Abstract
Background
Laryngeal cancer is one of the most common malignant tumors of the head and neck. Natural compounds in traditional Chinese medicine provide many valuable potential compounds for tumor chemotherapy. Esculetin, a coumarin derivative from several herbs, inhibits proliferation of many types of cancer cells, but its anticancer effect in laryngeal cancer is still not clear.
Material/Methods
We performed in vitro proliferation assay, invasion assay, and migration assay to assess the effect of esculetin against LC, and in vivo nude mouse xenograft animal model was used as well. Flow cytometry was conducted to analyze the effect of esculetin on cell cycle of LC cells, and Western blot analysis was used to assess the effect esculetin on the JAK-STAT signaling pathway.
Results
Esculetin remarkably inhibits proliferation, migration, and invasion of LC cells, and reduces in vivo xenograft tumor growth and tumor weight in a dose-dependent manner. Our molecular mechanism study demonstrated that esculetin significantly inhibits STAT3 phosphorylation and blocks translocation of STAT3 into the nucleus, and esculetin also blocks the cell cycle in G1/S phase.
Conclusions
In a summary, by inhibiting the STAT3 activation, esculetin shows potential anticancer effects against the laryngeal cancer.
MeSH Keywords: Antigen-Presenting Cells, Head and Neck Neoplasms, STAT3 Transcription Factor
Background
Laryngeal squamous cell carcinoma (or larynx cancer, LC) is a common malignant tumor of the head and neck. LC has the characteristics of concealed location, metastases at early stage, and poor prognosis. There is still no effective screening or early diagnosis method for LC. Laryngeal cancer accounts for 22% of all head and neck malignancies, and the incidence in men is higher than in women. There were approximately 20 000 new cases of laryngeal cancer in China in 2011, with an estimated 11 000 deaths and an incidence rate of 0.35–1.55 per 100 000 population [1]. Males, smokers, and people who drink alcohol, eat red meat, and have HPV infection are at highest risk. Gastroesophageal reflux and laryngitis reflux are also potential risk factors [2].
The treatment methods for laryngeal cancer include surgery, radiotherapy, chemotherapy, and biological target therapy. In recent years, great progress has been made in treating LC. Surgical treatment options have changed from the initial total laryngectomy to the various surgical methods of retaining or reconstructing the larynx, and radiotherapy and chemotherapy are also widely used to treat LC. These changes have important positive significance in improving the survival and life quality of LC patients [3,4].
Drug resistance to chemotherapeutics is a recurrent issue plaguing many cancer treatment regimens and increasing the demand for novel antitumor drugs and new targets of cancer therapy. Natural compounds from traditional Chinese herbs have great potential as leading compounds for cancer chemotherapy. Esculetin (Esc) is a coumarin derivate widely found in natural herbal plants, such as Cortex fraxini and Hymenodictyon excelsum [5]. Modern pharmacological studies showed that Esc has many pharmacological functions, such as inhibiting 5-lipoxygenase [6], and it has anti-inflammatory [7], anti-oxidation [8], and anti-tumor effects [9]. In vitro studies have demonstrated that Esc has antitumor effects, including non-small-cell lung carcinoma (NSCLC) cell lines (NCI-H358 and NCI-H1299) [10], human breast cancer cell line ZR-75-1 [11], human acute myelocytic leukemia cell Kasumi-1 [12], and human leukemia U937G1 cells [13]. Research suggests that Esc has cytotoxicity against many kinds of tumor cells, but the effect of Esc on LC has not been reported.
Signal transducer and activator of transcription-3 (STAT3) is an oncogene which is highly expressed in most tumor tissues and cells [14–16]. Over-expressed STAT3 has been found in various stages of LC development. Its expression and phosphorylation increased with the deterioration of LC [17]. Previous studies have demonstrated that STAT3 is an important mediators of vasculogenic mimicry of squamous cell carcinoma of the larynx, and suppression of the JAK-2/STAT-3 signaling pathway significantly inhibits invasion and vasculogenic mimicry of laryngeal squamous cell carcinoma in vitro [18]. Zhang et al. reported that the JAK2 inhibitor AG190 induces cell apoptosis and inhibits proliferation of LC Hep-2 cancer cells [19]. The above evidence suggests STAT3 is a new potential target for the treatment of LC.
This study explored the anti-laryngeal cancer activity of Esc in vivo and in vitro, as well as its effect on STAT3. Our findings may provide an experimental basis for further research and development of Esc for use in antitumor drugs.
Material and Methods
Cell culture and chemical compounds
Human laryngeal cancer cell lines Hep-2, TU-212, and M4e, and human tubular epithelial cell line HK2 were purchased from the American Type Culture Collection (Manassas, VA USA). The Hep-2, TU-212, and HK2 cells were cultured in RPMI Medium 1640 (Thermo Fisher, Waltham, MA, USA), and M4e cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco; Thermo Fisher, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA). Cells were cultured in a 37°C incubator containing 5% CO2. When growth was in logarithmic phase, cells were seeded onto 96-well plates for further study.
Esculetin was purchased from Sigma-Aldrich (99.99% purity) as 100 mM stock solution, and cisplatin was also purchased from Sigma-Aldrich. STAT3 inhibitor C188-9 was purchased from Selleck Chemicals (Houston, TX, USA) and the STAT3 activator colivelin was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). In an in vitro study, Esc, C188-9, and cisplatin were serially diluted with RPMI medium 1640 triple and triple. Final working concentrations were 0.0457, 0.1369, 0.4120, 1.229, 3.700, 11.10, and 33.29 μM and the highest working concentration was 100 μM.
MTT proliferation assay
The MTT assay followed the protocol of a previous study [20]. Cells in logarithmic growth phase were digested with 0.25% trypsin and diluted by medium containing 10% fetal bovine serum into a 5×104/ml cell suspension. Cells were inoculated in 96-well plates with 100 μl per well. Cells were cultured in an incubator at 37°C containing 5% CO2. We gradually added 100 μl Esc, C188-9, and cisplatin over 24 h; 5 parallel wells of each concentration were prepared, and 100 μl medium culture was added in the blank control group. The cells were cultured in for another 72 h, the supernatant was discarded, and 100 μl MTT was added MTT to each well and cultured for 4 h at 37°C. After discarding the supernatant and DMSO (Sigma-Aldrich), the OD value at 570 nm was measured by Labsystems WELLSCAN MK3 ELISA (Dragon, Finland) and the IC50 was calculated. STAT3 inhibitor C188-9 was used as a positive control in the MTT assay. HK2 cells were also used in MTT assay to evaluate the Esc cytotoxicity effect on normal human cells, and cisplatin was used as a positive control for cell cytotoxicity assay.
The growth inhibition rate (%)=(OD value of blank control group–OD value of Esc group)/OD value of blank control group×100%.
Determination of cellular reactive oxygen species
Reactive oxygen species (ROS) were assessed using a flow cytometer and DCFH-DA (Sigma-Aldrich) staining. The cells were incubated with 10 μM DCFH-DA at 37°C for 30 min. After incubation with fluorochrome, the cells were washed with phosphate-buffered saline and immediately analyzed by fluorescence microscopy (Observer A1 inverted microscope, ZEISS, Germany). To determine whether ROS production influences Esc cytotoxicity in LC cells, the LC cells were pretreated with 100 μM N-acetyl-l-cysteine (NAC, Sigma-Aldrich) for 2 h, then Esc (IC50 concentration) was added to assess the cell proliferation. LC cells without NAC pretreatment were used as control.
Migration assay (wound healing assay)
We used 6-well plates for scratch wound healing assay. At least 3 lines were drawn per well, then we added 6×105 cells to each well and covered them evenly with medium. A vertical line was drawn using a pipette tip (200 μl) after cells were cultured for 24 h. Suspended cells were washed 3 times with PBS. The serum-free medium and drug-free serum-free medium were added. Cells were cultured in an incubator and images were captured 24 h later.
Cell cycle detection
After cells in logarithmic growth were cultured for 24 h, medium or Esc with different concentrations were added to culture bottles, with 3 parallel samples in each group. Cells were collected after 48-h culturing, washed with PBS and fixed with 70% cold ethanol, and DNA was analyzed by flow cytometry.
Matrigel transwell migration
The invasion of LC cells was measured in Matrigel (Corning, NY, USA)-coated Transwell inserts (Merck Millipore, Darmstadt, Germany) that contained polycarbonate filters with a pore size of 8.0 μm. The inserts were coated with 60 μl Matrigel matrix. We added 100 μl cells with concentration of 5×105/mL into the upper Transwell chambers, and 600 μl medium containing 10% serum and drugs with different concentrations were added to the lower chambers. The cells were cultured for 6 h, then unmigrated cells in the chamber were swabbed after discarding medium. Cells were fixed by 4% polyformaldehyde for 10 min, then stained with crystal violet for 10 min. The filter membrane was photographed under an inverted microscope (200×) after sealing with neutral gum. Cells were counted using Image Pro Plus Version 6 software. We randomly selected 3 wells in each group and 5 vision fields of each well to calculate the average number of cells.
Xenografts in nude mice
The 0.2 mL Hep-2 cell suspension (concentration: 6×105/mL) was subcutaneously injected into the axilla of BALB/c male nude mice. Three groups were prepared, with 6 mice in each group, with a 0.5% sodium carboxymethyl cellulose (CMC-Na) solvent control group. Esc was administered at doses of 50 mg/kg and 100 mg/kg. Esc was administered orally for 14 days when the tumor size reached 100 mm3. Esc was added once a day. Tumor volume was measured with a vernier caliper every 3 days. The length of the longest axis (mm) and the length of the tumor were recorded, and the narrowest axis length was taken as the width of the tumor (mm). The volume of the tumor (mm3) was calculated by the following formula: [length (mm)×width (mm)2]/2. After 14 days of treatment, the mice were killed and the tumors were removed and weighed. The tumor inhibition rate was calculated by the following formula: tumor inhibition rate=(Wmodel/Wtreatment)/Wmodel×100%. W represents the weight of the tumor. In this study, inhibition rate >60% was defined as effective.
Western blot analysis
Western blot analysis was performed following the protocol from previous studies [21,22]. Briefly, Esc-treated cells or frozen tumor tissue in liquid nitrogen were used for preparing protein lysates. The frozen tissues were placed in a clean mortar and quickly ground into fine white dry powder. We added 1 ml precooled protein lysate to 100 mg tissues cultured 20 min on ice, then centrifuged at 4°C for 15 min at 12 000 rpm. Proteins were extracted from cytoplasm and nucleus, separately, by nuclear protein extraction kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein concentration was measured by BCA protein quantitative assay (Applygen, Beijing, China). We used 50 μg protein of each group to separate protein by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the separated protein was transferred to the polyvinylidene fluoride membrane at room temperature, then closed by 5% BSA for 1 h.
Rabbit anti-mouse primary antibody of STAT3, p-STAT3, Janus kinase-1 (JAK1), p-JAK1, JAK2, and p-JAK2 (1: 1000) were added and reacted overnight at 4°C, then β-actin antibody (1: 2000) was added as control. The horseradish peroxidase labeled antibodies (alkaline phosphatase-labeled goat anti-rabbit antibody, 1: 1000) were added and reacted at room temperature for 2 h. The hybrids of proteins were detected by chemiluminescence chromogenic kit after washing with ECL. Images were captured using the Pierce ECL substrate imprinting detection system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The integrated optical density of the strip was quantitatively measured using ImageJ software (Bethesda, MD, USA). Expressions of proteins were calculated as expression relative to β-actin.
Data analysis
Continuous variable data are represented as the mean ± standard deviation (SD). SPSS 19.0 software (IBM, Chicago, IL, USA) was used for statistical analysis. The difference between 2 groups was compared with the t test. The differences between multiple groups were compared with the ANOVA with a post hoc t test. P<0.05 was considered to have statistical significance.
Results
Esculetin significantly inhibits the proliferation of laryngeal cancer cells
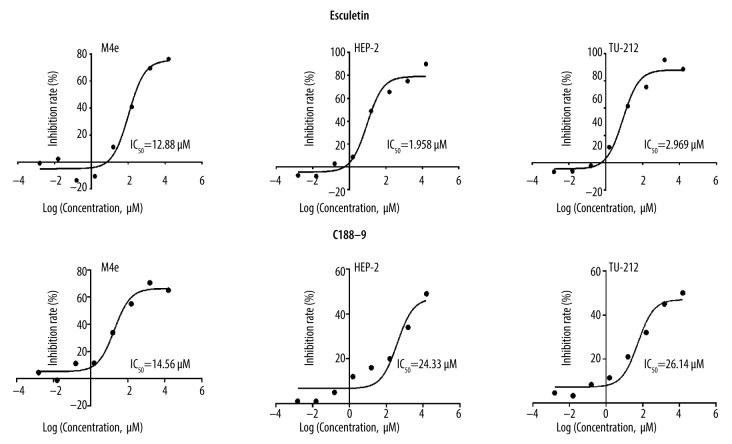
Cell proliferation assay is commonly used to measure the cytotoxicity of compounds. In the present study, we found that Esc could inhibit the proliferation of 3 LC cell lines, and its inhibitory effect increased with increasing Esc concentrations after 72-h intervention. The inhibitory effect of Esc on Hep-2 cells was the strongest among the 3 LC cell lines whose IC50 was 1.958 μM (Figure 1), so the Hep-2 cell line was selected for further study. The IC50 of the positive control STAT3 inhibitor C188-9 against all 3 LC cell lines was higher than 10 μM, while the cytotoxicity was weaker than that of esculetin. The IC50 of Esc for HK2 cells was higher than 100 μM, and the IC50 of cisplatin against LC cells was 7.47 μM for M4e cells, 2.15 μM for Hep-2 cells, and 3.58 μM for TU-212IC cells.
Figure 1.
The inhibitory effect of Esc and positive control C188-9 on LC cells (M4e, TU-212, and Hep-2 cells). The inhibitory effect of Esc on Hep-2 cells was the strongest and was selected for subsequent analysis.
Esculetin-induced cell cytotoxicity is dependent on ROS accumulation
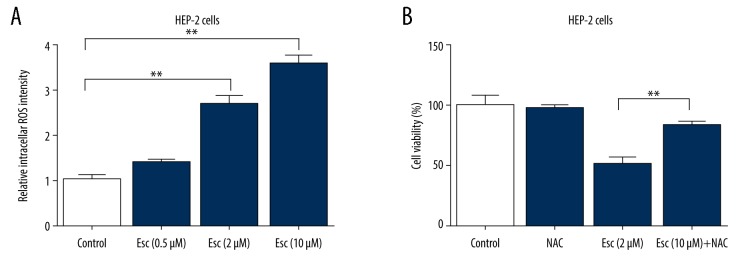
We investigated the role of ROS in mediating Esc-induced cytotoxicity in Hep-2 cells. As shown in Figure 2A, treatment with Esc for 72 h significantly increased intracellular ROS production in Hep-2 cells in a dose-dependent manner, as determined by fluorescence microscopy. Further interference research demonstrated that Esc cytotoxicity can be largely prevented by pretreatment with NAC (Figure 2B). These data suggest that the increase of cellular ROS was a critical factor underlying Esc-induced cell death.
Figure 2.
Esculetin-induced cytotoxicity is dependent on ROS accumulation. (A) Bar graphs show esculetin-induced cell intracellular ROS production. (B) Cell viability was detected by MTT assay. The cells were pretreated with NAC (100 μM) for 2 h, and then the cells were incubated with 2 μM esculetin for 72 h. The data represent the mean ±SD of 3 independent experiments, * P value represents significant differences at P<0.05.
Esculetin significantly inhibits the migration of laryngeal cancer cells
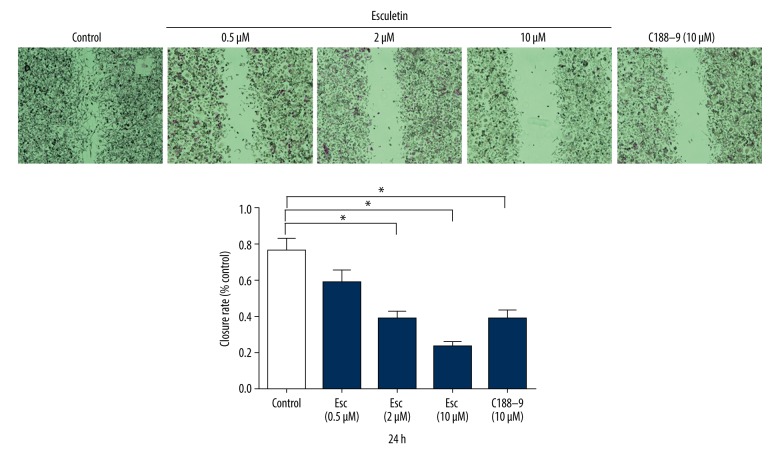
Wound healing assay was used to measure the effect of esculetin on migration ability of LC cells. The inhibitory migration rates of Hep-2 cells were observed at 24 h after Esc treatment. Typical wound healing at 24 h is shown in Figure 3. At 24 h after scratching, the cell migration rates of the Esc groups were all decreased compared with the control group. The 10 μM group had the highest migration inhibition rate (p<0.05, t test analysis). These results show that Esc inhibited the migration of LC Hep-2 cells. Compared with the positive control STAT3 inhibitor C188-9 at 10 μM, esculetin had a stronger inhibitory effect on LC cell migration.
Figure 3.
The inhibited migration rates of Hep-2 cells after treatment by Esc and positive control C188-9 (10 μM) for 24 h. The cell migration rates of the Esc groups were all decreased compared with the control group. The 10 μM concentration had the highest migration inhibition rate.
Effect of esculetin on the cell cycle of Hep-2
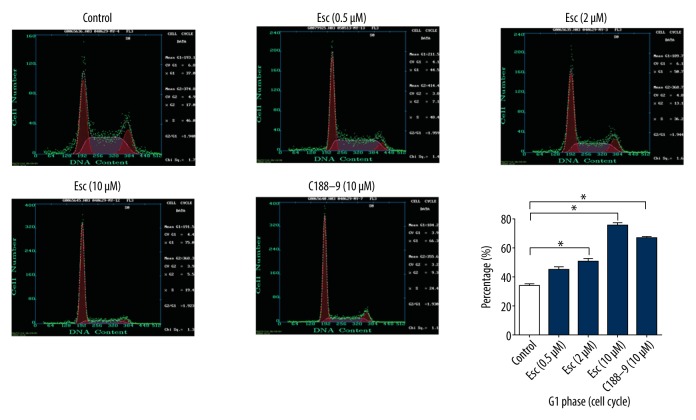
The cell cycle distribution was analyzed by flow cytometry. Flow cytometry detection of cell cycle showed that at 48 h after Esc and STAT3 inhibitor C188-9 were added into Hep-2 cells, the cell cycle was significantly blocked in G1 phase in Esc groups (0.5, 2, 10 μM) and C188-9 group compared with the blank control group, while the ratio of S/G2 phase cells decreased (Figure 4). At 10 μM, esculetin and C188-9 had almost same bioactivity on G1/S cell cycle arrest.
Figure 4.
Esc and positive control C188-9 caused G1 phase cell cycle arrest. The cell cycle was significantly blocked in G1 phase in Esc groups (0.5, 2, and 10 μM) compared with the blank control group. At 10 μM concentration, Esc and C188-9 had similar bioactivity to cause cell cycle arrest at G1.
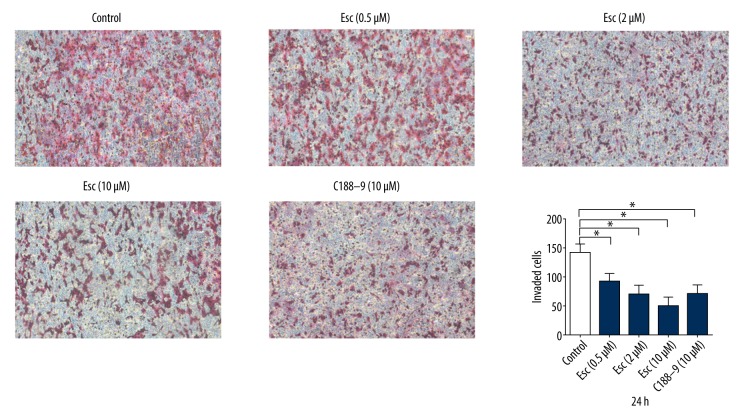
Esculetin significantly inhibits laryngeal cancer cell invasion ability
Compared with the control group, the number of migrated cells in Esc and STAT3 inhibitor C188-9 groups was significantly reduced (p<0.05). There was no significant difference between the control group and the 0.5 μM esculetin group, but the difference between the control group and the middle and high (2 and 10 μM) esculetin groups were significant. (p<0.05, Figure 5). Esculetin seems have higher invasion inhibitory ability than C188-9.
Figure 5.
Esc and positive control C188-9 decreased the number of invaded cells as determined by Transwell assay. The difference between control and middle and high groups (2 and 10 μM) were significant (p<0.05), and the invasion inhibition rate of Esc was higher than for positive control C188-9 at the same 10 μM concentration.
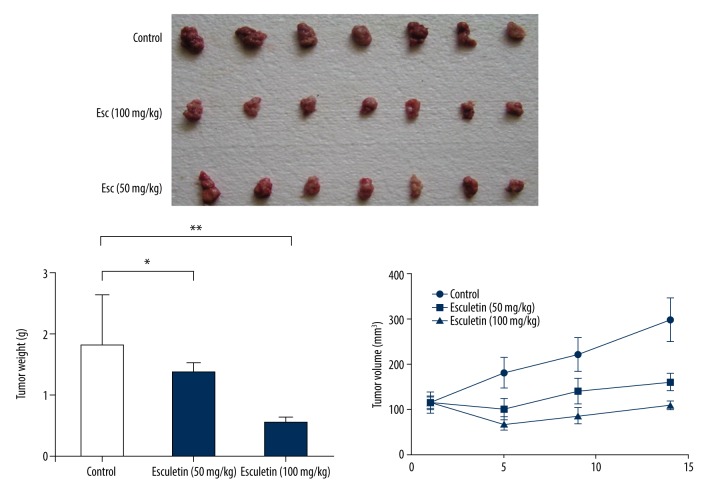
Esculetin significantly inhibits human laryngeal tumor xenografts in nude mice
The mice had normal reaction, smooth hair, and normal appetite within the first 7 days of administration. The total body weight decreased and the tumor growth increased in the control group from the eighth day onward. These symptoms occurred later (2 weeks after the administration) in Esc groups, and the symptoms of the mice were milder. The size of the tumors in the control group increased significantly. Compared with controls, the tumor volume of the Esc-treated group was significantly decreased (Figure 6).
Figure 6.
Esc significantly and dose-dependently inhibited the in vivo xenograft LC tumor growth and tumor weight. Tumor size in the control group was increased significantly. Compared with controls, the tumor volume of the Esc-treated group was significantly decreased.
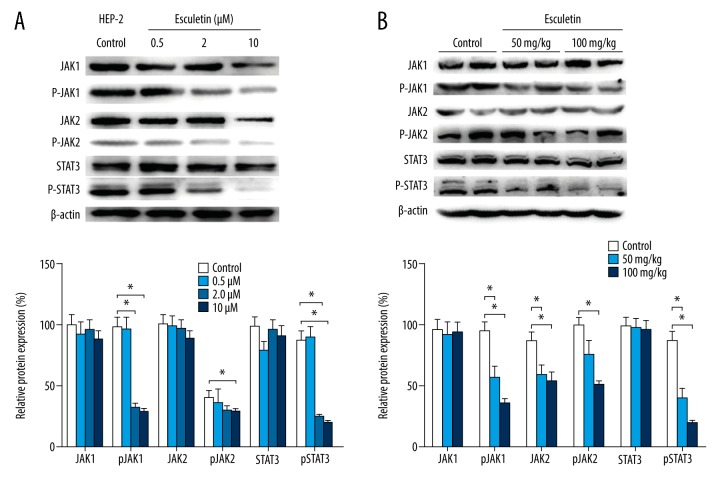
Esculetin blocks JAK-STAT3 signaling pathway in vitro and in vivo
JAK1/2 is the upstream signal of STAT3 [23]. The relative expressions of total JAK1 and total JAK2 in Hep 2 cells (Figure 7A) and tumors (Figure 7B) changed little after being treated with Esc, while the active form, the phosphorylated proteins (pJAK1 and pJAK2), decreased significantly and dose-dependably compared with the untreated control group (P<0.01). In addition, Esc remarkedly inhibited phosphorylation of STAT3 in a dose-dependent manner, while total STAT3 did not significantly change. Further analysis showed the level of p-STAT3 in Esc 2 and 10 μM decreased significantly (F=29.750, p<0.001; F=23.183, p=0.048) compared with the control group.
Figure 7.
The relative expressions of total JAK1, JAK2, and STAT 3 protein, and p-JAK1, p-JAK2, and p-STAT 3 after treatment with Esc in Hep 2 cell (A) and xenograft LC tumors in nude mice (B).
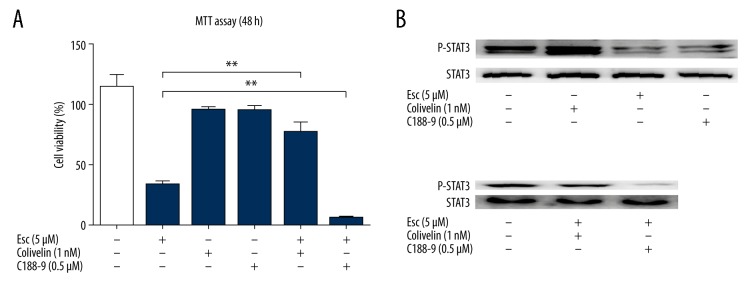
STAT3 activation reverses the anticancer effects of esculetin on Hep-2 cells
To further determine whether esculetin inhibits the proliferation of Hep-2 cells and is associated with modulating of STAT3, we used C188-9 (an inhibitor of STAT3) and colivelin (an activator of PI3K). The optimal concentration for C188-9 and colivelin was determined by pre-experiments. The working concentration was 1 nM for colivelin and 0.5 μM for C188-9, and at these concentrations, colivelin and C188-9 did not show cytotoxic effect on Hep-2 cells within 48 h, but both can effectively modulate STAT3 status (Figure 8). Pretreatment of cells with colivelin before esculetin reversed the proliferation inhibitory effects of esculetin, as evidenced by MTT assay (Figure 7). However, combination treatment with esculetin and C188-9 further enhanced the proliferation-inhibitory effect of esculetin. Western blot assay also demonstrated that esculetin combined with C188-9 decreased the STAT3 phosphorylation more effectively than single treatment (Figure 8B). Colivelin also neutralized the ability of Esc to inhibit phosphorylation of STAT3 (Figure 8B). These results together suggest that the anticancer effects of esculetin are mediated by inhibiting the STAT3 signaling pathway.
Figure 8.
The effects of colivelin and C188-9 on esculetin-induced cell proliferation inhibition and p-STAT3 phosphorylation. Hep-2 cells were treated with 5 μM of esculetin and (or) colivelin (1 nM), and C188-9 (0.5 μM) for 48 h. (A) Cell viability of Hep-2 was assessed by MTT assay. (B) STAT3 phosphorylation in Hep-2 cells was modulated by single compound and combination of 2 compounds. Data represent the mean ±SD (* P<0.05, ** P<0.01).
Discussion
In addition to surgery, chemotherapy is the main treatment of laryngeal squamous cell carcinoma (LC), but the adverse reactions of renal toxicity, ototoxicity, neurotoxicity, and myelosuppression of chemotherapy are also common. Furthermore, the drug resistance of chemotherapy reactions also makes the treatment of advanced laryngeal cancer less satisfactory. Cortex fraxini is commonly used in traditional Chinese medicine (TCM). The main chemical components of cortex fraxini are coumarin compounds. Cortex fraxini also contains phenols, saponins, tannins, and alkaloids. The aesculin, esculetin, fraxin, and fraxetin in cortex fraxini have obvious anti-inflammatory and analgesic effects [24]. Compelling studies have reported the in vitro and in vivo antitumor and immune regulation of Esc. Esc inhibits cell proliferation of lung cancer cells [25], melanoma cells [26], and human leukemia cells [27]. Esc induces apoptosis of oral squamous cell carcinoma cells through the EGFR/PI3K/Akt signaling pathway and nucleophosmin relocalization [28], and also exerts antitumor effect on human gastric cancer cells through the IGF-1/PI3K/Akt signaling pathway [29]. Kuo et al. demonstrated that Esc enhances the apoptosis effect of paclitaxel on human hepatoma cells HepG2, mediated by the ERK pathway [30].
In this study, the inhibitory effect of Esc on the proliferation of human LC cells was observed by MTT methods. Esc obviously inhibits human Hep-2 cells in a dose-dependent manner. Programmed cell death, or apoptosis, is an important way to control tumor proliferation [31]. Flow cytometry showed the cell cycle of Hep-2 cells was disturbed by Esc, the number of cells in G1 phase increased, and the number of cells in S/G2 phase decreased, which inhibited the proliferation of tumor cells in vitro. Turkekul et al. [32] demonstrated esculetin inhibits the survival of human prostate cancer cells by inducing apoptosis and caused G1 phase cell cycle arrest. These results are consistent with our research. Further analysis showed that downregulation of CDK2, CDK4, and cyclin D1 and upregulation of p53, p21Cip1, and p27Kip1 are involved in the process by which esculetin acts as a tumor suppressor in regulating the cell cycle in prostate cancer cells.
Our in vivo research shows that Esc has inhibitory effects on tumor mass and tumor volume in male tumor-bearing mice. Compared with the model group, Esc significantly inhibited the growth of tumors, reaching 80%. In a recent study performed in tumor-bearing mice, Esc exerted antitumor ability through enhancing the immune adhesion ability of erythrocytes to tumor cells [33], which demonstrates that erythrocyte immunity might be one of the mechanisms of by which Esc acts.
ROS are chemically reactive molecules that are constantly generated and eliminated during diverse biological and cellular reactions, and upregulated and redundant ROS are highly toxic to cells because of their peroxidative activity toward biological constituents [34]. Consistent with previous studies, we also demonstrated that Esc induces ROS production. After co-treatment with ROS scavenger, the LC cell proliferation inhibitory capability of Esc was significantly reduced, which suggests that ROS-mediated cytotoxicity is one of mechanisms for its anticancer effect.
Janus kinas (JAK) is a class of non-transmembrane tyrosine kinase that phosphorylate the cytokine receptors and a number of signal molecules containing specific SH2 domains. The JAK protein family includes 4 members: JAK1, JAK2, JAK3, and Tyk2 [35]. Signal transduction and activators of transcription (STAT) is a signal transducer or activator of transcription. Currently, 7 members of the STAT family have been found: STAT1–STAT4, STAT5a, STAT5b, and STAT3 [36]. Among them, the most conservative and functional section on sequences is the SH2 domain [37]. JAK catalyzes the phosphorylation of STAT protein on the receptor, and the activated STAT protein enters the nucleus in the form of 2 polymers, binding to the target gene and regulating the transcription of the gene [38]. The activation of STATs in normal cells is fast and transient, while STATs is persistently activated in tumor cells.
The JAK/STAT tyrosine kinase signal pathway was first discovered in interferon-induced signal transduction of cells. As an important pathway for cytokine signaling, JAK/STAT is activated by a variety of cytokines, growth factors, and receptors, and it participates in the processes of cell proliferation, differentiation, proliferation, apoptosis, angiogenesis, and immune regulation. Disrupted JAK-STAT signaling can lead to a variety of diseases, such as skin conditions, cancers, and disorders of the immune system.
STAT3 can be activated by a variety of growth factor and inflammatory signals, and research indicates STAT 3 is implicated in both primary and acquired resistance to chemotherapy [39]. Activation of STAT3 is mediated by a number of tyrosine kinases. We found that activated STAT3 is downregulated with decreased phosphorylation of JAK1 and JAK2 in Esc-treated LC cells or tissues. By co-treatment with STAT3 inhibitor or activator, our data suggest that Esc targeting these pathways in LC are likely via STAT3.
In addition to inhibiting phosphorylation of JAK1, JAK2, and STAT3, research demonstrated Esc can reverse the abnormal pattern of lipid peroxidation, antioxidants, and detoxification agents in 7,12-Dimethylbenz(a)anthracene (DMBA)-induced hamster buccal pouch carcinogenesis as compared to control hamsters [40]. Kim et al. also demonstrated that Esc suppresses tumor growth and metastasis by targeting the Axin2/E-cadherin axis in colorectal cancer cells and in a mouse model [41]. Taken together, these results suggest that there might be more tumor signaling pathways and targets involved in the antiangiogenic activity of Esc in cancer. Traditional Chinese medicine mainly focusses on the anti-inflammatory and analgesic effects of Esc. With more and more reported anticancer effects of Esc, future clinical research is essential.
Conclusions
In summary, our research shows Esc inhibits the proliferation, migration, and survival of human LC Hep-2 cells, and Esc induces apoptosis of LC cells and causes G1 phase cell cycle arrest. In vivo results also indicated Esc inhibits the growth of LC xenografts in nude mice. Esculetin exerts its antitumor effect on LC cancer through the JAK/STAT signaling pathway.
Abbreviations
- LC
larynx cancer
- Esc
esculetin
- STAT3
signal transducer and activator of transcription-3
Footnotes
Ethics statement
The animal protocol was approved by the Institutional Animal Care and Use Committee of Tianjin Medical University General Hospital, China.
Conflicts of interest
None.
Source of support: This study was supported by Tianjin Medical University General Hospital
References
- 1.Du L, Li H, Zhu C, et al. Incidence and mortality of laryngeal cancer in China, 2011. Chin J Cancer Res. 2015;27:52–58. doi: 10.3978/j.issn.1000-9604.2015.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. Cancer J Clin. 2017;67:31–50. doi: 10.3322/caac.21386. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JT. Individualized treatment of laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2017;143:367. doi: 10.1001/jamaoto.2016.4298. [DOI] [PubMed] [Google Scholar]
- 4.Garcia Lorenzo J, Montoro Martinez V, Rigo Quera A, et al. Modifications in the treatment of advanced laryngeal cancer throughout the last 30 years. Eur Arch Otorhinolaryngol. 2017;274:3449–55. doi: 10.1007/s00405-017-4639-z. [DOI] [PubMed] [Google Scholar]
- 5.Liang C, Ju W, Pei S, et al. Pharmacological activities and synthesis of esculetin and its derivatives: A mini-review. Molecules. 2017;22 doi: 10.3390/molecules22030387. pii: E387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon OS, Choi JS, Islam MN, et al. Inhibition of 5-lipoxygenase and skin inflammation by the aerial parts of Artemisia capillaris and its constituents. Arch Pharm Res. 2011;34:1561–69. doi: 10.1007/s12272-011-0919-0. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Chen X, Duan H, Hu Y, Mu X. Chinese herbal medicinal ingredients inhibit secretion of IL-6, IL-8, E-selectin and TXB2 in LPS-induced rat intestinal microvascular endothelial cells. Immunopharmacol Immunotoxicol. 2009;31:550–55. doi: 10.3109/08923970902814129. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Pei A, Chen J, et al. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J Neurochem. 2012;121:1007–13. doi: 10.1111/j.1471-4159.2012.07744.x. [DOI] [PubMed] [Google Scholar]
- 9.Park SS, Park SK, Lim JH, et al. Esculetin inhibits cell proliferation through the Ras/ERK1/2 pathway in human colon cancer cells. Oncol Rep. 2011;25:223–30. [PubMed] [Google Scholar]
- 10.Lee RH, Jeon YJ, Cho JH, et al. Esculetin exerts anti-proliferative effects against non-small-cell lung carcinoma by suppressing specificity protein 1 in vitro. Gen Physiol Biophys. 2017;36:31–39. doi: 10.4149/gpb_2016024. [DOI] [PubMed] [Google Scholar]
- 11.Chang HT, Chou CT, Lin YS, et al. Esculetin, a natural coumarin compound, evokes Ca(2+) movement and activation of Ca(2+)-associated mitochondrial apoptotic pathways that involved cell cycle arrest in ZR-75-1 human breast cancer cells. Tumour Biol. 2016;37:4665–78. doi: 10.1007/s13277-015-4286-1. [DOI] [PubMed] [Google Scholar]
- 12.Sawney S, Arora R, Aggarwal KK, Saluja D. Esculetin downregulates the expression of AML1-ETO and C-Kit in Kasumi-1 cell line by decreasing half-life of mRNA. J Oncol. 2015;2015 doi: 10.1155/2015/781473. 781473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Park C, Jin CY, et al. Involvement of extracellular signal-related kinase signaling in esculetin induced G1 arrest of human leukemia U937 cells. Biomed Pharmacother. 2008;62:723–29. doi: 10.1016/j.biopha.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Tong M, Wang J, Jiang N, et al. Correlation between p-STAT3 overexpression and prognosis in lung cancer: A systematic review and meta-analysis. PLoS One. 2017;12:e0182282. doi: 10.1371/journal.pone.0182282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji K, Zhang M, Chu Q, et al. The Role of p-STAT3 as a prognostic and clinicopathological marker in colorectal cancer: a systematic review and meta-analysis. PLoS One. 2016;11:e0160125. doi: 10.1371/journal.pone.0160125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Int J Cancer. 2016;138:2570–78. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu S, Song Y, Mei JY, et al. IL-17 and its receptors regulate JAK/STAT3 signaling pathway and promote angiogenesis in laryngeal carcinoma. Acta Universitatis Medicinalis Anhui. 2015;50:1464–67. [Google Scholar]
- 18.Hu A, Huang JJ, Jin XJ, et al. Curcumin suppresses invasiveness and vasculogenic mimicry of squamous cell carcinoma of the larynx through the inhibition of JAK-2/STAT-3 signaling pathway. Am J Cancer Res. 2015;5:278–88. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Zhang D, Luan X, et al. Inhibition of the signal transducers and activators of transcription (STAT) 3 signalling pathway by AG490 in laryngeal carcinoma cells. J Int Med Res. 2010;38:1673–81. doi: 10.1177/147323001003800512. [DOI] [PubMed] [Google Scholar]
- 20.Sen Z, Zhan XK, Jing J, et al. Chemosensitizing activities of cyclotides from Clitoria ternatea in paclitaxel-resistant lung cancer cells. Oncol Lett. 2013;5:641–44. doi: 10.3892/ol.2012.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Yang J, Li H, et al. Skimmin, a coumarin, suppresses the streptozotocin-induced diabetic nephropathy in wistar rats. Eur J Pharmacol. 2012;692:78–83. doi: 10.1016/j.ejphar.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Xin H, Li Y, et al. Skimmin, a coumarin from hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/819296. 819296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang J, Chu L, Li C, et al. JAK2 inhibitor blocks the inflammation and growth of esophageal squamous cell carcinoma in vitro through the JAK/STAT3 pathway. Oncol Rep. 2015;33:494–502. doi: 10.3892/or.2014.3609. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Wang X. [Progress of pharmacological research on icarii]. China Journal of Chinese Materia Medica. 2008;33:2732–36. [in Chinese] [PubMed] [Google Scholar]
- 25.Lacy A, O’Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 2004;10:3797–811. doi: 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- 26.Jeon YJ, Jang JY, Shim JH, et al. Esculetin, a coumarin derivative, exhibits anti-proliferative and pro-apoptotic activity in G361 human malignant melanoma. J Cancer Prev. 2015;20:106–12. doi: 10.15430/JCP.2015.20.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio V, Garcia-Perez AI, Tejedor MC, et al. Esculetin neutralises cytotoxicity of t-BHP but not of H2O2 on human leukaemia NB4 cells. Biomed Res Int. 2017;2017 doi: 10.1155/2017/9491045. 9491045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon YJ, Cho JH, Lee SY, et al. Esculetin induces apoptosis through EGFR/PI3K/Akt signaling pathway and nucleophosmin relocalization. J Cell Biochem. 2016;117:1210–21. doi: 10.1002/jcb.25404. [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Lu M, Yao Y, et al. Esculetin exerts antitumor effect on human gastric cancer cells through IGF-1/PI3K/Akt signaling pathway. Eur J Pharmacol. 2017;814:207–15. doi: 10.1016/j.ejphar.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Kuo HC, Lee HJ, Hu CC, et al. Enhancement of esculetin on Taxol-induced apoptosis in human hepatoma HepG2 cells. Toxicol Appl Pharmacol. 2006;210:55–62. doi: 10.1016/j.taap.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Monian P, Jiang X. The cellular apoptosis susceptibility protein (CAS) promotes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis and cell proliferation. J Biol Chem. 2016;291:2379–88. doi: 10.1074/jbc.M115.685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turkekul K, Colpan RD, Baykul T, et al. Esculetin inhibits the survival of human prostate cancer cells by inducing apoptosis and arresting the cell cycle. J Cancer Prev. 2018;23:10–17. doi: 10.15430/JCP.2018.23.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu RD, Shao T, Jia SH. [Effect of esculetin on immune function of red cell in tumor bearing mice]. Journal of Harbin University of Commerce (Natural Sciences Edition) 2016;32:146–53. [in Chinese] [Google Scholar]
- 34.Pan H, Wang BH, Lv W, et al. Esculetin induces apoptosis in human gastric cancer cells through a cyclophilin D-mediated mitochondrial permeability transition pore associated with ROS. Chem Biol Interact. 2015;242:51–60. doi: 10.1016/j.cbi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Vier J, Groth M, Sochalska M, Kirschnek S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis. 2016;7:e2103. doi: 10.1038/cddis.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee S, Biehl A, Gadina M, et al. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: Current and future prospects. Drugs. 2017;77:521–46. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borges S, Moudilou E, Vouyovitch C, et al. Involvement of a JAK/STAT pathway inhibitor: Cytokine inducible SH2 containing protein in breast cancer. Adv Exp Med Biol. 2008;617:321–29. doi: 10.1007/978-0-387-69080-3_30. [DOI] [PubMed] [Google Scholar]
- 38.Pencik J, Pham HT, Schmoellerl J, et al. JAK-STAT signaling in cancer: From cytokines to non-coding genome. Cytokine. 2016;87:26–36. doi: 10.1016/j.cyto.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Looyenga BD, Hutchings D, Cherni I, et al. STAT3 is activated by JAK2 independent of key oncogenic driver mutations in non-small cell lung carcinoma. PLoS One. 2012;7:e30820. doi: 10.1371/journal.pone.0030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selvasundaram R, Manoharan S, Buddhan R, et al. Chemopreventive potential of esculetin in 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch carcinogenesis. Mol Cell Biochem. 2018;448(1–2):145–53. doi: 10.1007/s11010-018-3321-0. [DOI] [PubMed] [Google Scholar]
- 41.Kim WK, Byun WS, Chung HJ, et al. Esculetin suppresses tumor growth and metastasis by targeting Axin2/E-cadherin axis in colorectal cancer. Biochem Pharmacol. 2018;152:71–83. doi: 10.1016/j.bcp.2018.03.009. [DOI] [PubMed] [Google Scholar]