Introduction
The association of paraneoplastic pemphigus (PNP) with malignancy is well established in the literature.1 PNP has been reported to occur in the setting of follicular dendritic cell sarcoma (FDCS). This neoplasm has been associated with several other autoimmune disorders, likely because of the role follicular dendritic cells play in facilitating humoral immunity.2 Pemphigus vulgaris (PV) may clinically resemble PNP, and a comprehensive evaluation may be necessary to differentiate these uncommon conditions in the setting of malignancy.3 Here we present a patient with significant, treatment-refractory mucositis, myasthenia gravis, and a history of PV in the setting of FDCS of the thymus.
Case report
A 72-year-old man presented with severe mucositis and a cutaneous eruption. He had a 10-year history of mucosal-dominant PV, with prior biopsy of the left buccal mucosa showing acantholysis with intraepithelial and suprabasal clefting and chronic superficial dermal inflammation. His disease was previously controlled on daily mycophenolate mofetil, 1500 mg, and prednisone, 10 mg. Two months before presentation, he was hospitalized for new-onset myasthenia gravis requiring plasma exchange and intravenous immunoglobulin (IVIG). Imaging found a suspicious right-sided mediastinal mass. Elective excision was planned pending clinical improvement.
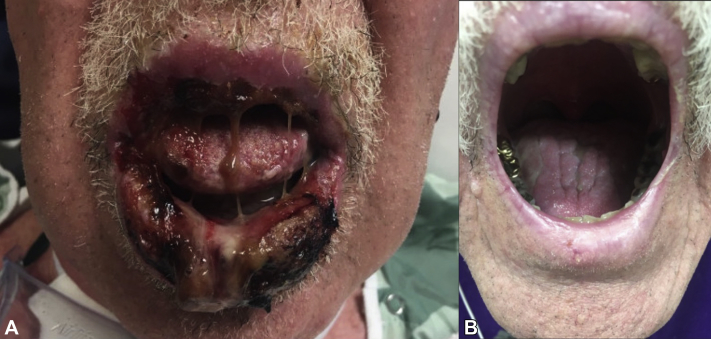
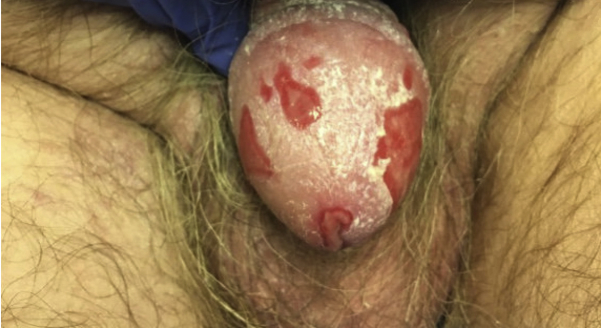
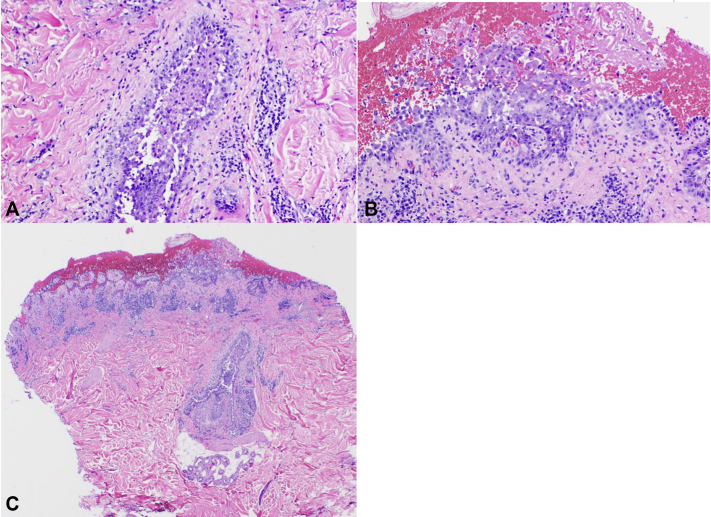
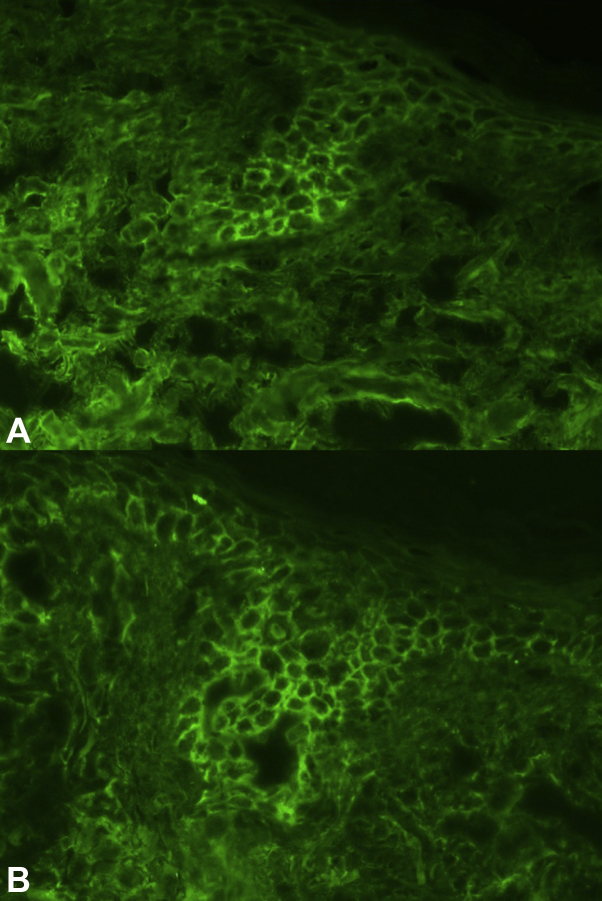
Physical examination found well-demarcated erosions of the oral mucosa, erythematous conjunctivae, arcuate erosions of the glans penis, and clustered flat-topped papules involving the trunk, extremities, and scalp (Figs 1, A and B and 2). Skin biopsy found intraepidermal vesiculation with suprabasilar acantholysis without interface dermatitis (Fig 3, A-C). Direct immunofluorescence (DIF) showed positive epidermal extracellular IgG and C3 without basement membrane zone (BMZ) involvement (Fig 4, A and B). Indirect immunofluorescence (IIF) demonstrated elevated cell surface IgG antibodies to monkey esophagus (1:40,960; positive>1:10) and intact human skin (1:20,480; positive>1:10). IgG cell surface antibodies were detected on rat bladder (1:40,960; positive>1:5) and mouse bladder (1:40,960; positive>1:5). BMZ staining was negative on rat and mouse bladder. Enzyme-linked immunosorbent assay (ELISA) revealed indeterminate Dsg1 (17 U/mL; positive>20) and elevated Dsg3 IgG antibodies (2,300 U/mL; positive>20). ELISA and immunoblotting were unable to be obtained for desmoplakin, envoplakin, or periplakin.
Fig 1.
PNP versus malignancy-exacerbated PV. Severe erosive stomatitis of the oral mucosa before (A) and after (B) treatment with rituximab, IVIG, high-dose prednisone, and mycophenolate mofetil.
Fig 2.
PNP versus malignancy-exacerbated PV. Well-demarcated erosions of the glans penis.
Fig 3.
A-C, Intraepidermal vesiculation with suprabasilar acantholysis on hematoxylin-eosin stain of affected skin.
Fig 4.
Direct immunofluorescence of epidermal extracellular (A) IgG and (B) C3 deposition in an area of affected skin.
The patient was started on high-dose prednisone and continued on mycophenolate mofetil. Despite this, his cutaneous lesions progressed. Because of the presence of suspicious mediastinal mass, thymectomy and partial pericardiectomy were performed. Histopathology confirmed FDCS without lymph node involvement. Unfortunately, the patient continued to have mucosal and skin lesions. He received 4 weekly infusions of rituximab (375 mg/m2), began monthly IVIG infusions (2 g/kg), and remained on high-dose prednisone (up to 80 mg daily) and mycophenolate mofetil (1500 mg twice a day) with topical corticosteroids for adjunctive therapy. The patient was discharged to a transitional facility and showed marked clinical improvement at 9 months (Fig 4). Trended IIF/ELISA supported treatment response (6 months posttreatment cell surface IgG antibodies to monkey esophagus, 1:20,480, and intact human skin, 1:1,280; 6 months posttreatment antibodies to Dsg1, 5 U/mL and Dsg3, 470 U/mL).
Discussion
Differentiating PNP from PV in the setting of underlying malignancy may represent a significant challenge. Thorough evaluation comprising clinical history, presentation, and laboratory evaluation may help narrow the differential, although definitive diagnosis may remain elusive. Our patient presented with acutely worsening mucositis and a cutaneous eruption in the setting of underlying thymic FDCS. His clinical progression was worrisome for a paraneoplastic process; however, definitively determining whether he had true PNP or PV exacerbated in the setting of malignancy is difficult to ascertain with certainty. We recommend maintaining a high index of suspicion for malignancy in the setting of an acute exacerbation of PV or treatment-refractory disease.
PV and PNP may present similarly. PNP is characterized by severe, treatment-refractory stomatitis with subsequent development of additional mucosal involvement.4 In addition, arcuate penile erosions may be more characteristic of PNP than PV.5 PV can present with severe mucositis if titers of anti-Dsg 3 are high.4 Histology may offer additional insights. Notably, the histology in PNP may vary widely by clinical morphology.3 PV classically demonstrates suprabasilar acantholysis and absence of interface change and lichenoid infiltrate.3 In our patient, severe stomatitis and arcuate erosive penile lesions raise suspicion for PNP, although histology was more supportive of PV.
Immunofluorescence studies may also be useful in distinguishing PNP from PV. Both exhibit IgG or C3 deposition on extracellular surfaces on DIF, whereas PNP may demonstrate concomitant involvement of the BMZ.4 However, these findings may vary.3 IIF studies using monkey esophagus with extracellular labeling have high sensitivity (∼90%) for PV and extracellular labeling detected on rat bladder is reported with a sensitivity and specificity of up to 86% and 98% to 100%, respectively in the setting of PNP.3 In our patient, IF was somewhat equivocal. DIF supported PV whereas IIF supported PNP.
ELISA may be useful to detect and monitor specific autoantigens implicated in PV and PNP. Those described in PNP include desmoplakin, periplakin, and envoplakin among others.6 Both PNP and PV may have Dsg1 and 3 autoantigens.7 Our patient had significantly elevated Dsg3 antibodies, which may support a diagnosis of PV but does not exclude PNP. We were unable to obtain ELISA or immunoblotting for desmoplakin, envoplakin, or periplakin.
We suspect FDCS contributed to worsening mucocutaneous disease and development of myasthenia gravis. The development of autoimmunity in the setting of FDCS is not unexpected. Tissue analysis from FDCS in patients with PNP has found B lymphocyte clones recognizing plakin family proteins and Dsg3.8 Management of PNP involves investigation, identification, and removal of the underlying neoplasm.1 Rituximab has been reported with initial efficacy after 4 weekly infusions (330 mg/m2) in a patient with myasthenia gravis, PNP, and FDCS; however, the response was not durable, and the patient unfortunately had worsening skin lesions and multiorgan failure leading to death.9 Our patient continues to improve clinically, supported by trended IIF/ELISA titers.
We present a case of refractory mucositis in the setting of FDCS of the thymus and myasthenia gravis. Although definitively concluding whether this represented PNP versus PV exacerbated in the setting of underlying malignancy is challenging, this case highlights the necessity for a high index of suspicion for neoplasm for patients presenting acutely with treatment-refractory or significantly worsening PV and highlights the therapeutic role of rituximab and IVIG in this case.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Paolino G., Didona D., Magliuolo G. Paraneoplastic pemphigus: insight into the autoimmune pathogenesis, clinical features and therapy. Int J Mol Sci. 2017;18(12) doi: 10.3390/ijms18122532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saygin C., Uzunaslan D., Ozguroglu M., Senocak M., Tuzuner N. dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88(2):253–271. doi: 10.1016/j.critrevonc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Joly P., Richard C., Gilbert D. sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 2000;43(4):619–626. doi: 10.1067/mjd.2000.107488. [DOI] [PubMed] [Google Scholar]
- 4.Mihai S., Sitaru C. immunopathology and molecular diagnosis of autoimmune bullous diseases. J Cell Mol Med. 2007;11(3):462–481. doi: 10.1111/j.1582-4934.2007.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sami N., Ahmed A.R. Penile pemphigus. Arch Dermatol. 2001;137(6):756–758. [PubMed] [Google Scholar]
- 6.Witte M., Zillikens D., Schmidt E. Diagnosis of autoimmune blistering diseases. Front Med (Lausanne) 2018;5:296. doi: 10.3389/fmed.2018.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amagai M., Nishikawa T., Nousari H.C., Anhalt G.J., Hashimoto T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen)are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J Clin Invest. 1998;102(4):775–782. doi: 10.1172/JCI3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Bu D.F., Li T. autoantibody production from a thymoma and a follicular dendritic cell sarcoma associated with paraneoplastic pemphigus. Br J Dermatol. 2005;153(3):558–564. doi: 10.1111/j.1365-2133.2005.06599.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Deng H., Mao M. Paraneoplastic pemphigus and myasthenia gravis associated with inflammatory pseudotumor like follicular dendiritc cell sarcoma: response to rituximab. Clin Case Rep. 2016;4(8):797–799. doi: 10.1002/ccr3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]