Abstract
The purpose of the present study was to characterize simulated microgravity and radiation-induced changes on retina and retinal vasculature and assess the accompanying early changes in immune cells and hematological parameters. To better understand the effects of spaceflight, we used a combination treatment designed to simulate both the radiation and low gravity aspects of space conditions. In order to simulate the broad energy spectrum of a large solar particle event (SPE) and galactic cosmic ray (GCR) radiation, we irradiated male C57BL/6J mice whole body with fully modulated beams of 150 MeV protons containing particles of energy from 0 to 150 MeV and a uniform dose versus depth profile. The mice were also hindlimb-unloaded (HLU) by tail suspension. Mice were unloaded for 7 days, irradiated at 50 cGy, unloaded for an additional 7 days and then sacrificed for tissue isolation at 4 days and 30 days after the combined treatment. Increases in the number of apoptotic cells were seen in radiation-only and radiation + HLU mice retina endothelial cells (ECs) compared to controls at both days 4 and 30 (p<0.05). The level of endothelial nitric oxide synthase (eNOS) was significantly elevated in the retina after radiation-only or radiation + HLU compared to controls at the 30-day time point (p<0.05). The most robust changes were observed in the combination group, suggesting a synergistic response of radiation and unloading. For hematopoietic parameters, our analysis indicated main effects for time and radiation at 4 days post treatment (11 days post irradiation) (p<0.05), but a smaller influence of HLU for both white blood cell and lymphocyte counts. The radiation +HLU group showed greater than 50% reduction of lymphocyte counts compared to controls. Radiation-dependent differences were also noted in specific lymphocyte subpopulations (T, B, natural killer cells). Our study found indications of an early impact of low-dose radiation and spaceflight condition on retina and immune populations.
Keywords: proton radiation, hindlimb unloading, retina, hematopoietic cells, spaceflight
INTRODUCTION
Microgravity and radiation are stressors unique to the spaceflight environment that can have an impact on the central nervous system (CNS) and immune system. They could potentially lead to significant risks to astronaut health both acutely, during the course of a mission, or chronically, leading to long-term, post-mission decrements in quality of life. A recent report shows that more than 50% of the astronauts returning from space have reported changes in their visual acuity (1). To date, the mechanisms behind these effects are not fully understood. Evidence also shows that exposure to space flight induces immune alterations in astronauts (2).
Studies have shown that both microgravity encountered by astronauts in space, as well as modeled microgravity on Earth, can induce many deleterious physiological effects including changes in ocular structure and function (3). After long-duration spaceflight, morphological changes in the optic nerve and surrounding tissues have been reported (4). Microgravity induces a significant head ward shift in body fluids, and an increase of intraocular pressure (IOP) (5). These changes within the CNS affect retinal structure and function (6). However, the cellular mechanisms of the unique physiological and pathological ocular responses are unknown. Microgravity-related immunological changes and immune dysregulation were also reported. Exposure to microgravity or simulated microgravity also induces changes in the numbers of several types of immune cells in the circulation (7, 8).
The space radiation environment consists of highly charged and energetic particles that include high-energy protons in the galactic cosmic ray (GCR) spectrum and are released from the sun during solar particle events (SPEs). During long-term deep space missions, it is anticipated that multiple SPEs will be encountered. Furthermore, an SPE dose may also exacerbate biological effects from the concurrent protracted GCR radiation exposure (9). These exposures are a significant radiation hazard to astronauts and spacecraft. Studies on mice that had been subjected to radiation and space flights (10–14) have shown that environmental conditions have profound impact on retinal endothelial health and retinal function. Our previous study documented that proton irradiation significantly induced oxidative stress associated-apoptosis in the retina at the dose of 0.5Gy (10). Cells of the immune system are also vulnerable to the effects of radiation (15). Many studies have demonstrated in rodent models that total body irradiation with protons can induce immune depression and that some abnormalities persist long-term (16–19). Combined exposure of space radiation and hindlimb suspension has significantly affected the number of circulating blood cells (20) and reduced the ability to control bacterial challenge in a mouse model due to impaired immune function (21).
Oxidative stress-induced ocular tissue damage resulting from reactive oxygen species (ROS) has been associated with a variety of pathological and environmental conditions, including radiation. Accumulating evidence suggests that ROS interfere with nitric oxide (NO) regulation causing endothelial dysfunction (22). NO, which is derived from L-arginine in an oxidizing reaction catalyzed by NO synthase (NOS), serves multiple functions including vasodilation, neurotransmission, and immune defense (23). In mammalian cells, three distinct isoforms of NOS have been identified: neural NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). Among these isoforms, eNOS is the major source of NO production in endothelial cells (ECs) (24). Our recently published paper showed significant increased expression of eNOS immunoactivity in retinal ECs following oxygen and proton irradiation compared to controls (25). This analysis indicated heavy ion charge particles may activate eNOS, promote apoptosis in retinal ECs, and contribute to endothelial dysfunction.
The purpose of the present study was to characterize early effects of combined hindlimb unloading (HLU) and radiation-induced changes on retina and retinal vasculature and assess the accompanying early changes in immune cells and hematological parameters. We hypothesized that simulated microgravity may enhance the effects of space-like radiation on retina vascular ECs, circulating blood cells and hematopoietic function.
MATERIAL & METHODS
Animals
Six-month old, male C57BL/6J mice, each weighing about 26–30 grams, were purchased from the Jackson Laboratory (Bar Harbor, ME). Upon arrival, animals were housed in cages (cage size of 17 × 9 × 6 in) within a BioZone VentiRack™ (BioZone, Inc., Fort Mill, SC). Animals were maintained under a constant ambient temperature of 68°F with a 12-hour day/night cycle throughout the study. Commercial pellet chow, Lab Diet 5LG4 (Lab Diet, St. Louis, MO)and hydrogel (ClearHjjO, Portland, ME) were available ad libitum. After acclimatization for approximately seven days, the mice were housed 1/cage and assigned to the following groups: i) control, ii) whole-body proton irradiation (IR), iii) hindlimb unloaded (HLU), iv) combination. There were 8 mice/group. The study followed recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the Institutional Animal Care and Use Committee (IACUC) of Loma Linda University. Mice were HLU for 7 days, then whole-body irradiated with proton at 50 cGy, followed by HLU for an additional 7 days and then sacrificed for tissue isolation at days 4 and 30 after the combined treatments (Figure 1). After the HLU and irradiation period, mice were returned to group housing, 4 per cage, until euthanasia. At the appropriate time point, mice were deeply anesthetized with 3% isoflurane followed by immediate exsanguination via inferior vena cava (IVC) puncture. Animals’ health status, food and water intakes were monitored on a daily basis.
Figure 1:
Diagram of experiment protocol. Mice were hindlimb unloaded for 7 days, irradiated with a single, whole-body dose of 50 cGy, hindlimb unloaded for an additional 7 days and then sacrificed for tissue isolation at 4 and 30 days after the combined exposure. Protons were delivered as a fully modulated beam of 150 MeV protons to give a broad energy distribution similar to a solar particle event (SPE) and galactic cosmic ray (GCR) radiation spectrum.
HLU
HLU is a widely accepted, ground-based animal model that simulates the mechanical unloading and body fluid shifts encountered in microgravity (26). Such changes in fluid perfusion could contribute to increases in intracranial pressure (ICP) or IOP (5). The cage floor was made with a grid panel to allow for animal and food waste to fall through the cage. For suspension, the tail was inserted into a plastic tube of a tail harness and attached to a loop of tape at the tip of the tail, and to a swivel fixed on a guide-wire running the length of the cage. The height of the bar is adjusted to maintain the animal in a 35 to 40 degree head down tilt with the hind legs elevated above the bottom of the cage. In this model, the forelimbs are used for locomotion and grooming. The control animals were not tail-suspended. Animals were observed daily for changes in appearance and activity.
Irradiation with protons
The mice were either sham irradiated or received whole-body proton irradiation at 50 c Gy (n = 8/group). The mice were restrained individually in 1.5-mm thick, rectangular plastic boxes (3 × 3 × 8.5 cm) with air holes. The proton beam was orientated vertically downward such that the mice were dorsally irradiated. SPE-like exposures were simulated by using a fully modulated 149.6 MeV/nucleonproton beam. The full modulation of the monoenergetic proton beam was produced by passing it through a rotating propeller-like modulator wheel with 21 thickness steps machined into the blades to range shift the single incident proton energy into 21 separate proton energies. A 2.0 cm thick plastic water range shifter was utilized in the irradiations to ensure dose uniformity over the entire subject. When the plastic water range shifter was combined with the 1.5 mm thick mouse box wall, the lowest Bragg peak energy incident on the test subject was 24.6 MeV, while the highest was 122.5 MeV. The superposition of 21 Bragg peaks created a uniform dose region (known as the spread-out Bragg peak) across the entire animal regardless of its orientation within the holder. Unirradiated controls were immobilized for the same length of time, positioned in the proton irradiation field without exposure (sham irradiation).
Eye and retina preparation
At 4 and 30 days post irradiation + HLU, mice were euthanized and the right eye from each mouse was placed individually in a sterile cryovial, snap frozen in liquid nitrogen and kept at −80°C prior to use. The left eyes were fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) for immunohistochemistry (IHC) assays.
Immunostaining assays and histology
Six μm paraffin-embedded sections were cut through each eye, and sections were roughly 100 μm apart providing 10 sections per eye for analysis. To characterize apoptosis, 5 sections were subjected to a terminal deoxynucleotidyltransferase dUTP nick-end labeling (TUNEL) staining. Retinal tissues were evaluated using the DeadEnde Fluorometric TUNEL system kit (catalog no. G3250, Promega Corp., Madison, WI). Sections were then stained with DyLight 488 Lycopersicon esculantum-Lectin (catalog no: DL-11721, Vector Laboratories,) for labeling vascular networks at a 1:100 dilution for 30 minutes at room temperature. Five tissue sections of each retina were examined using a BZ-X710 All-in-One inverted fluorescence microscope with structural illumination (Keyence Corp., Elmwood Park, NJ). TUNEL-positive cells were identified by green fluorescence, vascular endothelium were identified with red fluorescence; the nuclei of retinal cells were counterstained with diamidino-2-phenylindole (DAPI, blue). TUNEL-positive cells that were located within red lectin-labeled endothelium were identified as TUNEL-positive ECs. To characterize nitric oxide synthase (eNOS) function and associated oxidative damage in retina and retinal ECs, 5 ocular sections from each eye were incubated with rabbit anti-eNOS primary antibody (catalog no. ab5589, Abcam, Cambridge, UK) at 1:100 dilution at 37C for 1 hour, followed by a goat anti-rabbit IgG Dylight 594 secondary antibody (catalog no: 35561, Thermo Scientific, Hampton, NH) at 1:200 dilution for 1 hour at room temperature. Sections were then stained with DyLight 488 -Lectin (catalog no: DL-11721, Vector Laboratories, Burlingame, CA) at a 1:100 dilution for 30 minutes at room temperature. eNOS activity was identified by green fluorescence; vascular endothelium were identified with red fluorescence, the nuclei of retinal cells were counterstained with DAPI (blue fluorescence). eNOS-positive cells that were located within red lectin-labeled endothelium were identified as eNOS-positive ECs.
For quantitative analysis, the total number of TUNEL or eNOS-positive cells in the retinal vessels were counted in 5 sections of each eye. The area of selected region was measured on digital microphotographs using ImageJ counting plugin 1.41 software (National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/). The density profiles were expressed as mean number of apoptotic or eNOS positive cells/mm2. Similar procedures for density evaluation have been described in our previous papers (11, 25). The mean of the density profile measurements across 5 retina sections per eye was used as a single experimental value.
Hematological analysis in the blood
At the time of euthanasia, blood was obtained through IVC puncture with ethylenediaminetetraacetic acid (EDTA)-containing syringes. A Vet ABC Hematology Analyzer (Scil Animal Care, Gurnee, IL) was used to obtain white blood cell (WBC), major leukocyte population (lymphocyte, monocyte, granulocyte), red blood cell (RBC), platelet (PLT) counts and mean platelet volume (MPV). Values for hemoglobin (HGB) concentration, hematocrit (HCT, proportion of blood volume composed of RBC), mean corpuscular volume (MCV, average RBC volume), mean corpuscular hemoglobin (MCH, average mass of HGB/RBC), mean corpuscular HGB concentration (MCHC, average HGB/RBC), RBC distribution width (RDW, width of RBC based on cell number x cell size), and mean platelet volume (MPV, average platelet size) were obtained. Granulocyte, monocyte, and lymphocyte counts and percentages were also measured.
Spleen processing & leukocyte analysis
Spleens were cut into thirds and weighed. One third of the organ was immediately frozen in liquid nitrogen for further analysis. Two thirds were placed into 1 ml RPMI 1640 medium (Mediatech, Inc., Herndon, VA), homogenized using sterile wooden applicator sticks and filtered through 40 μm nylon filters (BD Biosciences, San Jose, CA). Once in single-cell suspensions, splenocytes were counted with the ABC Analyzer, as described above. Leukocyte counts were corrected for the mass.
Flow cytometry analysis of lymphocyte subpopulations in blood & spleen
A 2-tube custom-conjugate mixture with fluorescence-labeled monoclonal antibodies (mAb), a direct-staining procedure, was used to identify specific leukocyte types using a FACSCalibur™ flow cytometer (Becton Dickinson, Inc., San Jose, CA). The mAb were labeled with fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), allophycocyanin (APC), or peridinin chlorophyll protein (PerCP). For lymphocyte phenotyping, a 2-tube custom-conjugate mAb mixture (BD Pharmingen, San Diego, CA) was used to identify CD3+ for mature T-cells, CD19+ for B-cells, and NK1.1+ for natural killer (NK) cells. Analysis of 5,000–10,000 events/tube was performed using CellQuestTM software (v3.1, Becton Dickinson). The percentages obtained were used together with cell numbers from the hematology analyzer to obtain numerical data for each lymphocyte population.
Statistical analysis
The results obtained from IHC evaluation were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple-comparison test (SigmaPlot for Windows, version 13.0; Systat Software, Inc., Point Richmond, CA). The significance level was set at p<0.05. Data are shown as mean ± standard error (SEM).
The results obtained from hematological and splenocyte analysis were analyzed by three-way ANOVA to determine main effects and interaction using radiation, HLU and time points as the independent variables followed by Tukey’s honestly significant difference post hoc multiple-comparison test. The significance level was set at p<0.05. Data were shown as mean ± SEM. The terms “main effect” and “interaction” are common statistical terms used in describing ANOVA results. In our case, there were three independent variables: radiation, HLU and time. A significant main effect of radiation means that the effect of radiation was significant, independent of any impact of HLU or time. Similarly, a significant radiation × HLU interaction means that the effect of radiation was significantly dependent on the effect of the unloading.
RESULTS
Body weights
Mean animal mass for each group prior to exposure to radiation and/or unloading was as follows: control = 29.4 ± 0.6g, radiation only = 29.4 ± 0.9g, unloading only = 28.9 ± 0.8g, and combination = 28.2 ± 0.7g. At sacrifice (4 days post-exposure), masses were as follows: control = 31 ± 0.6g, radiation only = 29.7 ± 0.8g, unloading only =29.9 ± 0.5g, and combination = 29.8 ±0.5g; At 1 month time point, control = 31 ± 0.5g, radiation only = 31.5 ± 0.7g, unloading only =30.7 ± 1.0g, and combination = 29.5 ±1.1g. There were no significant group differences in pre-exposure mass compared to mass at time of sacrifice.
Apoptosis in retinal endothelial cell following proton irradiation and HLU at day 4 and 30 time points
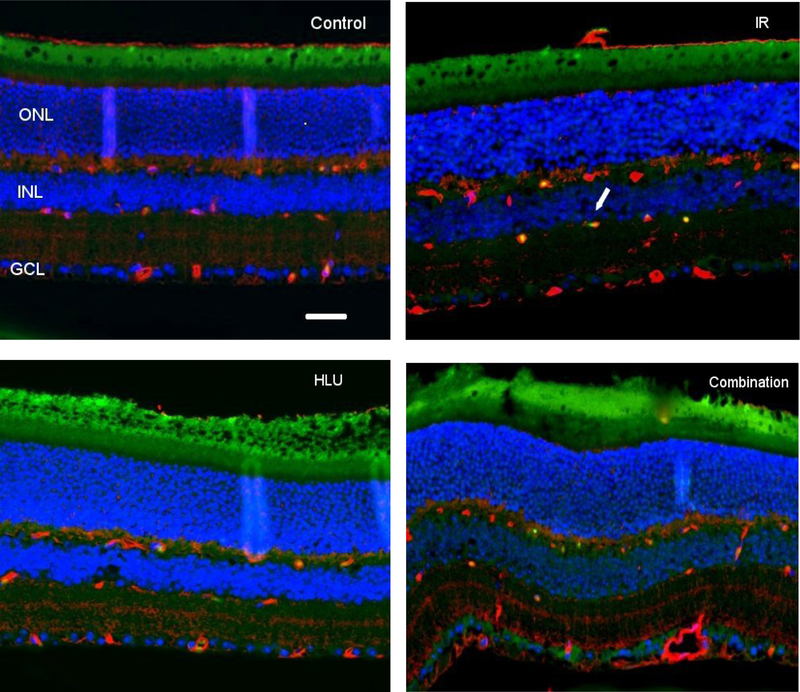
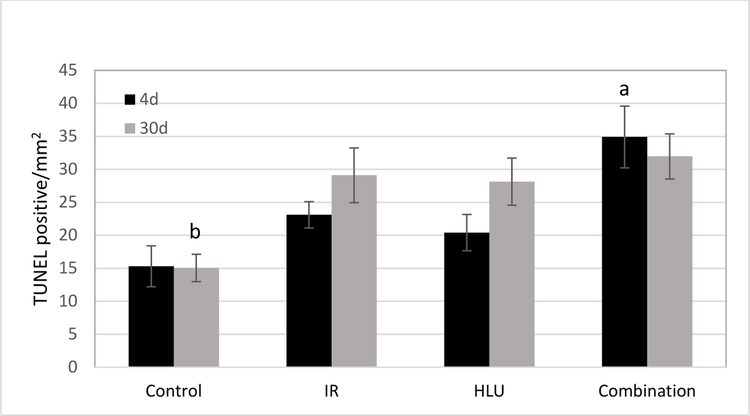
At 4 days post irradiation + HLU, an increase of TUNEL positive cells was noted in the retinal inner nuclear layer (INL) following proton radiation and in the combined treatment cohort (Figure 2). Our quantitative assessment revealed that TUNEL positive retinal endothelial cell density was the highest in the combination group at 4-day time point (p<0.05) compared to other groups. The counts for controls was significantly lower than all other groups at the 30-day time point (p<0.05) (Figure 3). Most robust changes are observed in proton+ HLU group compared to control.
Figure 2:
Apoptosis based on terminal deoxynucleotidyltransferase dUTP nick-end labeling (TUNEL) staining of retinal tissue. i) Control, ii) Proton irradiation (IR) at 50cGy, iii) Hindlimb unloading (HLU), iv) Combination. TUNEL-positive cells were identified with green fluorescence, endothelium was stained with lectin (red). The nuclei of photoreceptors were counterstained with DAPI (blue). TUNEL-positive cells that were located within red lectin-labeled endothelium were identified as TUNEL-positive endothelial cells (ECs). ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. Arrow: TUNEL-positive EC. In the control retinal tissue, only sparse TUNEL-positive cells were found. In the retina from mice exposed to 50 cGy of proton and HLU, TUNEL-positive labeling was apparent in the endothelial cells. Scale bar =50 μm. Green auto-fluorescent was noted in the outer layers.
Figure 3:
Immunoreactivity of TUNEL staining in the retinal endothelium following radiation and/or hindlimb unloading (HLU). Values are represented as mean density ± SEM for 8 mice/group. a significantly higher than all other groups at the 4-day time point (p<0.05). b Significantly lower than all other groups at the 30 day time point (p<0.05).
eNOS Immunoreactivity in retinal endothelial cells following proton and HLU
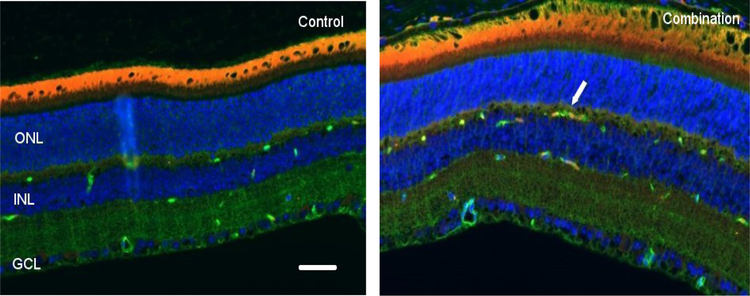
There were no significant differences among groups at the 4-day time point for eNOS expression. However, by day 30, increased eNOS staining was seen in the retinal INL after combined radiation and HLU treatment (Figure 4). Quantitative analysis revealed that eNOS immunoreactivity was significantly higher (p<0.05) in the combined treatment group compared to control and irradiated-only groups at the 30-day time point (Figure 5).
Figure 4:
Representative micrographs of retina sections evaluated for endothelial nitric oxide synthase (eNOS) expression at 30 days after proton radiation and/or hindlimb unloading (HLU). i) Control, ii) Combination. Endothelial NO synthase (eNOS) positive cells were identified with red fluorescence, endothelium was stained with lectin (green). The nuclei of photoreceptors were counterstained with DAPI (blue). eNOS-positive cells that were located within red lectin-labeled endothelium were identified as eNOS-positive endothelial cells (ECs). ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. Arrow: eNOS-positive EC. In the non-irradiated retinal tissue, only sparse eNOS-positive cells were found. In the retina from mice exposed to 50 cGy of proton radiation combined with HLU, eNOS-positive labeling was apparent in the ECs. Scale bar =50 μm. Red auto-fluorescent was noted in the outer layers.
Figure 5:
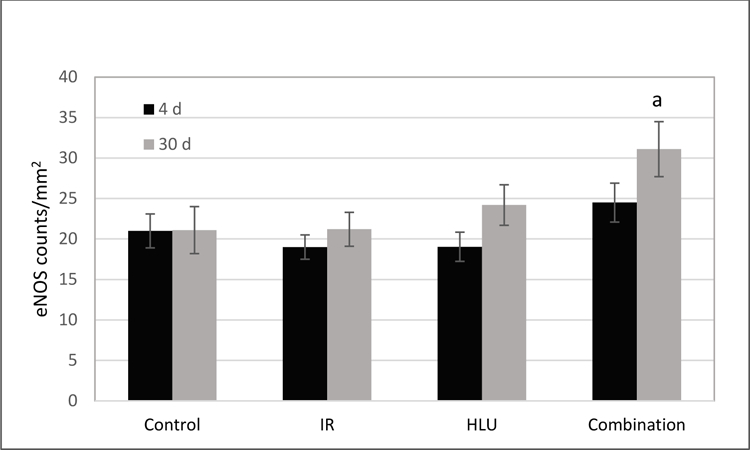
Endothelial NO synthase (eNOS) immunoreactivity following proton irradiation and /or hindlimb unloading (HLU). Values are represented as mean density of eNOS-positive endothelial cells ± SEM for 8 mice/group. a significantly higher than control and radiation-only groups (p<0.05).
Blood and spleen analysis
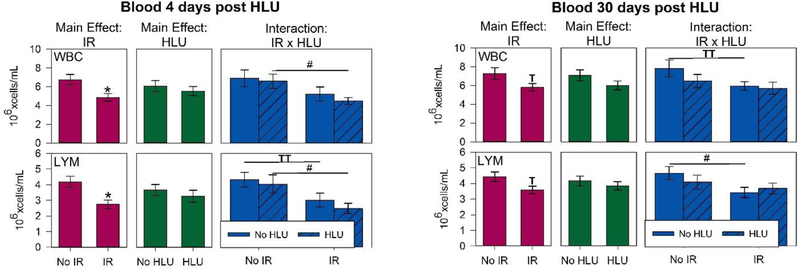
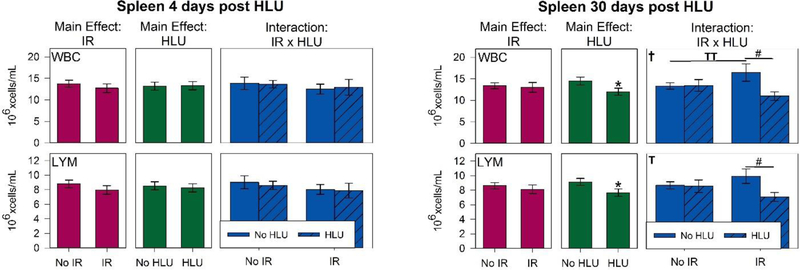
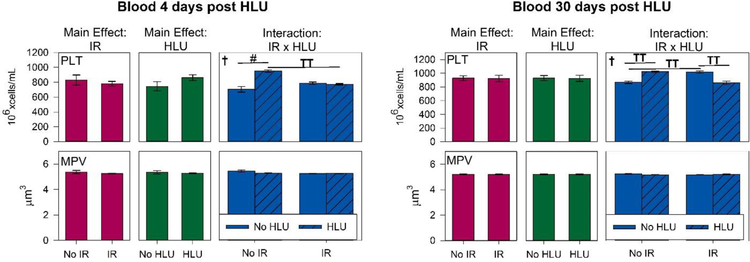
There were significant main effects of radiation (p<0.05) for WBC and lymphocyte counts in the blood (Figure 6A). Our quantitative analysis showed significant interactions in WBC and lymphocyte counts between radiation and unloading at day 4 (p < 0.05), suggesting that unloading significantly augmented the response to radiation. Post hoc analysis showed the WBC and lymphocyte counts were greatly reduced following irradiation at both 4- and 30-day time points with smaller influences of HLU on both total white blood cells and lymphocytes. With the exception of day 30 for lymphocyte, the proton +HLU group had consistently lower counts than other groups. The proton +HLU group was over 50% lower in lymphocyte counts compared to control and HLU groups by day 4 (p<0.05). For platelet counts, there were no significant difference between groups for both time points, except on day 4, HLU group had significantly higher counts compared to controls (p<0.05) and strong trend increase compared to proton + HLU group (p=0.07) (Figure 7). There were no significant differences in RBC and other platelet measurements and time points between groups (data not shown).
Figure 6:
White blood cell (WBC) and lymphocyte (LYM) counts in the blood (6A) and spleen (6B) after irradiation and/or hindlimb unloading (HLU). Mice were euthanized and blood was collected in ethylenediaminetetraacetic acid (EDTA)-coated syringes via inferior vena cava (IVC) at 4 or 30 days post-unloading (11 and 37 days post-irradiation, respectively). Blood and spleens were analyzed via an ABC Vet Automated Hematology Analyzer. Three-Way ANOVA: * = P<0.05, main effect of proton irradiation (IR) or HLU, †=P<0.05, IR x HLU interaction. T =P<0.1, IR x HLU interaction. Post hoc Tukey test: # =p<0.05, TT =P<0.1. Note the strong radiation dependence at 4 days post treatment, but smaller influence of HLU for both WBC and lymphocytes.
Figure 7:
Platelet (PLT) counts and mean platelet volume (MPV) in the blood after irradiation and/or hindlimb unloading (HLU) at 4 or 30 days post-unloading (11 and 37 days post-irradiation, respectively). Three-Way ANOVA: †=P<0.05, IR x HLU interaction. T =P<0.1, IR x HLU interaction. Post hoc Tukey test: # =p<0.05, TT =P<0.1. Note the strong HLU dependence at 4 days post treatment, but smaller influence of radiation for PLT counts.
For spleen, at day 4, there were no significant main effects of radiation or unloading on the counts of any major immune cell subset (Figure 6B). There was a significant interaction of radiation and HLU by day 30 for WBCs and lymphocytes (p<0.05). The combination group has the lowest counts. There were no significant difference in RBC, and platelet measurements between groups (data not shown).
Major lymphocyte populations in blood
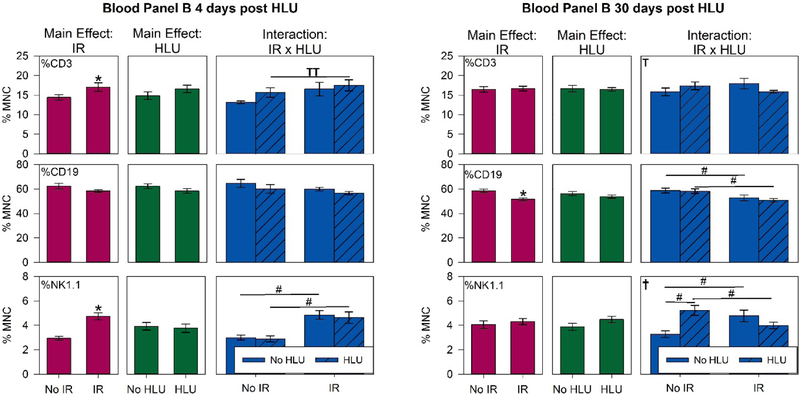
Since lymphocytes are the most radiosensitive of all leukocyte subsets, we characterized T (CD3+), B (CD19+), and NK (NK1.1) cell subsets in the blood and spleen using flow cytometry. By day 4, there were significant main effects of radiation (p<0.05) for T and NK cell counts in the blood (Figure 8). The proton and proton + HLU groups have a higher proportion of NK cells compared to control (p<0.05). There was no effect on B cell counts at this time point. By day 30, the proportion of B cells was significantly lower in the proton and proton +HLU groups compared to controls (p<0.05). Our results showed that there was a strong interaction between radiation exposure and HLU especially for NK cells in the blood at both 4-and 30-day time points. Only weak effects were seen for spleen (data not shown).
Figure 8:
Major lymphocyte subpopulations in the blood. T (CD3+), B (CD 19+) and NK (NK1.1) cell counts are presented as percentages of all mononuclear cells (MNC). Data were obtained using fluorescence-labeled monoclonal antibodies and flow cytometry Mice were euthanized and blood was collected in ethylenediaminetetraacetic acid (EDTA)-coated syringes via inferior vena cava (IVC) at 4 or 30 day post-unloading (11 and 37 days post-irradiation, respectively). Blood samples were analyzed using FACSCaliburTMflow cytometer. Three-Way ANOVA: * = P<0.05, main effect of proton irradiation (IR) or HLU. †=P<0.05, IR x HLU interaction. T =P<0.1, IR x HLU interaction. Post hoc Tukey test: # =P<0.05, TT =P<0.1.
DISCUSSION
It is an important contribution for risk assessment to determine whether the low dose radiation response is modulated by simulated microgravity. The objective of the present study was to use a ground-based animal model to assess the biological effects of the spaceflight condition, combining space-like radiation exposure and microgravity. This study design more accurately modeled environmental stressors inherent to the spaceflight environment, providing a more actual risk assessment for astronauts. To our knowledge, this is the first study to examine the impact of the simulated space flight on retinal oxidative damage.
Combined exposure of ionizing radiation and HLU induces cellular and immune response significantly at 4 and 30 days post exposure. A short latency between exposures to tested stressors and the onset of oxidative changes in retinal endothelial cells and some hematopoietic parameters were observed at 4 days after proton irradiation and HLU. Time points chosen for our study were based on the reports that ionizing radiation results in rapid depletion of peripheral blood WBCs to a minimum at 4 days post radiation followed by a restoration by 14 to 30 days (27, 28).
One of the mechanisms involved in the response to environmental stress including spaceflight and radiation exposure is acute and chronic oxidative stress (29). Persistent up-regulation of oxidative stress has been demonstrated in the retina of mice following spaceflight and radiation exposure (11, 25, 30), and many of these changes have been implicated in functional consequences including visual impairment (31, 32). Similarly, exposure to simulated microgravity also results in enhanced ROS production that may contribute to unloading–induced oxidative stress (33, 34). The CNS is sensitive to oxidative injury due to high concentrations of oxidizable, unsaturated lipids and low levels of antioxidant defenses. The retina contains a high level of polyunsaturated fatty acids, making it susceptible to lipid peroxidation (35). In many cases, cell death induced by oxidative damage has been identified as occurring via the process of apoptosis (36). Oxidative stress has also been implicated in the pathogenesis of many ocular lesions and diseases. A large body of evidence supports that increases in oxidative stress in retinal endothelial cells and microvasculature is a key factor for the development of retinal vascular diseases (37–39). Our study demonstrates that HLU and radiation both induce apoptosis in the mouse retina. There were synergistic effects of HLU and proton radiation on increased apoptosis at the early time point. At the later time point, apoptotic damage due to combined or individual stressors are similar, which may indicate biological regulation of damage response and repair.
Exposure to microgravity induces inflammatory responses and modulates immune functions. Altered immune responses increase ROS production that can promote microgravity–associated oxidative stress (40). In-flight data also suggest that oxidative stress is an observed outcome in a long-duration spaceflight (41). Our data showed that eNOS expression was significantly increased following combined exposure to simulated space radiation and HLU at the 30-day time point, but not significantly altered at the 4-day time point. At the early time point, several other potential sources of ROS and factors in neuronal tissues may contribute to cellular response following simulated space radiation or microgravity, including xanthine oxidase, mitochondrial enzymes, and enzymes involved in NO synthesis or arachidonic acid metabolism and NADPH oxidase (42). In our further study, to determine the extent of oxidative stress in the retina following radiation exposure and unloading, the production of other stress markers will be assessed. The increased number of apoptotic cells found in this study supports the notion that apoptotic mechanisms are involved in retina cell death.
Our study showed effects of low-dose radiation and simulated microgravity on immune populations. There were significant or strong trend decreases in the WBC counts, as well as the lymphocyte population after exposure to proton irradiation on days 4 and 30 in the blood, but not in the spleen. These findings indicate that body compartment (blood versus spleen) may make a difference in leukocyte survival after oxidative stress (43). Radiation-induced reduction in leukocyte population counts by day 30 post exposure were noted, suggesting a relatively long-term impact of radiation effect. Obtained results were consistent with results of previous studies using larger doses of acutely delivered protons (44, 45).
When broad energy spectrum proton radiation at 50 cGy and HLU are used in a combined motif, a synergistic (or additive) effect of radiation dose and HLU is not consistent with many of our measured parameters, and the effects of HLU on hematological assessment appear to be mild for most parameters. Others also reported that combined treatment with HLU did not exacerbate the deleterious effects of SPE-like proton radiation in circulating immune cells (20). However, we observed a strong interaction between radiation and HLU treatment for their effects on the splenic lymphocytes at day 30.
It is also well known that immune cells respond to ionizing radiation with distinct characteristics that depend on the radiation dose and time (16). Different cell populations are known to have different sensitivity to radiation. Furthermore, radiation and unloading-induced effects on lymphocytes but not on monocytes/macrophages and granulocytes suggests that survival and/or DNA repair mechanisms in lymphocytes were less efficient after radiation exposure. The lymphocytes, especially, T, B, and NK cells are essential for optimal immune defenses (46). Analysis of lymphocyte subpopulations showed that radiation alone, or in combination with HLU, significantly reduced B cell counts and elevated NK counts in the blood on day 30. This could result in a proportional shift in favor of cell populations involved in innate immunity.
In contrast to leukocytes, proton radiation at 50 cGy and unloading had little effect on erythrocytes or platelet counts. This may indicate their different response in radiosensitivity, cycling kinetics, DNA repair capacity and other innate characteristics. Hematopoietic progenitors are heterogeneous in their ability to repair damage and repopulate after exposure to ionizing radiation (47). Although some of the observed changes in hematopoietic populations were relatively small, it remains to be determined whether these changes will increase over time and whether the findings have a long-term impact on immune function and their ability to maintain homeostasis.
In conclusion, our data showed proton irradiation alone or combined with simulated microgravity has significant impact on retinal EC survival and some measured immune parameters based on numerical changes in immune cell populations. Reduction in lymphocytes and WBC could compromise immune function and increase the risk of development of immune diseases. Late and long term effects and functional consequences of observed changes will be determined in our further studies.
ACKNOWLEDGMENTS
This study was supported by National Space Biomedical Research Institute (NSBRI) grant #RE03701 through NASA cooperative agreement NCC 9–58, NASA 80NSSC18K0310 and LLUMC Department of Radiation Medicine.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R, Polk JD. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118(10):2058–69, 2011. [DOI] [PubMed] [Google Scholar]
- 2.George K, Rhone J, Beitman A, Cucinotta FA. Cytogenetic damage in the blood lymphocytes of astronauts: effects of repeat long-duration space missions. Mutat Res 756(1–2):165–9, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Taibbi G, Cromwell RL, Kapoor KG, Godley BF, Vizzeri G. The effect of microgravity on ocular structures and visual function: a review. Suv Ophthalmol 58: 155–63, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Patel N, Pass A, Mason S, Gibson CR, Otto C. Optical Coherence Tomography Analysis of the Optic Nerve Head and Surrounding Structures in Long-Duration International Space Station Astronauts. JAMA Ophthalmol 136 (2):193–200, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Morgan WH, Balaratnasingam C, Lind CR, Colley S, Kang MH, House PH, et al. Cerebrospinal fluid pressure and the eye. Br. J. Ophthalmol doi: 10.1136/bjophthalmol-2015-306705. [DOI] [PubMed] [Google Scholar]
- 6.Zhao D, He Z, Vingrys AJ, Bui BV, Nguyen CT. The effect of intraocular and intracranial pressure on retinal structure and function in rats. Physiol Rep 2015. August;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Zhu X, Zhao L, Yang X, Cao F, Huang Y, Mu P. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. Effects of simulated weightlessness on biological activity of human NK cells induced by IL-2. 31(10):1297–300, 2015. [PubMed] [Google Scholar]
- 8.Chen Y, Xu C, Wang P, Cai Y, Ma H. Effect of Long-Term Simulated Microgravity on Immune System and Lung Tissues in Rhesus Macaque. Inflammation. 40(2):589–600, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Chancellor JC, Scott GB, Sutton JP. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life (Basel), 4(3), 491–510, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao XW, Green LM, Mekonnen T, Lindsey N, Gridley DS. Gene expression analysis of oxidative stress and apoptosis in proton-irradiated rat retina. In Vivo 24: 425–30, 2010. [PubMed] [Google Scholar]
- 11.Mao XW, Pecaut MJ, Stodieck LS, Ferguson VL, Bateman TA, Bouxsein M, Jones TA, Moldovan M, Cunningham CE, Chieu J and Gridley DS. Space Flight Environment Induces Mitochondrial Oxidative Damage in Ocular Tissue. Rad Res 180:340–50, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Mayer M, Kaiser N, Layer PG, Frohns F. Cell Cycle Regulation and Apoptotic Responses of the Embryonic Chick Retina by Ionizing Radiation. PLoS One. 2016;11(5):e0155093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frizziero L, Parrozzani R, Midena G, Miglionico G, Vujosevic S, Pilotto E, Midena E. Hyperreflective intraretinal spots in radiation macular edema on spectral domain optical coherence tomography. Retina 36(9):1664–9, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Vinogradova IuV, Tronov VA, Liakhova KN, Poplinskaia VA, Ostrovskiĭ MA. Damage and functional recovery of the mouse retina after exposure to ionizing radiation and methylnitrosourea. Radiats Biol Radioecol 54(4):385–92, 2014. [PubMed] [Google Scholar]
- 15.Gridley DS, Rizvi A, Luo-Owen X, Makinde AY, Coutrakon GB, Koss P, Slater JM, Pecaut MJ. Variable hematopoietic responses to acute photons, protons and simulated solar particle event protons. In Vivo 22(2):159–69, 2008. [PubMed] [Google Scholar]
- 16.Gridley DS, Pecaut MJ, Dutta-Roy R and Nelson GA: Dose and dose rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: Part I. Immunol Lett 80(1): 55–66, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Gridley DS and Pecaut MJ: Whole-body irradiation and long term modification of bone marrow-derived cell populations by low- and high-LET radiation. In Vivo 20(6B): 781–789, 2006. [PubMed] [Google Scholar]
- 18.Kajioka EH, Andres ML, Li J, Mao XW, Moyers MF, Nelson GA, Slater JM and Gridley DS: Acute effects of whole-body proton irradiation on the immune system of the mouse. Radiat Res 153(5 Pt 1): 587–594, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Pecaut MJ, Gridley DS and Nelson GA: Long term effects of low dose whole-body proton irradiation on immunity: Shielded vs. unshielded. Aviat Space Environ Med 74(2): 115–124, 2003. [PubMed] [Google Scholar]
- 20.Romero-Weaver AL, Lin LY, Carabe-Fernandez A, Kennedy AR. Effects of Solar Particle Event-Like Proton Radiation and/or Simulated Microgravity on Circulating Mouse Blood Cells. Gravit Space Res 2:42–53, 2014. [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Holmes V, Zhou Y, Ni H, Sanzari JK, Kennedy AR, Weissman D. Hindlimb suspension and SPE-like radiation impairs clearance of bacterial infections. PLoS One 9(1):e85665, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep 7, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, et al. eNOS activation and NO function: Structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J. Endocrinol 210: 271–284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao XW, Boerma M, Rodriguez D, Campbell-Beachler M, Jones T, Stanbouly S, Sridharan V, Wroe A, Nelson GA. Acute Effect of Low-Dose Space Radiation on Mouse Retina and Retinal Endothelial Cells. Radiat Res 190(1):45–52, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects J Appl Physiol, 92: 1367–1377, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Pecaut MJ, Gridley DS. The impact of mouse strain on iron ion radio-immune response of leukocyte populations. Int J Radiat Biol 86(5):409–19, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Chang J, Li X, Pathak R, Sridharan V, Jones T, Mao XW, Nelson G, Boerma M, Hauer-Jensen M, Zhou D, Shao L. Low doses of oxygen ion irradiation cause long-term damage to bone marrow hematopoietic progenitor and stem cells in mice. PLoS One 12(12):e0189466, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbin ME Zhao W Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol 80:251–259, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Cheng LB, Li KR, Yi N, Li XM, Wang F, Xue B, Pan YS, Yao J, Jiang Q, Wu ZF. miRNA-141 attenuates UV-induced oxidative stress via activating Keap1-Nrf2 signaling in human retinal pigment epithelium cells and retinal ganglion cells. Oncotarget 8(8):13186–13194, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang T, Chang Q, Cai J, Fan J, Zhang X, Xu G. Protective Effects of Melatonin on Retinal Inflammation and Oxidative Stress in Experimental Diabetic Retinopathy. Oxid Med Cell Longev 2016:3528274. [DOI] [PMC free article] [PubMed]
- 32.Berkowitz BA, Kern TS, Bissig D, Patel P, Bhatia A, Kefalov VJ, Roberts R. Systemic Retinaldehyde Treatment Corrects Retinal Oxidative Stress, Rod Dysfunction, and Impaired Visual Performance in Diabetic Mice. Invest Ophthalmol Vis Sci 56(11):6294–303,2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao XW, Nishiyama NC, Pecaut MJ, Campbell-Beachler M, Gifford P, Haynes KE, Becronis C and Gridley DS. Simulated microgravity and low-dose/low-dose-rate radiation induces oxidative damage in the mouse brain. Rad Res 185: 647–57, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury P, Soulsby ME, Scott JL. Effects of aminoguanidine on tissue oxidative stress induced by hindlimb unloading in rats. Ann Clin Lab Sci 39(1):64–70,2009. [PubMed] [Google Scholar]
- 35.Ohie SE, Opere CA, Leday AM. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res 579:22–36, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Gobbel GT, Bellinzona M, Vogt AR, Gupta N, Fike JR, Chan PH. Response of postmitotic neurons to X-irradiation: implications for the role of DNA damage in neuronal apoptosis. J Neurosci 18: 147–155, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbajal JM, Schaeffer RC. H2O2 and genistein differentially modulate protein tyrosine phosphorylation, endothelial morphology, and monolayer barrier function. Biochem Biophys Res Commun 249: 461–6, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 35: 1491–9, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal 7: 1581–7, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Wise KC, Manna SK, Yamauchi K, Ramesh V, Wilson BL, Thomas RL, Sarkar S, Kulkarni AD, Pellis NR, Ramesh GT. Activation of nuclear transcription factor-kappaB in mouse brain induced by a simulated microgravity environment. In Vitro Cell Dev Biol Anim 41(3–4):118–23, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Markin AA, Zhuravleva OA. Lipid peroxidation and antioxidant defense system in rats after a 14-day space flight in the “Space-2044” spacecraft. Aviakosm Ekolog Med 27:47–50; 1993. [PubMed] [Google Scholar]
- 42.Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol 100:739–743, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Gridley DS, Freeman TL, Makinde AY, Wroe AJ, Luo-Owen X, Tian J, Mao XW, Rightnar S, Kennedy AR, Slater JM, Pecaut MJ. Comparison of proton and electron radiation effects on biological responses in liver, spleen and blood. Int J Radiat Biol 87(12):1173–81, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Luo-Owen Xian, Pecaut Michael J., Rizvi Asma and Gridley Daila S. Low-Dose Total-Body g Irradiation Modulates Immune Response to Acute Proton Radiation. Rad. Res 177: 251–264, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Gridley DS, Pecaut MJ, Green LM, Sanchez MC, Kadhim MA. Strain-related differences and radiation quality effects on mouse leukocytes: gamma-rays and protons (with and without aluminum shielding). In Vivo 25(6):871–80, 2011. [PubMed] [Google Scholar]
- 46.Gridley DS, Luo-Owen X, Rizvi A, Makinde A, Pecaut M, Mao XW, Slater JM. Low-dose photon and simulated solar particle event proton effects on Foxp3+ T regulatory cells and other leukocytes. Technol Cancer Res Treat. 9(6):637–49, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Wagwemaker G: Heterogeneity of radiation sensitivity of hemopoietic stem cell subsets. Stem Cells 13(Suppl 1): 257–260, 1995. [DOI] [PubMed] [Google Scholar]