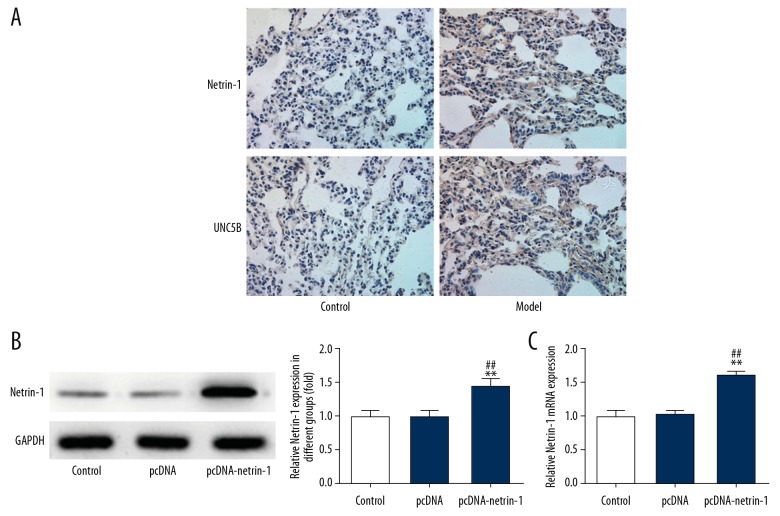
As shown in Figure 2, levels of inflammatory factors (TNF-α, IL-1β, and IL-6) were increased in the model group, while the expression of netrin-1 and UNC5B was decreased compared with the control group. The results of immunohistochemistry (Figure 3A) verified the results of the Western blot (Figure 2). Therefore, sepsis triggers inflammation in the lungs of rats and downregulates the expression of netrin-1 and its receptor UNC5B.
Figure 2.
Effect of sepsis on the expression of inflammatory factors, netrin-1, and its receptor UNC5B in lung tissues. (A) The expression of TNF-α, IL-1β, and IL-6 was detected by Western blot analysis. ** P<0.01 vs. control group. (B) The expression of netrin-1 and UNC5B was detected by Western blot analysis. ** P<0.01 vs. control group.
Figure 3.
Expression of netrin-1 and UNC5B in lung tissues and verification of Netrin-1 transfection effect. (A) The expression of netrin-1 and UNC5B in lung tissues was detected by Immunohistochemistry. (B) The transfection effect of netrin-1 was evaluated by Western blot. ** P<0.01 vs. control group. ## P<0.01 vs. pcDNA group. (C) The transfection effect of netrin-1 was evaluated by RT-qPCR analysis. ** P<0.01 vs. control group. ## P<0.01 vs. pcDNA group.
Netrin-1 alleviates the expression of inflammatory factors in LPS-induced BEAS-2B cells
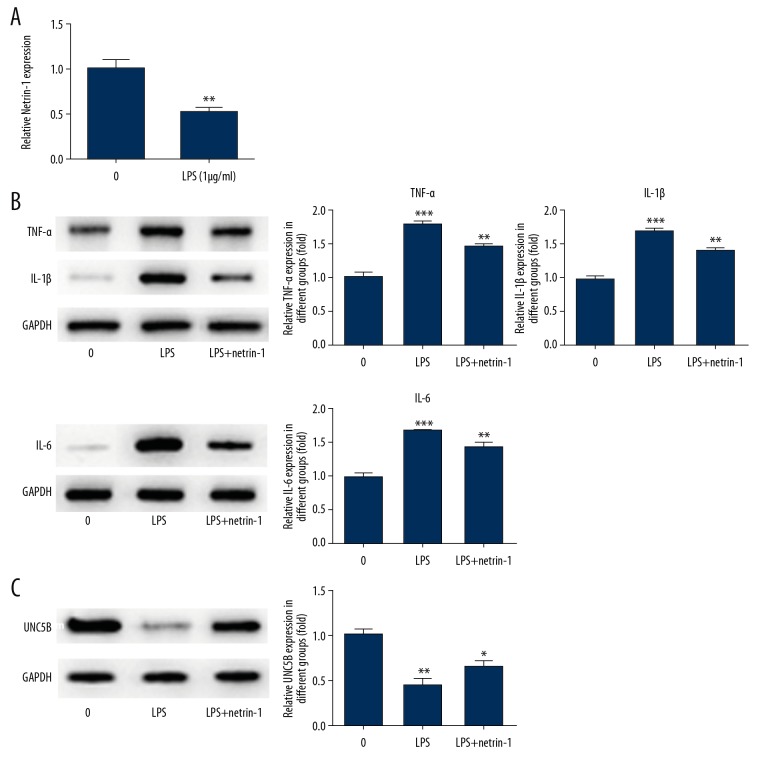
As shown in Figure 3B and 3C, the expression of netrin-1 was upregulated in the pcDNA-netrin-1 group compared with the control group and pcDNA group. Compared with the 0 μg/ml group (no LPS acting on cells), the expression of netrin-1 was decreased in the LPS-induced group (Figure 4A). The expression of TNF-α, IL-1β, and IL-6 was increased in the LPS-induced group compared with the 0 μg/ml group. The expression of TNF-α, IL-1β, and IL-6 was reversed with the transfection of pcDNA-netrin-1 and was lower than that in the LPS-induced group (Figure 4B). These experimental results show that netrin-1 can reduce the inflammation response.
Figure 4.
Netrin-1 alleviated the expression of inflammatory factors and UNC5B in LPS-induced BEAS-2B cells. (A) Netrin-1 was decreased in BEAS-2B cells treated with LPS. ** P<0.01 vs. 0 μg/ml group. (B) The expression of TNF-α, IL-1β, and IL-6 was detected by Western blot analysis after Netrin-1 transfection. ** P<0.01 and *** P<0.001 vs. control group. (C) The expression of UNC5B was detected by Western blot analysis after netrin-1 transfection. * P<0.05 and ** P<0.01 vs. control group.
Netrin-1 promotes the expression of UNC5B in LPS-induced BEAS-2B cells
As shown in Figure 4C, the expression of UNC5B was lower in the LPS-induced group compared with the control group. However, the expression of UNC5B was reversed with the transfection of pcDNA-netrin-1 and higher than that in the LPS-induced group. These results show that netrin-1 can improve the expression of UNC5B in LPS-induced BEAS-2B cells.
Discussion
Here, we explored whether netrin-1 can relieve the inflammation of ALI in sepsis. Netrin-1 and UNC5B were decreased in sepsis, and netrin-1 was reduced in LPS-induced BEAS-2B cells. Netrin-1 overexpression alleviated inflammation and upregulated UNC5B expression in LPS-induced BEAS-2B cells.
The early phase of sepsis is characterized by excessive inflammation, regulated by the systemic production of inflammatory cytokines, including IL-1, IL-6, and TNF-α [14,18]. The pro-inflammatory factor TNF-α can induce lung endothelial cell activation, leukocyte migration, granulocyte degranulation, and capillary leakage which result in edema to further hinder alveolar cell perfusion and oxygen exchange, thus causing ALI [19]. We found that the levels of IL-1, IL-6, and TNF-α in the lung tissue of sepsis rats were increased. And, the pathological changes of lung tissue supported the previous findings. The lung W/D ratio was increased in ALI of sepsis rats compared with normal rats, showing that inflammatory edema developed in the lung tissue.
Netrin-1 is the earliest isolated and named intracellular secreted soluble protein in the netrin family, which is highly expressed in the nervous system of many species [20]. Many studies have demonstrated that netrin-1 inhibits inflammation in renal ischemic reperfusion injury, intestinal disease, diabetic nephropathy, and corneal disease [21–24]. Ly et al. [25] found that netrin-1 expression was related to infection and inflammatory factors. The receptor UNC5B of netrin-1 is highly expressed in leukocytes. When netrin-1 binds to its receptor, UNC5B, it inhibits the migration and aggregation of leukocytes in the vascular lumen. Exogenous netrin-1 can inhibit apoptosis of renal proximal tubule epithelial cells, stimulate their proliferation, and inhibit the production of inflammatory cytokines such as inflammatory cytokines and chemotropic cytokines. The anti-inflammation role of netrin-1 in renal ischemia reperfusion injury in mice depends on UNC5B [26–28]. Mirakaj et al. [29] found that lung injury resulted in downregulated expression of netrin-1 in lung tissue and aggravated lung injury in mice with knockout of netrin-1. In this experiment, netrin-1 and UNC5B changed during ALI induced by sepsis, which verified that netrin-1 reduced the lung inflammation damage caused by sepsis depending on UNC5B. The expression of IL-1, IL-6, and TNF-α was increased and expression of netrin-1 and UNC5B was decreased in LPS-induced BEAS-2B cells. In addition, netrin-1 overexpression relieved the inflammatory response by decreasing the expression of IL-1, IL-6, and TNF-α, and netrin-1 overexpression promoted the expression of UNC5B in LPS-induced BEAS-2B cells.
Conclusions
In conclusion, this study shows that netrin-1 binding to its receptor UNC5B protects against the ALI induced by sepsis by inhibiting inflammation. These findings suggest that netrin-1 is a potential therapeutic option in sepsis.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the Science and Technology Development Foundation of Bengbu Medical College (No. BYKY18160)
References
- 1.Peake SL, Anthony D, Michael B, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 2.Sharawy N, Lehmann C. New directions for sepsis and septic shock research. J Surg Res. 2015;194(2):520–27. doi: 10.1016/j.jss.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Proudfoot A, McAuley D, Griffiths M, et al. Human models of acute lung injury. Dis Model Mech. 2011;4(2):145–53. doi: 10.1242/dmm.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Lin XJ, Xiao JB, et al. Sonchus oleraceus Linn protects against LPS-induced sepsis and inhibits inflammatory responses in RAW264.7 cells. J Ethnopharmacol. 2019;236:63–69. doi: 10.1016/j.jep.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 5.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(04):337–49. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 6.Sevransky J, Levy M, Marini J. Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: An evidence-based review. Crit Care Med. 2004;32(11 Suppl):S548–53. doi: 10.1097/01.ccm.0000145947.19077.25. [DOI] [PubMed] [Google Scholar]
- 7.Marik PE. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol Ther. 2018;189:63–70. doi: 10.1016/j.pharmthera.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Gomaa AMS, El-Mottaleb NAA, Aamer HA. Antioxidant and anti-inflammatory activities of alpha lipoic acid protect against indomethacin-induced gastric ulcer in rats. Biomed Pharmacother. 2018;101:188–94. doi: 10.1016/j.biopha.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 9.Rungsung S, Singh TU, Rabha DJ, et al. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine. 2018;110:333–43. doi: 10.1016/j.cyto.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Liu Z, Hu L, et al. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp Cell Res. 2018;370(1):13–23. doi: 10.1016/j.yexcr.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Serafini T, Kennedy TE, Galko MJ, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78(3):409–24. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 12.He J, Zhao Y, Deng W, et al. Netrin-1 promotes epithelial sodium channel-mediated alveolar fluid clearance via activation of the adenosine 2B receptor in lipopolysaccharide-induced acute lung injury. Respiration. 2014;87(5):394–407. doi: 10.1159/000358066. [DOI] [PubMed] [Google Scholar]
- 13.Wu JY, Feng L, Park HT, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2016;410(6831):948–52. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewandowska L, Matuszkiewicz-Rowinska J, Jayakumar C, et al. Netrin-1 and Semaphorin 3A predict the development of acute kidney injury in liver transplant patients. PLoS One. 2014;9(10):e107898. doi: 10.1371/journal.pone.0107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuexing T, Hui W, Renhua S, et al. Urinary netrin-1 and KIM-1 as early biomarkers for septic acute kidney injury. Ren Fail. 2014;36(10):1559–63. doi: 10.3109/0886022X.2014.949764. [DOI] [PubMed] [Google Scholar]
- 16.Xie Z, Huang L, Enkhjargal B, et al. Recombinant Netrin-1 binding UNC5B receptor attenuates neuroinflammation and brain injury via PPARγ/NFκB signaling pathway after subarachnoid hemorrhage in rats. Brain Behav Immun. 2018;69:190–202. doi: 10.1016/j.bbi.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sackett SD, Otto T, Mohs A, et al. Myeloid cells require gp130 signaling for protective anti-inflammatory functions during sepsis. FASEB J. 2019;33(5):6035–44. doi: 10.1096/fj.201802118R. [DOI] [PubMed] [Google Scholar]
- 19.Kenji O. [Regulation of inflammatory responses by endothelial cells – understanding the molecular mechanism(s) and its therapeutic application to sepsis]. Masui. 2008;57(3):311–20. [in Japanese] [PubMed] [Google Scholar]
- 20.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M, et al. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78(3):425–35. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am J Physiol Renal Physiol. 2013;304(7):F948–57. doi: 10.1152/ajprenal.00580.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aherne CM, Collins CB, Eltzschig HK. Netrin-1 guides inflammatory cell migration to control mucosal immune responses during intestinal inflammation. Tissue Barriers. 2013;1(2):e24957. doi: 10.4161/tisb.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed R, Jayakumar C, Ranganathan PV, et al. Kidney proximal tubular epithelial-specific overexpression of Netrin-1 suppresses inflammation and albuminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am J Pathol. 2012;181(6):1991–2002. doi: 10.1016/j.ajpath.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han Y, Shao Y, Lin ZR, et al. Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest Ophthalmol Vis Sci. 2012;53(3):1285–95. doi: 10.1167/iovs.11-8722. [DOI] [PubMed] [Google Scholar]
- 25.Ly NP, Komatsuzaki K, Fraser IP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102(41):14729–34. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang WW, Reeves WB, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol. 2008;294(4):F739–47. doi: 10.1152/ajprenal.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Reeves W, Ramesh G. Netrin-1 increases proliferation and migration of renal proximal tubular epithelial cells via the UNC5B receptor. Am J Physiol Renal Physiol. 2009;296(4):F723–29. doi: 10.1152/ajprenal.90686.2008. [DOI] [PubMed] [Google Scholar]
- 28.Tadagavadi RK, Wang WG. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185(6):3750–58. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 29.Mirakaj V, Thix C, Laucher S, et al. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med. 2010;181:815–24. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]