Abstract
Purpose
ErbB3 and its ligand neuregulin-1 (NRG1), are widely expressed in head and neck squamous cell carcinoma (HNSCC) and associated with tumor progression. A “window-of-opportunity” study () was conducted to evaluate the pharmacodynamic effects of CDX-3379, an anti-ErbB3 monoclonal antibody, in patients with HNSCC.
Experimental Design
Twelve patients with newly diagnosed, operable HNSCC received two infusions of CDX-3379 (1000 mg) at a two-week interval prior to tumor resection. The primary study objective was to achieve ≥ 50% reduction in tumor ErbB3 signaling (phosphorylation of ErbB3; pErbB3) in ≥ 30% of patients. Other potential tumor biomarkers, pharmacokinetics, safety, and tumor measurements were also assessed.
Results
pErbB3 was detectable in all tumors prior to treatment and decreased for 10/12 (83%) patients following CDX-3379 dosing, with ≥ 50% reduction in 7/12 (58%, p=0.04, 95% CI=27.7%, 84.8%). Target trough CDX-3379 serum levels were achieved in all patients. CDX-3379 treatment-related toxicity was grade 1–2 and included diarrhea, fatigue, and acneiform dermatitis. 5/12 (42%) patients had shrinkage in tumor burden, including a marked clinical response in a patient with HPV-negative oral cavity HNSCC. All patients with tumor shrinkage had tumors that expressed both NRG1 and ErbB3 and demonstrated reduced pErbB3 with CDX-3379 treatment.
Conclusions
This study demonstrates that CDX-3379 can inhibit tumor ErbB3 phosphorylation in HNSCC. CDX-3379 was well tolerated and associated with measurable tumor regression. A phase 2 study () has been initiated to evaluate CDX-3379 in combination with cetuximab for patients with advanced HNSCC.
Keywords: ErbB3, head and neck squamous cell carcinoma; neuregulin; HER3; CDX-3379
Introduction
Despite the advent of EGFR-targeted therapies and immune checkpoint inhibitors, the median overall survival for patients with advanced HPV- head and neck squamous cell carcinoma (HNSCC) remains less than one year. The best data to date for first line palliative therapy is with the use of the EXTREME regimen (cetuximab in combination with platinum plus 5-fluorouracil chemotherapy followed by cetuximab) [1]. In 2016, two monoclonal antibodies (pembrolizumab, nivolumab) targeting the programmed death-1 (PD-1) receptor were approved for the treatment of patients with platinum-refractory, recurrent/metastatic HNSCC [1, 2]. Nivolumab improved overall survival compared to single-agent, investigator-choice chemotherapy, with a lower toxicity rate in a randomized phase III trial [3]. Despite this exciting therapeutic advance, the response rate is only 13%, with a median progression free survival of 2 months and median overall survival of 7.5 months. As such, there remains a pressing unmet clinical need to identify novel therapeutic targets.
Human epidermal growth factor receptor 3 (ErbB3 or HER3) is a member of the EGFR human epidermal growth factor (HER/ErbB) family of receptor tyrosine kinases (RTK), which also includes EGFR, ErbB2 (HER2), and ErbB4 (HER4). The ErbB family of RTKs plays a role in the pathogenesis of many types of cancers, including lung, breast, colorectal, and head and neck. ErbB3 lacks detectable tyrosine kinase activity, and consequently needs to form heterodimers with other ErbB receptors to become active [4]. In the presence of the ErbB3 ligand, NRG1 or NRG2, ErbB3 preferentially dimerizes with ErbB2, subsequently undergoing phosphorylation and enabling downstream signaling. NRG1 is expressed in the majority (>80%) of HPV-negative and positive HNSCC tumors [5] and membranous ErbB3 expression is strongly associated with poor prognosis in HNSCC [6]. While EGFR targeting with monoclonal antibodies such as cetuximab have demonstrated clinical benefit, response rates are modest, suggesting contribution of additional tumor drivers and/or resistance mechanisms. We and others reported that signaling through ErbB3 stimulates tumor growth and mediates cetuximab resistance in preclinical HNSCC models [7]. These data suggest ErbB3 may play a role in the pathogenesis of HNSCC.
CDX-3379 (previously known as KTN3379) is a human anti-ErbB3 monoclonal antibody engineered with half-life extending YTE substitutions in the Fc region and reduced ADCC activity [8].Preclinically, significant tumor growth inhibition was observed in response to CDX-3379 treatment in HNSCC xenograft models [5]. In a prior phase 1/1b study () [9], CDX-3379 monotherapy (up to 20 mg/kg every 3 weeks) was well tolerated without identification of a maximum tolerated dose. Target CDX-3379 serum trough levels of 50 μg/mL, which elicited maximal antitumor activity in preclinical models, were achieved with doses >10 mg/kg every three weeks. Treatment with the combination of CDX-3379 and cetuximab was associated with a durable complete response (CR) in a patient with HNSCC who had previously progressed on cetuximab [9].
The present “window-of-opportunity” study was conducted to evaluate the effect of CDX-3379 on tumor ErbB3 signaling (phosphorylation of ErbB3; pErbB3) in patients with newly diagnosed HNSCC. The CDX-3379 dose level (1000 mg every 2 weeks) was chosen to exceed the established target serum trough levels that led to maximal antitumor efficacy in preclinical models. Pre- and post-treatment tumor samples were evaluated for changes in ErbB3 phosphorylation and proliferation (Ki67). Additional molecular analyses included expression of NRG1, NRG2, ErbB3 and phosphatase and tensin homolog (PTEN) as potential biomarkers of response to CDX-3379. Clinical assessments within the brief pre-surgical timeframe included safety and radiographic tumor assessments.
Materials and Methods
Patients
This study was open to adult patients ≥18 years of age with newly diagnosed HNSCC of the oral cavity, oropharynx, hypopharynx or larynx who were planned for surgical resection. Other criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, adequate bone and marrow function, and contraceptive requirements. Exclusion criteria for the study included any prior or concurrent therapy for HNSCC, systemic steroids within 7 days of first study dose (allowable exception of inhaled or topical corticosteroids), another invasive malignancy within 2 years prior to enrollment (excepting localized cancers), or uncontrolled intercurrent illness.
The study was conducted at the University of Pittsburgh Medical Center and University of California San Francisco Medical Center, was approved by institutional review boards and the United States Food and Drug Administration and was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice. All patients gave written informed consent before any protocol-specific procedures. This study is registered with ClinicalTrials.gov ().
Study design, treatments and procedures
In this single-arm, open-label “window of opportunity” study, eligible, consented patients with newly diagnosed, operable HNSCC received treatment with CDX-3379 in the window prior to planned surgical resection. CDX-3379, at a dose of 1000 mg, was administered as a 60-minute intravenous infusion, at a two-week interval. Surgery was scheduled as soon as possible and ideally within 14 days after the second dose of CDX-3379. A third dose of CDX-3379 was permitted in the event of a scheduling delay resulting in surgery at ≥ 3 weeks following the second dose.
Safety assessments performed prior to and throughout study treatment included physical exam, vital signs, electrocardiogram (ECG), echocardiogram or multigated acquisition scan (MUGA), and routine chemistry, hematology, coagulation and urinalysis panels. Toxicity was assessed through 30 days post-treatment and graded per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Radiographic imaging (CT or MRI) was performed pre-treatment and post-treatment prior to resection. Tumor measurements were assessed by investigators according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1)[10]. Blood samples were obtained for pharmacokinetic and immunogenicity analyses prior to each CDX-3379 infusion, on the day of surgery, and at 30 days post-treatment. Pharmacokinetic samples were also obtained at 0, 1 and 4 hours after completion of each infusion, with additional samples after the first infusion at 24 hours and day 8.
Tissue acquisition and correlative studies
Biomarker expression analysis was conducted on pre- and post-treatment biopsy and surgical resection samples. Given the sensitivity of phosphorylated proteins to method preparation, post-treatment biopsy samples were collected on the day of surgery before devascularization or resection of the tumor specimen to provide matched pre- and post-treatment samples with consistent preparation and fixation methods for pErbB3 analysis. Tumor tissue was also obtained from the resection specimens to ensure sufficient amount of tissue for remaining analyses.
Three to four tissue samples were taken either by incisional biopsy or punch biopsy, immediately fixed in 10% neutral buffered formalin (NBF) for 6 to 72 hours, and then paraffin-embedded. Hematoxylin and eosin staining (H&E) was used to confirm the presence of tumor.
ErbB3 phosphorylation (pErbB3) VeraTag® assays were performed per established methods [5, 11]. The following antibodies were used: biotinylated HER3 antibody (mouse monoclonal, B9A11; Monogram) and VeraTag-labeled phosphoErbB3 antibody (phospho-HER3/ErbB3 [Tyr1289] monoclonal antibody: Cell Signaling Technology). Released fluorescent VeraTags were quantified and reported in units of relative fluorescence per square millimeter (RF/mm2). Samples with assay values below the level of detection were artificially set at the assay limit of detection (0.14 RF/mm3), which was defined by taking the lowest value reported in the assay and dividing by 2.5.
Quantitative analysis for NRG1, NRG2 and ErbB3 mRNA was performed using an in situ hybridization (ISH) protocol, RNAScope®, modified for quantitative immuno-fluorescence (QIF) as described previously [5, 12].
AQUA® method was used to measure Ki67 protein expression within defined tumor areas and tumor cell nuclei based on co-localization with cytokeratin and DAPI as described previously [5]. An AQUA score for each field of view was calculated as the signal intensity of Ki-67 in the nuclear compartment divided by the area of the nuclear compartment and is reported in arbitrary units. Data for each tumor sample were reported as an average Ki67 AQUA score of all of the field of views analyzed for the sample.
Quantification of PTEN in post treatment/surgical resection specimens was also performed using the AQUA method, with the following modifications. Antigen retrieval was performed using Tris-ethylenediaminetetraacetic acid (EDTA) antigen retrieval buffer at pH 8 (20 minutes at 97°C in the Lab Vision PT Module). The primary antibody (PTEN clone 138G6, rabbit monoclonal antibody, Cell Signaling Technology, #9559) and mouse monoclonal anti-human cytokeratin antibody (1:100) (Dako, clone AE1/AE3) were diluted in 0.3% BSA/TBS and incubated overnight at 4°C. This was followed by application of a goat anti-mouse antibody, Alexa Fluor 546 (Life Technologies, Cat #: A11003), which was diluted 1:100 in an anti-rabbit horse radish peroxidase-labelled polymer reagent (Rabbit Envision, Dako). These steps were followed by Cy5 tyramide application and mounting with DAPI as described above.
Pharmacokinetics
CDX-3379 concentrations in subject serum samples were determined using an immunoassay with electrochemiluminescent (ECL) readout on the MesoScale Discovery (MSD) at Intertek Pharmaceutical Services, San Diego, CA. Briefly, an anti-YTE antibody is passively adsorbed to a standard MSD plate. After washing, and blocking, CDX-3379 in samples, standards and controls is captured and detected with a biotinylated monoclonal antibody specific for CDX-3379, which is conjugated with biotin. Streptavidin-sulfoTAG is added and the signal is developed after addition of tripropylamine and application of electrical current. Relative luminescent unit (RLU) signal is proportional to the amount of CDX-3379 in samples and QCs. Assay sensitivity is 7.8 ng/mL.
Noncompartmental analysis was performed in cycle 1 using Phoenix WinNonlin 7.0 (Certara USA, Princeton, NJ). Area under the curve (AUC) was estimated from serum concentrations in the first cycle using linear trapezoidal, linear interpolation. The elimination rate constant of the terminal phase was estimated from least squares regression of the time versus concentration profile using uniformly weighted data.
Immunogenicity
A qualitative solid phase extraction with acid dissociation assay using direct ECL detection is used to detect antibodies to CDX-3379 in human serum. Briefly, subject samples positive for CDX-3379 and controls are incubated with CDX-3379-Biotin to bind anti-drug antibodies (ADA) prior to transfer to a blocked Streptavidin coated plate, where ADA-CDX-3379-Biotin complexes are allowed to bind. After incubation, unbound material is washed away, and acid dissociation releases ADA, which are then neutralized, transferred and passively adsorbed to a 96 well standard MSD plate. Following incubation, washing and blocking, CDX-3379-Sulfo-Tag is added as the secondary detection reagent. MSD Read Buffer containing TPA is added to the wells and the plate is read on an MSD Sector™ Imager 2400. The RLU signal generated from positive controls and test samples is proportional to the amount of anti-CDX-3379 antibody found in the sample. A screening cut-point was determined from 50 normal human serum samples based on parametric method and a 5 % false positive rate.
Specificity of screened positive samples is confirmed by immunodepletion with drug product using an inhibition cut-point based on a 1 % false positive rate
Statistical methods
The primary study objective was to assess change in pErbB3 in tumor tissue before and after exposure to CDX-3379. Secondary objectives were to assess the effect of CDX-3379 on the Ki67 proliferative index in tumor tissue obtained pre- and post-treatment; identify candidate biomarkers for CDX-3379 pharmacodynamic response or resistance; evaluate the safety, tolerability, pharmacokinetics (PK), and immunogenicity of CDX-3379; and changes in tumor measurements before and after exposure to CDX-3379. The relationship between pErbB3 and the tumor proliferation marker Ki67, tumor measurements or other biomarker results, were also evaluated.
A sample size of 29 patients was calculated to provide 80% power to determine if ≥30% of patients demonstrated a ≥50% decrease in tumor pErbB3 levels following treatment with CDX-3379. However, enrollment was discontinued after the primary study objective was met in the first 12 study patients. Statistical analysis for this measurement was calculated using the Clopper-Pearson exact confidence interval.
Results
Patient Characteristics
Twelve patients with newly diagnosed HNSCC were enrolled in this study between October 26, 2015 and June 29, 2016. Of the 12 enrolled patients, 10 were male while 2 were female, and median age was 56.5 years (range 45–61). Nine (75%) had HPV-negative and 3 (25%) had HPV-positive tumors. The most frequent primary tumor site was oral cavity (5; 42%), followed by oropharynx (4; 31%) and larynx (3, 25%). Median time from diagnosis to first CDX-3379 dose was 31 days (range 14, 48). Stage IVA disease was most prevalent (10, 83%); 1 patient presented with Stage III and 1 with Stage II disease (Table 1).
Table 1.
Patient Baseline and Tumor Characteristics
| All treated patients (N=12) | |
|---|---|
| Days from diagnosis to treatment, median (range) | 31 (14, 48) |
| Age, median (range) | 56.5 (45 – 61) |
| Male | 10 (83%) |
| ECOG Performance Status | |
| ECOG 0 | 11 (92%) |
| ECOG 1 | 1 (8%) |
| Primary Head and Neck Tumor Type | |
| Oral Cavity | 5 (41.7%) |
| Oropharynx | 4 (33.3%) |
| Larynx | 3 (25.0%) |
| Histological/Cytological Diagnosis | |
| Squamous Cell Carcinoma | 12 (100%) |
| Stage | |
| II | 1 (8%) |
| III | 1 (8%) |
| IVA | 10 (83%) |
| HPV status | |
| Negative | 9 (75%) |
| Positive | 3 (25%) |
HPV, human papillomavirus
Data shown as n (%) unless otherwise specified.
Study Treatments and Tolerability
All patients received 2 doses of CDX-3379. No treatment-related serious adverse events were reported. The most common treatment-related toxicities reported were diarrhea, fatigue, and dermatitis acneiform (Table 2), all of which were mild or moderate. No study patients discontinued or required dose modifications due to toxicity.
Table 2.
Treatment-Related Toxicity
| All treated patients (N=12) |
|||
|---|---|---|---|
| Grade 1 | Grade 2 | Total | |
| Any treatment-related adverse event | 5 (42%) | 3 (25%) | 8 (67%) |
| Diarrhea | 5 (42%) | 1 (8%) | 6 (50%) |
| Fatigue | 1 (8%) | 1 (8%) | 2 (17%) |
| Dermatitis acneiform | 2 (17%) | - | 2 (17%) |
| Mucosal inflammation | 1 (8%) | - | 1 (8%) |
| Folliculitis | 1 (8%) | - | 1 (8%) |
| Tinea cruris | 1 (8%) | - | 1 (8%) |
| Myalgia | 1 (8%) | - | 1 (8%) |
| Dysgeusia | - | 1 (8%) | 1 (8%) |
| Headache | 1 (8%) | - | 1 (8%) |
| Oropharyngeal pain | 1 (8%) | - | 1 (8%) |
| Sinus Congestion | 1 (8%) | - | 1 (8%) |
| Flushing | 1 (8%) | - | 1 (8%) |
All treatment-related toxicity was ≤ grade 2. Data are shown as n (%).
Pharmacokinetics and Immunogenicity
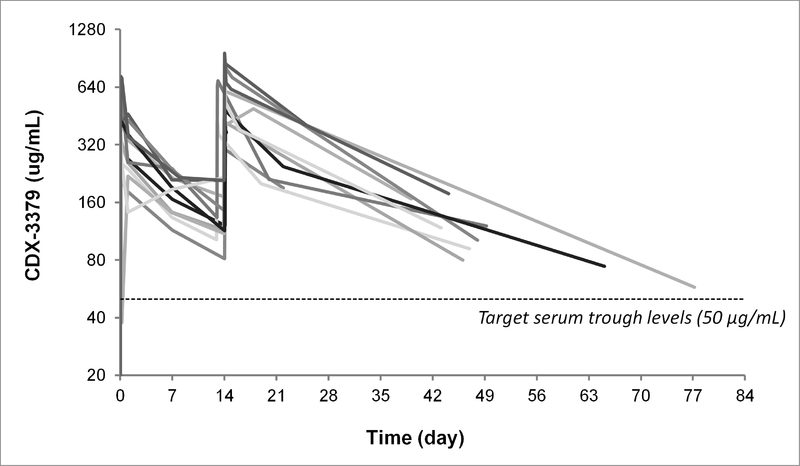
Based on phase 1 clinical study data [9] with doses up to 20 mg/kg (comparable to single doses of up to 1,828 mg) every 3 weeks, it was determined that a 1000 mg dose every 2 weeks would achieve trough serum concentrations of CDX-3379 exceeding those that resulted in maximal antitumor activity in preclinical models (50 μg/mL). As shown in Figure 1, CDX-3379 serum levels in all patients exceeded the target PK trough levels for up to 77 days. Mean half-life in cycle 1 was calculated as 11.5 days, with mean clearance of 365 mL/day (Supplementary Table S1). A specific anti-CDX-3379 antibody response was observed at day 15 in one patient who was negative at baseline, but the pre-resection sample collected 4 days later was found to be non-specific. No apparent impact on PK parameters was noted in association with this transient immunogenicity.
Figure 1. Pharmacokinetics.
CDX-3379 serum concentration over time is individually displayed for each patient. All 12 patients received CDX-3379 (1000 mg/kg) at a two-week interval for a total of two doses. Dotted line represents target trough concentration of 50 μg/mL which resulted in maximal antitumor efficacy in preclinical models.
Molecular Biomarkers in Tumor Samples
Pre-treatment and post-treatment tumor samples were obtained for all 12 patients. Surgical resection occurred at a median of 27.5 days (range 18, 35) after first CDX-3379 dose, corresponding to a median of 13.5 days (range 4, 21) after last CDX-3379 dose.
Post-treatment tumor biopsy samples collected prior to surgery were used to determine pErbB3 levels, with the exception of patient 108–02, where the surgical resection samples were used due to insufficiency of the post-treatment sample. All pre-treatment tumor samples had detectable levels of pErbB3 (range 0.41–1.04 RF/mm2). Decreased pErbB3 was observed in most post-treatment tumor samples, with a ≥ 50% decrease achieved in 7/12 (58%, p=0.04, 95% CI=27.7%, 84.8%) patients and 4/12 (33%) having post-treatment pErbB3 levels below the limit of detection of the assay (Table 3, Figure 2b). The 2 patients (103–02 and 105–01) without a decrease in pErbB3 had post-treatment samples taken at 20 & 21 days post last CDX-3379 dose.
Table 3.
Molecular Analysis of Tumor Specimens and Tumor Measurements
| Patient | HPV Status | pErbB3a (RF/mm2) |
Ki67b |
NRG1c | NRG2c | ErbB3c | PTENc | Tumor Measurement (% Change)d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | % Change | Pre | Post | % Change | |||||||
| 112–02 | − | 0.45 | BLQ | −69% | 4820 | 6450 | 34% | ++ | − | + | + | −26 |
| 102–02 | + | 0.54 | 0.46 | −15% | 5266 | 264 | −95% | ++ | ++ | ++++ | + | −16 |
| 108–02 | + | 0.60 | BLQ | −77% | 2525 | 1858 | −26% | + | − | + | Loss | −6 |
| 101–02 | − | 0.73 | 0.39 | −47% | 198 | 446 | 125% | +++ | − | + | + | −5 |
| 111–02 | − | 0.74 | BLQ | −81% | 5035 | 4187 | −17% | + | − | +++ | Loss | −4 |
| 104–02 | − | 0.42 | 0.21 | −51% | 2931 | 3332 | 14% | − | + | + | + | 0 |
| 107–01 | − | 1.02 | 0.64 | −37% | 6024 | 3197 | −47% | + | − | ++ | + | 0 |
| 110–01 | − | 1.03 | BLQ | −86% | 6422 | 541 | −92% | + | − | + | + | 0 |
| 103–02 | − | 0.59 | 0.73 | 22% | 531 | 1064 | 100% | + | − | + | + | 11 |
| 105–01 | − | 0.41 | 0.41 | 0% | 6200 | 8881 | 43% | + | − | ++++ | + | 13 |
| 113–02 | − | 0.70 | 0.35 | −50% | 751 | 6396 | 752% | ++ | − | − | + | 17 |
| 109–02 | + | 1.04 | 0.48 | −54% | 6629 | 7160 | 8% | + | − | +++ | + | 26 |
ErbB3 phosphorylation (pErbB3) determined using quantitative VeraTag® of pre- and post-treatment biopsies for all patients except 108–02 (post-treatment results determined using resection sample). Data represent change from pre- to post-treatment samples.
Ki67 expression assessed by IHC (AQUA®) in pre- and post-treatment samples.
NRG1, NRG2, ErbB3, and PTEN assessed using post-treatment tumor resection samples for all patients except 102–02, 103–02, and 104–02 (pre-treatment biopsies were evaluated). mRNA expression levels of NRG1, NRG2, ErbB3 evaluated by quantitative RNAScope® assays (−, not different from the negative control probe; +, expression level of 1.2 – 2.5; ++, expression level of 2.6–3.8; +++, expression level of 3.9–5.2; ++++, expression level >5.2). PTEN expression assessed by IHC (AQUA®).
Percent change from pre- to post-treatment in the sum of the longest diameters of RECIST 1.1 target lesions. BLQ, below the limit of quantification
Figure 2. Phospho-tyrosine 1289-ErbB3 Expression by VeraTag®.
(A) Change in pErbB3 level in individual patient tumor samples. Post-treatment pErbB3 levels were below the assay level of detection for four patients (108–01, 110–02, 111–02 and 112–02); values were set at the assay level of detection of 0.14. (B) Mean tumor pErbB3 levels for all patients (n=12) before and after CDX-3379 treatment.
Of the 12 evaluable pre- and post-treatment tumor tissue sample pairs, 5/12 (42%) showed a decrease in Ki67 levels post-treatment, with two post-treatment tumor samples (102–02, HPV-positive and 110–01, HPV-negative) showing a > 90% reduction in Ki67 in response to CDX-3379 treatment (Table 3, Supplementary Figure S1a). However, this decrease was not statistically significant overall (Supplementary Figure S1b). Decreases in Ki67 after CDX-3379 treatment did not associate with changes in pErbB3 (Table 3).
Tumor tissue was evaluated for NRG1, NRG2 and ErbB3 mRNA and PTEN protein expression (Table 3). Analysis of NRG1, NRG2 and ErbB3 mRNA expression was deemed more quantitative than immunohistochemistry and requires less tissue than Western blotting. In addition, the large number of NRG1 isoforms are more reliably captured by mRNA-based methods. Furthermore, ErbB3 mRNA and protein expression were shown to correlate in a panel of HNSCC cell lines (Supplementary Figure S2).
Tumor resection samples were used preferentially, although pre-treatment biopsy samples were utilized for 3 patients. ErbB3 mRNA was detectable in 11/12 (92%) of tumor samples. One tumor sample (113–02) was negative for ErbB3 mRNA expression, but had detectable pretreatment pErbB3, which may reflect assay limitations. Most patient samples expressed either NRG1 (11/12), or NRG2 (2/12), consistent with the observation that pErbB3 was detectable in all pretreatment tumor samples Loss of PTEN staining in tumor tissue was observed in 2/12 (17%) tumors. No clear relationships between NRG, ErbB3 or PTEN expression and changes in Ki67 or tumor measurements were observed (Table 3, Supplementary Figure S3)
Tumor Assessments and Molecular Correlations
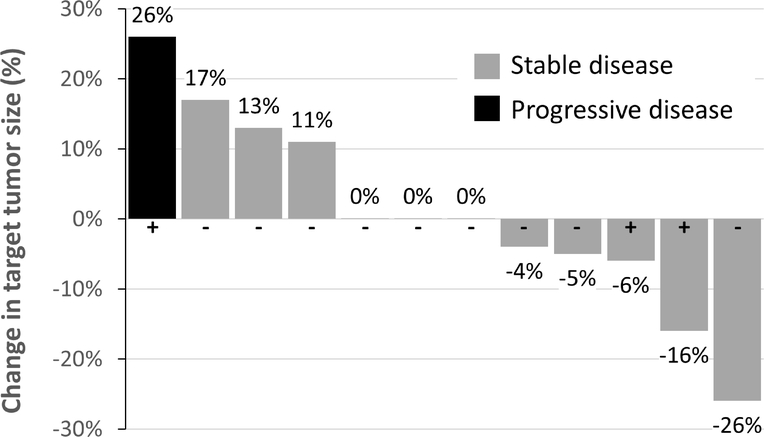
Post-treatment radiographic assessments were performed at a median of 20.5 (range 15–26) days after the first CDX-3379 dose. At the post-treatment assessment, decreased tumor burden was observed for 5/12 (42%) patients, with shrinkage of the sum of target lesion diameters ranging from −4% to −26% (Table 3, Figure 3). Three patients had no change in tumor size, while three had slight tumor growth, for a total of 11/12 (92%) of patients with RECIST stable disease during the pre-operative period. One patient with HPV-positive disease experienced a 26% increase in target lesion burden, resulting in progressive disease.
Figure 3. Waterfall Plot of Change in Tumor Burden.
Percent change from baseline in the sum of longest diameters of target lesion(s) for each study patient. Radiographic assessments were performed at a median (range) of 20.5 (15–26) days from first CDX-3379 dose. HPV status is denoted by + and − signs.
Notably, one 45-year-old male patient with Stage T4aN1M0 HPV-negative oral cavity HNSCC experienced a marked clinical response in association with CDX-3379 treatment [13]. At baseline, the tumor on physical examination was large and fungating and was associated with eating difficulties and significant pain requiring analgesics. Within 48 hours of the first dose, the patient noted tumor shrinkage, a marked decrease in pain (from 8/10 to 2/10), and an improved ability to eat. The primary tumor site was not readily evaluable radiographically due to interference of dental amalgam, however nodal metastasis decreased by 26%, and the primary tumor size decreased by 92% as assessed on physical examination on day 16. Tumor pErbB3 expression decreased below the assay limit of quantification (Table 3). Additional molecular characterization of this patient’s tumor is described in a separate manuscript [13].
Although the magnitude of tumor shrinkage did not clearly associate with changes in tumor pErbB3 and NRG1 (Table 3), all five patients showing a measurable reduction in tumor burden also had a reduction in tumor pErbB3 with treatment, including 3 (60%) with reductions in pErbB3 below the limit of quantification. In contrast, a reduction below the limit of quantification was achieved in only 1 (14%) of the remaining 7 patients who had no change in tumor size. Similarly, moderate to high NRG1 expression was detected for three (60%) of the five patients with tumor regression, and 1 (14%) of the remaining patients (Table 3). No correlations were observed between change in tumor burden and Ki67, HPV status, or other biomarkers (Supplementary Figure S3).
Discussion
Window-of-opportunity trials provide a powerful model to assess biomarker alterations induced upon drug treatment, by comparing baseline tumor tissue with those obtained after drug treatment. This window of opportunity study set out to evaluate whether CDX-3379 would inhibit ErbB3 phosphorylation in human tumors prior to surgery. In addition, we assessed toxicity and any clinical activity prior to tumor resection in these newly diagnosed, operable HNSCC patients. Potential biomarkers of response were also assessed.
There were no treatment-related serious adverse events, and drug treatment was very well tolerated. Treatment with two 1000 mg doses of CDX-3379 at a 2-week interval achieved trough serum levels above the target level for maximal activity in preclinical models. pErbB3 was detectable in all tumor samples collected prior to dosing, indicating the ErbB3 signaling pathway was active in these tumors. Consistent with this, either NRG1 or NRG2 mRNA was also detected in all tumors. Following CDX-3379 treatment, the majority of patients’ tumors demonstrated a significant reduction in ErbB3 phosphorylation (Figure 2b), with 58% (p=0.04, 95% CI=27.7%, 84.8%) achieving a reduction of ≥50%. These data indicate that, at the dose and schedule used in this study, sufficient concentrations of CDX-3379 were achieved in tumors to inhibit ErbB3 activation. Despite the short treatment duration, we observed tumor shrinkage on post-treatment radiographic assessment in 42% of patients, including 2 out of 3 HPV-positive patients. Another HPV-positive patient had progressive disease despite a 54% reduction in pErbB3, suggestive of an additional tumor driver in addition to ErbB3. Interestingly, one HPV-negative patient experienced a dramatic clinical response after a single dose.
We did not see an obvious relationship between tumor shrinkage and pharmacodynamic changes in pErbB3, suggesting that other tumor drivers may compensate for loss of ErbB3 activity. However, all patients with measurable reductions in tumor burden had tumors expressing both NRG1 and ErbB3 and reduced pErbB3 with CDX-3379 treatment, consistent with the clinical hypothesis. Comparison of NRG1, NRG2 and ErbB3 expression data with changes in pErbB3, Ki-67 or tumor measurements did not reveal significant correlations, although we may be limited by the small sample size and brief duration of treatment (Supplementary Figure S3). Two patients with PTEN loss showed some of the largest pErbB3 reduction changes and measurable tumor shrinkage, which is of potential interest considering that PTEN loss has previously been associated with resistance to cetuximab [14]. Consistent with previous data, we observed that the majority of HNSCC tumors express NRG1 but not NRG2. In our study, moderate to high NRG1 expression appeared more frequent in patients with measurable tumor regression, a hypothesis-generating observation compatible with the theoretical mechanism. However, our analysis is limited by the fact that NRG1 was assessed primarily in post-treatment tissue as well as the small sample size and short duration of treatment. Ongoing genomic and functional correlative studies are aimed at understanding how CDX-3379 treatment affects tumor biology, with the ultimate goal of identifying biomarkers that predict response.
Ki67 is a nuclear non-histone protein expressed in proliferating human tissue and is commonly used as a biomarker of treatment activity in window of opportunity studies. However, a treatment-induced decrease in Ki67 in HNSCC tumors has not been demonstrated to correlate with treatment activity, and Ki67 may not be a surrogate biomarker in all cancers [15–18]. In this clinical study, Ki67 decreases were observed in a subset of tumors following CDX-3379 treatment. However, the changes did not correlate with changes in pErbB3 or tumor measurements. Hence, the utility of Ki67 as a surrogate endpoint for clinical benefit in HNSCC is not supported by this study.
To our knowledge, this is the first example of an anti-ErbB3 monoclonal antibody demonstrating meaningful target inhibition in a relevant clinical setting and single agent activity in HNSCC. One major strength of this study is the ability to evaluate pre- and post-treatment samples on all patients. Limitations of this study include the relatively small number of treated patients, and the short duration of treatment with CDX-3379 which hampers assessment of CDX-3379 antitumor efficacy and duration of response.
Overall, this window of opportunity study demonstrated the feasibility of this type of trial design in the evaluation of single agent activity of compounds being developed and explored HNSCC as a target indication for the ErbB3/NRG signaling pathway. This study demonstrated biologic activity of CDX-3379 at the target serum exposure level based on significant decreases in post-treatment pErbB3 levels as well as antitumor effects. The current development focus for CDX-3379 is the investigation of rational combination treatment strategies by dual ErbB blockage in NRG1-expressing tumors. A phase 2 clinical study of combination CDX-3379 and cetuximab treatment in patients with HPV-negative HNSCC has been initiated at multiple clinical sites to further evaluate the potential for clinical benefit in this patient population.
Supplementary Material
Statement of Translational Relevance.
ErbB3 signaling is a known resistance mechanism to anti-EGFR therapies including cetuximab. The ErbB3 ligand neuregulin-1 (NRG1) is expressed in the majority of HNSCC, suggesting that ErbB3 signaling is active in this tumor type and may contribute to tumor growth and resistance to therapy. A window-of-opportunity study was conducted in HNSCC patients to characterize the biologic activity of CDX-3379, a human anti-ErbB3 monoclonal antibody, and other potential biomarkers of response. Most tumors expressed NRG1 and had detectable ErbB3 phosphorylation (pErbB3) prior to treatment. CDX-3379 modulated tumor pErbB3 at target trough concentrations and tumor shrinkage was observed in a subset of patients, including a marked clinical response. These data indicate CDX-3379 inhibits ErbB3 in patients with HNSCC tumors and provide the rationale for further development of CDX-3379 in HNSCC and potentially other ErbB3- and NRG-expressing tumors.
Acknowledgement
This work does not represent the views of the Department of Veterans Affairs nor the US Government.
Financial support: This study was funded by Celldex Therapeutics (previously Kolltan Pharmaceuticals).
Conflict of interest: Umamaheswar Duvvuri has received a grant from Kolltan Pharmaceuticals and is supported by a grant from the Department of Veterans Affairs IO1-BX003456 and NIH grant RO1-DE028343. Jonathan George and Seungwon Kim report no conflicts of interest. Veronique Neumeister reports employment with Indivumed Inc. Ahmed Chenna reports employment with LabCorp. Diego Alvarado, Richard Gedrich, Thomas Hawthorne and Theresa LaVallee report employment with Celldex Therapeutics (previously Kolltan Pharmaceuticals), including stock ownership and/or patents. Theresa LaVallee additionally reports stock or other ownership interest in Astrazeneca and a consulting role with GLG Consulting. Jennifer Grandis reports financial support from the American Cancer Society and NIH grants R01DE023685 and R35CA231998 and serves as a scientific inventor and advisor to STAT3 Therapeutics. Julie Bauman’s effort is supported in part by a VFoundation Translational Grant and NIH 5P50CA097190. She also reports clinical research grants from Kolltan, Celldex, Bristol-Myer Squibb, Aveo, and AstraZeneca.
References
- 1.Argiris A, et al. , Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Front Oncol, 2017. 7: p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkins E, et al. , FDA Approval Summary: Pembrolizumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma with Disease Progression on or After Platinum-Containing Chemotherapy. Oncologist, 2017. 22(7): p. 873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris R, et al. , Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med, 2016. 375(19): p. 1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sierke SL, et al. , Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. Biochem J, 1997. 322 ( Pt 3): p. 757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarado D, et al. , ErbB activation signatures as potential biomarkers for anti-ErbB3 treatment in HNSCC. PLoS One, 2017. 12(7): p. e0181356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takikita M, et al. , Membranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinoma. J Transl Med, 2011. 9: p. 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand TM, et al. , Human Papillomavirus Regulates HER3 Expression in Head and Neck Cancer: Implications for Targeted HER3 Therapy in HPV(+) Patients. Clin Cancer Res, 2017. 23(12): p. 3072–3083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 8.Dall’Acqua WF, Kiener PA, and Wu H, Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem, 2006. 281(33): p. 23514–24. [DOI] [PubMed] [Google Scholar]
- 9.Falchook GS, et al. , Safety, pharmacokinetics (PK), pharmacodynamics (Pd), and antitumor activity in a phase 1b study evaluating anti-ErbB3 antibody KTN33 79 in adults with advanced tumors alone and with targeted therapies. Journal of Clinical Oncology, 2016. 34(15_suppl): p. 2501–2501.27247217 [Google Scholar]
- 10.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A, et al. , Profiling the HER3/PI3K pathway in breast tumors using proximity-directed assays identifies correlations between protein complexes and phosphoproteins. PLoS One, 2011. 6(1): p. e16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordeaux JM, et al. , Quantitative in situ measurement of estrogen receptor mRNA predicts response to tamoxifen. PLoS One, 2012. 7(5): p. e36559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faden D, et al. , Genomic correlates of exceptional response to ErbB3 inhibition in head and neck squamous cell carcinoma. JCO Precision Oncology 2018. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eze N, et al. , PTEN loss as a predictive biomarker in head and neck squamous cell cancer (HNSCC) patients treated with cetuximab (C). Journal of Clinical Oncology, 2017. 35(15_suppl): p. e17520–e17520. [Google Scholar]
- 15.Maugeri-Sacca M, et al. , Presurgical window of opportunity trial design as a platform for testing anticancer drugs: Pros, cons and a focus on breast cancer. Crit Rev Oncol Hematol, 2016. 106: p. 132–42. [DOI] [PubMed] [Google Scholar]
- 16.Gross ND, et al. , Erlotinib, erlotinib-sulindac versus placebo: a randomized, double-blind, placebo-controlled window trial in operable head and neck cancer. Clin Cancer Res, 2014. 20(12): p. 3289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauman JE, et al. , Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI Insight, 2017. 2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curry J, et al. , Metformin effects on head and neck squamous carcinoma microenvironment: Window of opportunity trial. Laryngoscope, 2017. 127(8): p. 1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.