Abstract
Background
Neutrophil and albumin are respective indicators of inflammation and malnutrition. Whether combining those 2 markers can predict acute prognosis in patients with ST-segment elevation myocardial infarction (STEMI) remains unknown. This study aimed to investigate the prognostic value of neutrophil percentage to albumin ratio (NPAR) for in-hospital mortality in STEMI patients.
Material/Methods
There were 1024 patients hospitalized with acute STEMI retrospectively enrolled in this study. Demographic, clinical, and admission laboratory data were extracted from medical record. NPAR was calculated as neutrophil percentage numerator divided by albumin in the admission blood samples. In-hospital mortality was designed as the primary outcome in the study, major adverse cardiac events (MACE) and cardiac death were recorded as the secondary clinical outcomes.
Results
The rates of in-hospital mortality, MACE, and cardiac death in high NPAR group were significantly higher than those in the low NPAR group (P<0.001, P=0.004, P<0.001). The Kaplan-Meier analysis showed worse outcomes in higher NPAR group (P<0.001). NPAR levels and age independently predicted in-hospital mortality. A NPAR value >1.9 was identified as an effective cut point in STEMI for in-hospital mortality (P<0.001, sensitivity 82%, specificity 52%).
Conclusions
Admission NPAR was independently correlated with in-hospital mortality in patients with STEMI.
MeSH Keywords: Albumins, Hospital Mortality, Myocardial Infarction, Neutrophils, Prognosis
Background
Acute ST-segment elevation myocardial infarction (STEMI) is a quite critical condition of coronary heart disease which needs timely revascularization therapy. Recent studies have highlighted a fall in acute mortality following STEMI in parallel with greater use of primary percutaneous coronary intervention (PPCI), antithrombotic therapy and secondary prevention [1], nevertheless, mortality remains substantial. The in-hospital mortality of unselected patients with STEMI in the national registries of the European countries varies between 4% and 12% [2].
Inflammatory processes are believed to actively trigger cardiovascular disease development and final clinical events. Neutrophils drive the early inflammatory response following a myocardial infarction, and high neutrophil count was demonstrated as an important marker for cardiovascular mortality risk prediction [3]. In myocardial infarction patients undergoing mechanical revascularization, a higher neutrophil percentage is related to worse short-term prognosis [4]. Besides, malnutrition which is reflected by reduced serum albumin level has been reported to be closely linked to adverse clinical outcomes and mortality in patients with acute myocardial infarction [5]. However, whether both markers can be combined together to predict the acute outcomes remains unknown.
We developed and tested a simple index of the neutrophil percentage to albumin ratio (NPAR) for predicting in-hospital mortality of STEMI.
Material and Methods
Study design and population
We retrospectively evaluated consecutive 1306 patients with acute STEMI from China Beijing Friendship Hospital Database (CBD) who were admitted to the department of cardiology, Beijing Friendship Hospital (Beijing, P.R. China) from January 2013 to February 2017. The protocol was approved by the ethical committee of Beijing Friendship Hospital (No. 2017-P2-013-01) and the study began in November 2017. Patients were included in the study if they fulfilled the following criteria according to previous study [6]: STEMI was defined as typical symptoms (chest pain >30 minutes) and ST elevation (greater than or equal to 1 mm in at least consecutive 2 leads on ECG). In total, 282 patients were excluded from this study because they had complications of active infectious disease, active carcinoma, hematological proliferative diseases, chronic inflammatory disease, active hepatobiliary disorders, steroid therapy for autoimmune disease, or had no admission laboratory parameters recorded. Finally, a total of 1024 patients were included in the study.
Laboratory analysis and NPAR calculation
Peripheral blood samples were drawn from patients immediately on admission. Routine complete blood count and blood biochemistry parameters were measured by automatic analyzer. Neutrophil percentage, indicated by the percentage of neutrophil in white blood cells, was automatically calculated by the analyzer. Serum albumin level was measured using bromocresol green method with album kits and AU5800 biochemistry analysis system (Beckman Coulter Company) according to manufacturer’s instruction within 30 minutes from blood collection. NPAR was calculated as neutrophil percentage numerator (i.e., 70% was recorded 70) divided by albumin using the same blood samples drawn on admission. The total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, and high-sensitivity C-reactivity protein levels were from the fasting blood samples which were taken on the next morning after admission. Serum levels of cardiac markers including NT-proBNP, cardiac troponin I (cTnI) and creatinine kinase-MB (CK-MB) were measured on admission and every 24 hours until the peaks occurred, and their levels went down according to the hospital protocol.
Data collection and definitions
The patients’ demographic information and cardiovascular risk factors including previous coronary heart disease, hypertension, diabetic mellitus, dyslipidemia, and smoking history were retrospectively collected from medical records. The systolic blood pressure, diastolic blood pressure, heart rate and Killip class were defined as the first data recorded on admission. The Simpson method was used to calculate the left ventricular ejection fraction (LVEF) for quantitative assessment of the left ventricular systolic function before discharge. As the primary outcome, in-hospital mortality was defined as all-cause deaths during hospitalization. Cardiac death was defined as previously reported including death from acute MI, heart failure, arrhythmia and unexplained sudden death [6]. A composite of cardiovascular mortality, repeat target vessel revascularization, re-infarction, malignant ventricular arrhythmias, and stroke was defined as major adverse cardiac events (MACE).
Revascularization procedure and medications
Loading dose of antiplatelet medications (300 mg aspirin, 300 to 600 mg clopidogrel) were used before PPCI according to established guidelines. PPCI was performed according to the guidelines with standard techniques and appropriate strategies, based on the discretion of the attending physician. No thrombolytic therapy was performed in the study population. During hospitalization period and after discharge, antiplatelets, statins, β-blockers, and angiotensin-converting enzyme inhibitors were administered to all patients according to guidelines unless contraindicated.
Statistical analysis
Qualitative variables were expressed as percentages and quantitative variables as the mean±standard deviation or the median (interquartile range). Continuous variables were tested using Kolmogorov-Smirnov statistics to check the normal distribution assumption. Differences among tertile groups and pairwise comparisons were analyzed with variance analysis and the Tukey post hoc test or the Mann-Whitney U test as appropriate. Univariate logistic regression analysis was used to identify the predictors of in-hospital mortality. A forward stepwise multivariate logistic regression analysis was used to identify independent predictors of in-hospital mortality, which included variables with P<0.05 from the univariate logistic regression or of clinical importance. Receiver operating curve test was performed to define the cutoff value of NPAR for in-hospital mortality. A P value <0.05 was defined statistically significant. The Kaplan -Meier method was used to create event-free survival curves. The comparisons of ROC curves were performed using the Delong test with MedCalc (version 11.4.2.0). Other statistical analyses were performed with SPSS (version 24.0, Chicago, IL, USA).
Results
We divided the recruited patients into 3 groups according to tertiles of admission NPAR. Of the 1024 patients, 341 were in the tertile 1 NPAR group (mean age 61.1±12.2 years, 78% male); 342 were in the tertile 2 NPAR group (mean age 63.3±13.6 years, 75.4% male) and 341 were in the tertile 3 NPAR group (mean age 67.8±12.1 years, 67.4% male). Detailed comparing information on the clinical, laboratory and demographic parameters among tertile groups were presented in Table 1.
Table 1.
Demographic, clinical, and laboratory characteristics of the tertile groups according to the NPAR.
| Characteristics | Tertile 1, n=341 <1.75 |
Tertile 2, n=342 1.75–2.05 |
Tertile 3, n=341 >2.05 |
P1 value | P2 value |
|---|---|---|---|---|---|
| Age, years | 61.1±12.2 | 63.3±13.6 | 67.8±12.1 | <0.001 | <0.001 |
| Male gender, n (%) | 266 (78.0) | 258 (75.4) | 229 (67.4) | 0.004 | 0.002 |
| PPCI, n (%) | 179 (59.7) | 172 (56.8) | 185 (66.3) | 0.056 | 0.099 |
| Medical histories | |||||
| CHD, n (%) | 106 (31.5) | 77 (22.5) | 101 (29.9) | 0.021 | 0.658 |
| Hypertension, n (%) | 194 (57.4) | 205 (60.1) | 210 (61.9) | 0.478 | 0.228 |
| DM, n (%) | 91 (26.8) | 92 (26.9) | 105 (31.0) | 0.391 | 0.236 |
| Dyslipidemia, n (%) | 148 (43.8) | 149 (43.7) | 136 (40.1) | 0.545 | 0.334 |
| Smoke, n (%) | 228 (67.1) | 213 (62.3) | 189 (55.8) | 0.010 | 0.002 |
| SBP, mmHg | 127.4±22.1 | 125.3±19.9 | 122.1±23.1 | 0.006 | 0.004 |
| DBP, mmHg | 75.2±12.6 | 74.0±12.7 | 70.5±12.9 | <0.001 | <0.001 |
| HR, bpm | 75.8±14.2 | 76.2±14.5 | 77.5±19.0 | 0.777 | 0.510 |
| Killip ≥III n (%) | 20 (5.9) | 26 (6.8) | 60 (17.9) | 0.001 | <0.001 |
| Laboratory index | |||||
| WBC, ×109/L | 8.3±2.8 | 9.1±3.2 | 9.8±3.1 | <0.001 | <0.001 |
| Neutrophil, ×109/L | 4.7 (3.8–6.2) | 6.6 (5.0–8.4) | 7.9 (6.1–9.8) | <0.001 | <0.001 |
| Hemoglobin, g/L | 144.2±16.7 | 138.7±18.7 | 129.9±20.1 | <0.001 | <0.001 |
| platelets, ×109/L | 233.0±67.4 | 232.3±75.3 | 218.2±70.3 | 0.009 | 0.019 |
| RDW, % | 12.5 (11.7–13.3) | 12.7 (11.9–13.6) | 12.9 (12.1–13.7) | <0.001 | <0.001 |
| PDW, % | 13.2±2.8 | 14.0±2.6 | 14.4±2.8 | <0.001 | <0.001 |
| Glucose, mmol/L | 8.0 (6.4–10.6) | 7.7 (6.4–10.4) | 8.2 (6.9–11.4) | 0.034 | 0.076 |
| Creatinine, μmol/L | 74.4 (66.4–86.5) | 78.6 (69.0–94.6) | 78.0 (66.0–99.0) | 0.002 | 0.004 |
| ALT, U/L | 24.0 (18.0–35.0) | 23.0 (17.0–36.0) | 24.0 (17.0–38.0) | 0.728 | 0.981 |
| Albumin, g/L | 42.2±3.8 | 40.3±3.6 | 35.9±4.0 | <0.001 | <0.001 |
| T-BIL, μmol/L | 11.3 (8.3–15.3) | 12.3 (9.2–17.3) | 11.9 (9.0–17.0) | 0.009 | 0.026 |
| TC, mmol/L | 4.5±1.0 | 4.4±1.1 | 4.3±1.1 | 0.035 | 0.045 |
| LDL-C, mmol/L | 2.6±0.7 | 2.6±0.8 | 2.5±0.8 | 0.180 | 0.182 |
| HDL-C, mmol/L | 1.1 (0.9–1.2) | 1.1 (0.9–1.2) | 1.0 (0.9–1.2) | 0.115 | 0.064 |
| TG, mmol/L | 1.5 (1.1–2.2) | 1.4 (1.0–1.9) | 1.2 (0.9–1.7) | <0.001 | <0.001 |
| HsCRP, mg/dL | 4.3 (1.7–13.3) | 7.4 (2.7–17.6) | 10.7 (3.5–27.3) | <0.001 | <0.001 |
Data are expressed as count (percentage) for categorical variables, mean±standard deviation or median (interquartile range) for numerical variables. P1 is the P value acquired from comparing 3 groups. P2 is the P value acquired from comparing the tertile 1 group with the tertile 3 group. PPCI – primary percutaneous coronary intervention; CHD – coronary heart disease; DM – diabetic mellitus; SBP – systolic blood pressure; DBP – diastolic blood pressure; HR – heart rate; WBC – white blood cells; RDW – red blood cell distribution width; PDW – platelet distribution width; ALT – alanine aminotransferase; T-BIL – total bilirubin; TC – total cholesterol; LDL-C – low density lipoprotein cholesterol; HDL-C – high density lipoprotein cholesterol; TG – triglycerides; hs-CRP – high-sensitivity C-reactivity protein.
In-hospital deaths occurred in 45 patients (4.4%) in our study, cardiac deaths in 40 patients (3.9%) and MACE in 70 patients (6.8%). Patients with high NPAR had higher values of Peak NTproBNP, Peak CK-MB, Peak cTnI and lower values of LVEF compared to tertile 1 NPAR group (P<0.05, Table 2). Concordantly, in-hospital mortality, cardiac death and MACE in the tertile 3 NPAR group were significantly higher compared to the tertile 1 NPAR group (8.8% versus 2.1%, 8.3% versus 1.8%, 10.9% versus 5.0%; P<0.001, P<0.001, P=0.004, Table 2). Kaplan-Meier curves showed that a worse outcome occurred in the higher NPAR group (log-rank P<0.001, Figure 1).
Table 2.
In-hospital events, myocardial injury, and cardiac function of STEMI patients according to the tertiles of NPAR.
| Characteristics | Tertile 1, n=341 <1.75 |
Tertile 2, n=342 1.75–2.05 |
Tertile 3, n=341 >2.05 |
P1 value | P2 value |
|---|---|---|---|---|---|
| In-hospital death, % | 7 (2.1) | 8 (2.3) | 30 (8.8) | <0.001 | <0.001 |
| Cardiac death, % | 6 (1.8) | 6 (1.8) | 28 (8.3) | 0.001 | <0.001 |
| MACE, % | 17 (5) | 16 (4.7) | 37 (10.9) | 0.001 | 0.004 |
| Cardiac markers | |||||
| Peak NTproBNP, pg/mL | 1386.0 (565.0–3390.5) | 1868.0 (780.5–5062.3) | 3772.5 (1354.5–12725.0) | <0.001 | <0.001 |
| Peak CK-MB, ng/mL | 71.4 (14.8–187.0) | 90.0 (22.4–229.0) | 114.1 (23.7–254.0) | 0.010 | 0.003 |
| Peak cTnI, ng/mL | 10.9 (3.5–28.6) | 14.0 (5.3–35.1) | 17.3 (6.0–42.0) | 0.002 | <0.001 |
| LVEF | 0.59±0.09 | 0.57±0.10 | 0.55±0.11 | <0.001 | <0.001 |
Data are expressed as count (percentage) for categorical variables, mean ± standard deviation or median (interquartile range) for numerical variables. P1 is the P value acquired from comparing 3 groups. P2 is the P value acquired from comparison of the tertile 1 group with the tertile 3 group. MACE – major adverse cardiac events; CK-MB – creatinine kinase-MB; cTnI – cardiac troponin I; LVEF – left ventricular ejection fraction.
Figure 1.
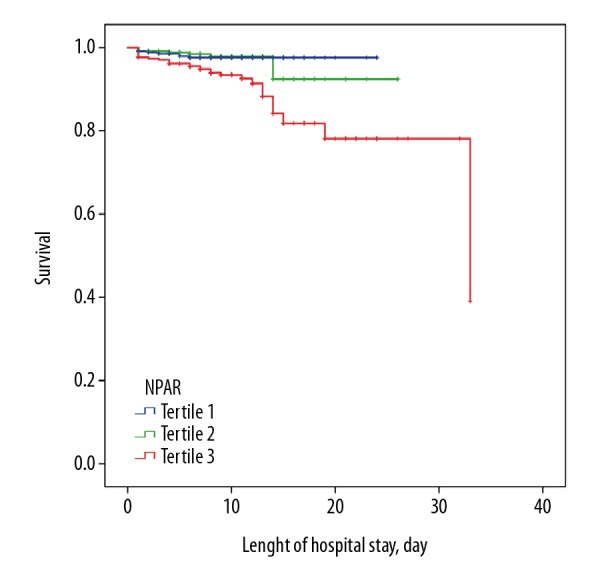
Kaplan-Meier curves of the tertile groups. The Kaplan-Meier analysis showed that a worse outcome occurred in the higher NPAR group (log-rank P<0.001). NPAR – neutrophil percentage to albumin ratio.
Six risk factors of statistical significance identified by univariate analysis (age, male gender, admission glucose, creatinine, hemoglobin and NPAR) were further analyzed with multivariate logistic regression. High NPAR levels were found to be significantly associated with the adjusted risk of in-hospital mortality (odds ratio [OR] 4.928, 95% confidence interval [CI] 1.136–21.372, P=0.033, Table 3) on multivariate analysis. Another independent risk factor of in-hospital mortality was age (OR 1.07, 95% CI 1.02–1.13, P=0.013, Table 3). A NPAR value >1.9 was demonstrated to be an valid cut-point in patients with STEMI for in-hospital mortality through receiver operating characteristic curve (ROC) analysis (area under the curve, AUC=0.72, sensitivity 82%, specificity 52%, 95% CI 0.62–0.81, P<0.001; Figure 2), and the comparisons of ROC curves found NPAR was a better predictor than either albumin (P=0.024) or neutrophil percentage (P=0.001) alone.
Table 3.
Effects of multiple variables on in-hospital mortality in univariate and multivariate logistic regression analysis.
| Variables | Unadjusted OR | 95% CI | P value | Adjusted OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Age | 1.094 | 1.066–1.123 | <0.001 | 1.071 | 1.015–1.129 | 0.013 |
| Male gender | 0.432 | 0.250–0.747 | 0.003 | 1.197 | 0.356–4.017 | 0.772 |
| PPCI | 2.667 | 0.886–8.030 | 0.081 | 1.937 | 0.593–6.325 | 0.274 |
| CHD | 1.679 | 0.960–2.934 | 0.069 | |||
| Hypertension | 1.571 | 0.877–2.815 | 0.129 | |||
| DM | 2.035 | 1.177–3.518 | 0.011 | |||
| Smoke | 0.282 | 0.158–0.500 | <0.001 | |||
| SBP | 0.992 | 0.980–1.005 | 0.241 | |||
| Killip Class | 5.445 | 4.026–7.364 | <0.001 | |||
| WBC | 1.074 | 0.987–1.168 | 0.096 | |||
| Neutrophil | 1.095 | 1.003–1.197 | 0.043 | |||
| Hemoglobin | 0.969 | 0.955–0.983 | <0.001 | 0.975 | 0.944–1.007 | 0.120 |
| Platelets | 1.001 | 0.997–1.005 | 0.509 | |||
| RDW | 0.985 | 0.897–1.082 | 0.750 | |||
| PDW | 1.029 | 0.931–1.139 | 0.572 | |||
| Glucose | 1.074 | 1.016–1.136 | 0.012 | 1.078 | 0.970–1.197 | 0.162 |
| Creatinine | 1.003 | 1.001–1.004 | 0.001 | 0.993 | 0.976–1.010 | 0.399 |
| Albumin | 0.877 | 0.829–0.928 | <0.001 | |||
| T-BIL | 0.987 | 0.943–1.033 | 0.571 | |||
| TC | 0.737 | 0.535–1.016 | 0.063 | |||
| LDL-C | 0.670 | 0.434–1.033 | 0.070 | |||
| TG | 0.727 | 0.479–1.104 | 0.135 | |||
| HsCRP | 1.030 | 1.008–1.053 | 0.008 | |||
| NPAR | 3.673 | 1.944–6.939 | <0.001 | 4.928 | 1.136–21.372 | 0.033 |
OR – odds ratio; CI – confidence interval; PPCI – primary percutaneous coronary intervention; CHD – coronary heart disease; DM – diabetic mellitus; SBP – systolic blood pressure; HR – heart rate; WBC – white blood cells; RDW – red blood cell distribution width; PDW – platelet distribution width; T-BIL – total bilirubin; TC – total cholesterol; LDL-C – low density lipoprotein cholesterol; TG – triglycerides; hs-CRP – high-sensitivity C-reactivity protein; NPAR – neutrophil percentage to albumin ratio.
Figure 2.
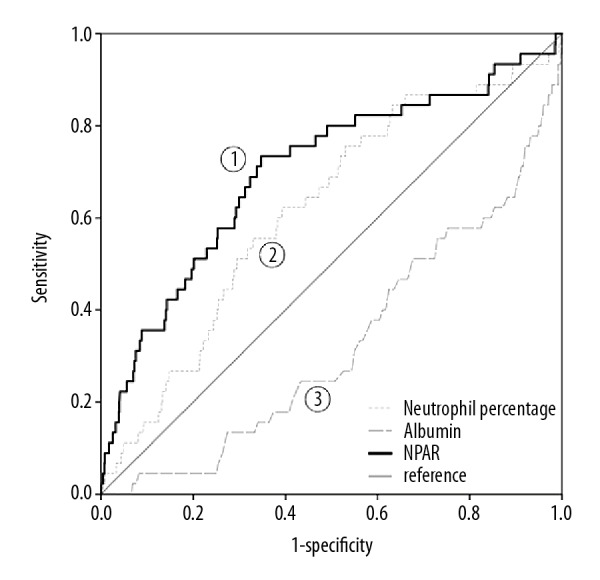
Receiver operating characteristic curve of NPAR value. NPAR value was an effective marker for predicting in-hospital mortality in STEMI by receiver operating characteristic curve analysis. AUC – area under the curve; CI – confidence interval. AUC 1=0.72, 95% CI 0.62–0.81, P<0.001; AUC 2=0.62, 95% CI 0.53–0.70, P=0.007; AUC 3=0.33, 95% CI 0.25–0.41, P<0.001.
Discussion
This study demonstrated that the admission NPAR value in patients with acute STEMI was an independent predictor of in-hospital mortality. In-hospital mortality rates of STEMI patients undergoing primary PCI varied from 4% to 12% [2], and the mortality rate (4.4%) in our study was compatible with prior reports. As far as we know, there has been few studies focused on the relation of NPAR with in-hospital mortality in patients with STEMI.
Even in the era of reperfusion therapy, in-hospital mortality in patients with STEMI remains a considerable variability [2]. Individualized and timely risk assessment for each patient allows a more accurate decision-making of treatment strategy and medical resource allocation. To carry out individualized therapeutics, doctors should be aware of the risk factors with regard to morbidity and mortality in STEMI patients. Both neutrophil percentage and serum albumin tests are timely, easy, inexpensive, routine examination techniques that give us information about the blood contents. The combination of neutrophil percentage and serum albumin provides a quite fast evaluation of risks for the patients with STEMI. Furthermore, even under the condition lacking further imaging or more laboratory test, as long as the complete blood count and biochemistry test can be performed, NPAR will still work as an effective tool for instant risk assessments. Our finding may provide additional convenience in some special situations, for example, underdeveloped areas.
Inflammatory or stress status correlated with adverse cardiovascular events and complications occurring in STEMI patients. Some easily acquirable laboratory index related to inflammation have been recently studied in various cardiovascular diseases, and they were demonstrated to be predictive for short- or long-term mortality [7]. For example, neutrophil to lymphocyte ratio, monocyte to high-density lipoprotein ratio, platelet-leukocyte aggregates, red cell distribution width are markers related to inflammation in STEMI which attracted the attention of investigators [6,8–11]. In our study, we included neutrophil percentage into the NPAR value as an acute inflammatory response marker. Neutrophils intermediate the early inflammatory response following a myocardial infarction, and they were found most valuable to predict cardiovascular mortality among all leukocyte subsets [12], partly because of the serum ingredients produced by neutrophil such as gelatinases (matrix metalloproteinase [MMP]-2 and -9), collagenases (MMP-1, -8, -7, -13) and elastase [13]. Similarly, neutrophil counts, percentage, and the delta neutrophil index were also included in the predictive index for STEMI prognosis in other studies [4,9,14–16].
A low albumin level was an independent predictor of new-onset heart failure and in-hospital mortality in patients with acute coronary syndrome, and the underlying mechanisms may be malnutrition and inflammation [5]. Albumin has a half-life of more than 10 days [17], thus we included it into the NPAR value to reflect recent status. In previous studies, neutrophil to albumin ratio was demonstrated as an independent predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation [18]. Another study demonstrated that the delta neutrophil index-to-serum albumin ratio on emergency department admission was a promising prognostic marker for 28-day mortality in patients with severe sepsis [19]. In the area of cardiology, we demonstrated that the NPAR value, as a combination of acute inflammation and recent malnutrition marker, was effective in short-term prognostic judgment. Similarly, a combined effect of serum albumin and C-reactive protein was investigated in patients with coronary artery disease who underwent their first intervention, and it was associated with long-term adverse cardiac events [20].
The difference in the proportions of patients with PPCI was not significant among tertile groups, which meant the effects on clinical outcomes among tertile groups by PPCI therapy seemed not significant. However, the factor of PPCI was not statistically significant on univariate analysis. This might result from the limitation of sample size. Nevertheless, it was a very strong protective factor, and was included in multivariate logistic regression model. After the adjustment with six risk factors, NPAR remained an independent predictor of in-hospital mortality on multivariate analysis.
Limitations
NPAR only reflects the aspects of malnutrition and inflammation. It is a very simple index which can be fast calculated. Neutrophil to lymphocyte ratio, platelet-leukocyte aggregates, red cell distribution width, monocyte to high-density lipoprotein ratio are all markers reflecting inflammation. The advantage of those kinds of markers including NPAR is that they are laboratory objective variables and can be easily obtained from first biochemical blood examination, which is routinely performed in cases of STEMI in clinical practice. However, the severity and mortality of STEMI vary according to many factors such as advanced age, Killip class, time delay to treatment, treatment strategy, history of myocardial infarction, diabetic mellitus, renal failure and so on. Generally speaking, the more key variates a model includes, the more precisely it predicts. The newly developed laboratory stratification model [21] is also used to judge STEMI acute outcomes. The Global Registry of Acute Coronary Events score includes several retrospective factors [22], and the Thrombolysis In Myocardial Infarction (TIMI) risk index is defined by age, systolic blood pressure, and heart rate [23]. Those models can be used more precisely to predict the prognosis in acute coronary syndrome. Besides, because there was no thrombolytic therapy performed in the study population, the clinical applicability of NPAR in patients undergoing thrombolytic therapy remains unclear.
Conclusions
High admission NPAR was identified as an independent predictor of in-hospital mortality in patients with STEMI. Our results suggested that STEMI patients with high NPAR value should be carefully monitored and considered for intensive care because of the close association with early mortality. Further studies are still required to confirm and illuminate clinical implications of the findings.
Acknowledgement
We appreciate Ms. Guoliang Zhao, Shanshan Wu, and Na Zeng for the data collection and interpretation for the study.
Abbreviations
- PPCI
primary percutaneous coronary intervention
- CHD
coronary heart disease
- DM
diabetic mellitus
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HR
heart rate
- WBC
white blood cells
- RDW
red blood cell distribution width
- PDW
platelet distribution width
- ALT
alanine aminotransferase
- T-BIL
total bilirubin
- TC
total cholesterol
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoprotein cholesterol
- TG
triglycerides
- hs-CRP
high-sensitivity C-reactivity protein
- MACE
major adverse cardiac events
- NT proBNP
N-terminal pro-B-type Natriuretic Peptide
- CK-MB
creatinine kinase MB
- cTnI
cardiac troponin I
- LVEF
left ventricular ejection fraction
- OR
odds ratio
- CI
confidence interval
- NPAR
neutrophil percentage to albumin ratio
Footnotes
Conflicts of interests
None.
Source of support: This work was supported by the National Natural Science Foundation (No. 81603425), the Seed Plan Program of Beijing Friendship Hospital (YYZZ2017A06), the Beijing Talents Fund (No. 2016000021469G221), and the Rising Star Program from Beijing Friendship Hospital (No.YYQDKT2015-25)
References
- 1.Townsend N, Wilson L, Bhatnagar P, et al. Cardiovascular disease in Europe 2016: An epidemiological update. Eur Heart J. 2016;37(42):3182–83. doi: 10.1093/eurheartj/ehw468. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen SD, Laut KG, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: Current status in 37 ESC countries. Eur Heart J. 2014;35(29):1957–70. doi: 10.1093/eurheartj/eht529. [DOI] [PubMed] [Google Scholar]
- 3.Shah AD, Denaxas S, Nicholas O, et al. Neutrophil counts and initial presentation of 12 cardiovascular diseases: A CALIBER Cohort Study. J Am Coll Cardiol. 2017;69(9):1160–69. doi: 10.1016/j.jacc.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiarresga AJ, Ferreira RC, Feliciano J, et al. Prognostic value of neutrophil response in the era of acute myocardial infarction mechanical reperfusion. Rev Port Cardiol. 2004;23(11):1387–96. [PubMed] [Google Scholar]
- 5.Gonzalez-Pacheco H, Amezcua-Guerra LM, Sandoval J, et al. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol. 2017;119(7):951–58. doi: 10.1016/j.amjcard.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 6.Gul M, Uyarel H, Ergelen M, et al. Prognostic value of total bilirubin in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2013;111(2):166–71. doi: 10.1016/j.amjcard.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Rasoul S, Ottervanger JP, de Boer MJ, et al. Predictors of 30-day and 1-year mortality after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis. 2009;20(6):415–21. doi: 10.1097/MCA.0b013e32832e5c4c. [DOI] [PubMed] [Google Scholar]
- 8.Karatas MB, Canga Y, Ozcan KS, et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am J Emerg Med. 2016;34(2):240–44. doi: 10.1016/j.ajem.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 9.Pan W, Zhao D, Zhang C, et al. Application of neutrophil/lymphocyte ratio in predicting coronary blood flow and mortality in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention. J Cardiol. 2015;66(1):9–14. doi: 10.1016/j.jjcc.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Wang S, Yuan L, et al. Increased mean platelet volume is associated with higher in-hospital mortality rate in patients with acute myocardial infarction. Clin Lab. 2017;63(1):163–67. doi: 10.7754/Clin.Lab.2016.160627. [DOI] [PubMed] [Google Scholar]
- 11.Ren F, Mu N, Zhang X, et al. Increased platelet-leukocyte aggregates are associated with myocardial no-reflow in patients with ST elevation myocardial infarction. Am J Med Sci. 2016;352(3):261–66. doi: 10.1016/j.amjms.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 12.o Hartaigh B, Bosch JA, Thomas GN, et al. Which leukocyte subsets predict cardiovascular mortality? From the LUdwigshafen RIsk and Cardiovascular Health (LURIC) Study. Atherosclerosis. 2012;224(1):161–69. doi: 10.1016/j.atherosclerosis.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Montecucco F, Liberale L, Bonaventura A, et al. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep. 2017;19(3):11. doi: 10.1007/s11883-017-0646-1. [DOI] [PubMed] [Google Scholar]
- 14.Akpek M, Kaya MG, Lam YY, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110(5):621–27. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Ren L, Lei L, et al. The relationship between neutrophil counts on admission and angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Acta Cardiol. 2016;71(2):241–46. doi: 10.2143/AC.71.2.3141856. [DOI] [PubMed] [Google Scholar]
- 16.Huang YL, Hu ZD. Lower mean corpuscular hemoglobin concentration is associated with poorer outcomes in intensive care unit admitted patients with acute myocardial infarction. Ann Transl Med. 2016;4(10):190. doi: 10.21037/atm.2016.03.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrerizo S, Cuadras D, Gomez-Busto F, et al. Serum albumin and health in older people: Review and meta-analysis. Maturitas. 2015;81(1):17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Tawfik B, Mokdad AA, Patel PM, et al. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs. 2016;27(9):879–83. doi: 10.1097/CAD.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 19.Hwang YJ, Chung SP, Park YS, et al. Newly designed delta neutrophil index-to-serum albumin ratio prognosis of early mortality in severe sepsis. Am J Emerg Med. 2015;33(11):1577–82. doi: 10.1016/j.ajem.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Cinar T, Cagdas M, Rencuzogullari I, et al. Prognostic efficacy of C-reactive protein/albumin ratio in ST elevation myocardial infarction. Scand Cardiovasc J. 2019;53(2):83–90. doi: 10.1080/14017431.2019.1590628. [DOI] [PubMed] [Google Scholar]
- 21.Yanishi K, Nakamura T, Nakanishi N, et al. A simple risk stratification model for st-elevation myocardial infarction (STEMI) from the combination of blood examination variables: Acute Myocardial Infarction-Kyoto Multi-Center Risk Study Group. PLoS One. 2016;11(11):e0166391. doi: 10.1371/journal.pone.0166391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–53. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 23.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–37. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]


