Abstract
Objective:
To investigate whether endometrial thickness and endometrial blood flow on the day of hCG administration is a predictor of intrauterine insemination (IUI) success.
Method:
A cross-sectional prospective clinical study with simple randomized sampling; Patient: 100 women experiencing the IUI cycle; Interventions: a comparison was made between pregnant and non-pregnant patients in terms of the endometrial thickness and pattern as well as the color Doppler flow on the day of hCG administration and also cycle parameters. Main outcome measures: endometrial thickness and patterns as well as the blood flow in color Doppler.
Results:
With the overall pregnancy rate being 38%, it was demonstrated that the endometrial blood flow was significantly greater in the cycle pregnancy obtained on the day of hCG administration, yet it was realized that the endometrial thickness and pattern of sonography did not have any predictive values for endometrial receptivity . In a multivariate analysis, the pregnancy rate was affected by the following variables: the duration of infertility, the women's age, the type number of IUI cycles, the number of injections to stimulate dominant follicles, and the sperm count. In the current study, the variability was realized to be of no predictive values for the IUI outcome, yet the endometrial flow in color Doppler was found to be positively connected with the pregnancy outcome.
Keywords: Color Doppler, eendometrial thickness, endometrial blood flow
Introduction
Statement of the problem
During ovulatory cycles, endometrial blood flow and endometrial thickness are variable.[1] After mensturation endometrium is thin and becomes thicker gradually. Many studies suggest affecting factors on endometrial thickness and endometrial blood flow like age, etiology of infertility, estradiol level.[2,3]
Several methods are employed for the treatment of infertility, with the oldest one being intrauterine insemination (IUI) as well as artificial insemination;[3,4] the major sings of IUI are the cervical factor, male infertility, and difficulties impeding the movement of the sperms toward an oocyte.[4]
Some ultrasound factors, including uterine endometrial thickness and the blood flow have been examined to evaluate for uterine receptivity.[5] A handful of studies have highlighted the blood content as a major yardstick for the acceptance by the uterus; some studies have also identified uterine blood and the endometrial flow as parameters affecting the enhancing of the probability of pregnancy in the course of in vitro fertilization (IVF) cycles.[6] Although many researches have emphasized the ultrasound pattern and endometrial thickness on the hGG injection day, there is no agreement about the efficacy of such factors throughout the IUI cycles.[7] The aim of this current study was to determine the effect of endometrial thickness and endometrial blood flow on pregnancy in intrauterine insemination cycles.
Material and Method
Study type: This study is a cross-sectional prospective one where IUI candidate patients have been selected in a randomized simple sampling method.
The current study includes 100 patients aged 21--38 who visited Shahihd Akbar Abadi Infertility Center from September 2015 to August 2018. The stimulation protocol was individually chosen on the basis of parameters such as age, the history of infertility, diagnosis, and the medical status. If the diameter of the biggest follicle in the follicular phase was smaller than 10, the patient would be subjected to COH via the administering of gonadotropin with follicle stimulating hormone (FSH) or HMG. The primary dose of gonadotropin amounted to 150--450 iu, and it changed on the basis of the FSH level, the patient's age, and antral follicles. If there were two follicles bigger than 16 mms, 10,000 units of hGG would be prescribed; on the hCG administration day, the patients were exposed to transvaginal ultrasound and Doppler in order to evaluate endometrium, endometrial thickness, and the flow pattern of blood vessels; 36--42 h following the hCG injection time, IUI was carried out. Afterwards, the blood samples were collected on day 14 following IUI, and the headline more than 15 got considered to be positive. Clinical pregnancy was identified on the basis of the detecting of the fetal heart rate at the 6th week of pregnancy using transvaginal ultrasound, and the patients were divided into two pregnant and non-pregnant groups; the mentioned groups were juxtaposed with each other in terms of different factors such as the stimulation duration, endometrial thickness, age, the infertility duration, the number of injections, ultrasound, and the endometrial vascular flow pattern. Following hCG administration, endometrial thickness, the vascular flow, and its pattern were measured using transvaginal ultrasound and Doppler, 15 min following the patients had depleted their bladder and taken some rest (ultrasound with Siemens G50, probes 4/9 MHz). The entire patients had undergone ultrasound from 9 a.m. to 11 a.m. by the same person.
Subjects were categorized into three categories in terms of endometrial thickness:
ET ≥7
7 <ET <14
ET ≤14
The endometrial ultrasound pattern was divided into three parts:
The triple-line, inclusive of the central hyperecho lines encircled by two hypoecho layers.
The average ISO echo scope and the central echogenic line encircled by roughly unknown boundaries.
Hyperechoic endometrium as well as homogeny.
The endometrial blood flow was explained in the three ways as follows:
Lack of flow
Low blood flow
Noticeable vascular flow
Inclusion criteria were as follows:
The patients’ consent.
IUI candidates (cervical problems, unexplained infertility, cervical mucus, or having past issues in the cervix), male factor, and polycystic ovarian syndrome (PCO);
Being 21--38 years old.
Exclusion criteria included:
Endometrial polyps;
Uterine anomalies;
Any uterine changes that disorient the endometrial view.
Results
The current research was comprised of 100 women with infertility problems who underwent IUI; 83 patients (83%) were aged less than 35 and 17 patients (17%) aged over 35. 75 patients were inflicted with primary infertility, and 25 patients were admitted to the center due to secondary causes. The subjects were also assessed in terms of infertility causes, and based on the results, 54 patients (54%) were reported for male reasons, 42 cases (42%) for polycystic ovary, 3 patients (3%) for being over the average age, and one case for an unidentified cause.
Blood samples were taken to assess βhCG criteria, and values over 15 were regarded as pregnant; serum samples of 36 subjects with βhCG criteria were positive, and those patients were regarded as pregnant. Ultrasound was performed on the patients for 6 weeks, and in case of the existence of fetal Heart rate (FHR), they would be diagnosed with clinical pregnancy. Reasoning from this fact, the fetal heart rate was verified for all 36 cases, and the certain diagnosis of pregnancy was established.
The average infertility duration was 1/6 ± 3/7 years for pregnant women and 2/9 ± 4/2 for non-pregnant ones; the independent t-test demonstrated no considerable difference between them (P = 0/310) [Figure 1]. 28 pregnant women had less than six injections, and seven subjects had over six injections to treat the related infertility; in the group connected with non-pregnant women, 55 subjects received less than six injections, while nine individuals received over six injections. Data collected were analyzed using the Chi-square test, and no significant difference was indicated between the two groups (P = 0/240) [Figure 2]. The values of base FSH were 3/5 ± 6/3 in pregnant women, and 5/5 ± 6/1 in non-pregnant ones. Data analysis using the independent t-test indicated no meaningful difference in the basal FSH serum of the two groups (P = 0/176). Six women (6/16%) in the pregnant group and 11 (2/17%) individuals in the non-pregnant group were over 35 years old. Data analysis using the Chi-square test indicated no meaningful difference between the two groups, either (P = 0/614).
Figure 1.
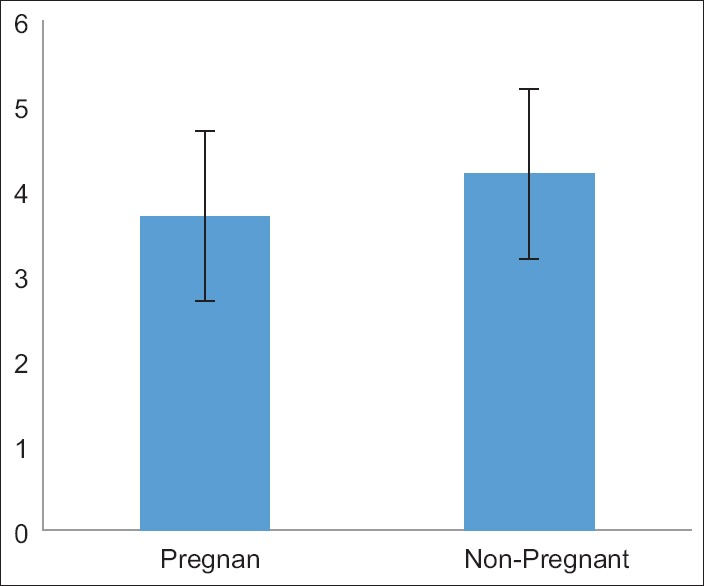
The duration of infertility
Figure 2.
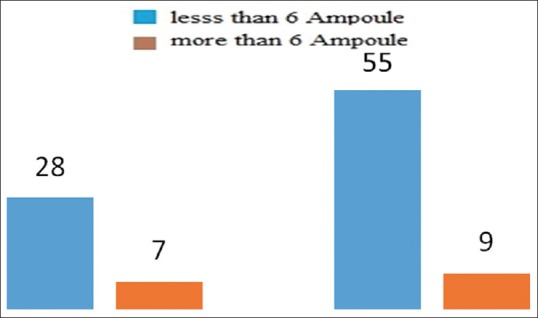
Number of shots taken (blue: less than 6; red: more than 6 shots)
In the end, the two pregnant and non-pregnant groups of patients were juxtaposed with each other in terms of the endometrial blood flow, endometrial thickness, and the pattern of endometrial ultrasound. Using the three following ways, endometrial thickness was defined in the pregnant women group, where 21 (58/3%) of subjects existed with the endometrial thickness of less than 7, 14 (38/9%) individuals with the endometrial thickness of between 7 and 14, and an individual with the thickness of over 14; in the non-pregnant group, there were 23 patients (35/9%) with the endometrial thickness of less than 7 and 41 patients (64/1%) with the endometrial thickness of 7--14 [Figure 3]. Data analysis via the Chi-square test demonstrated meaningful differences in the endometrial thickness between the two groups (P = 0/029)
Figure 3.
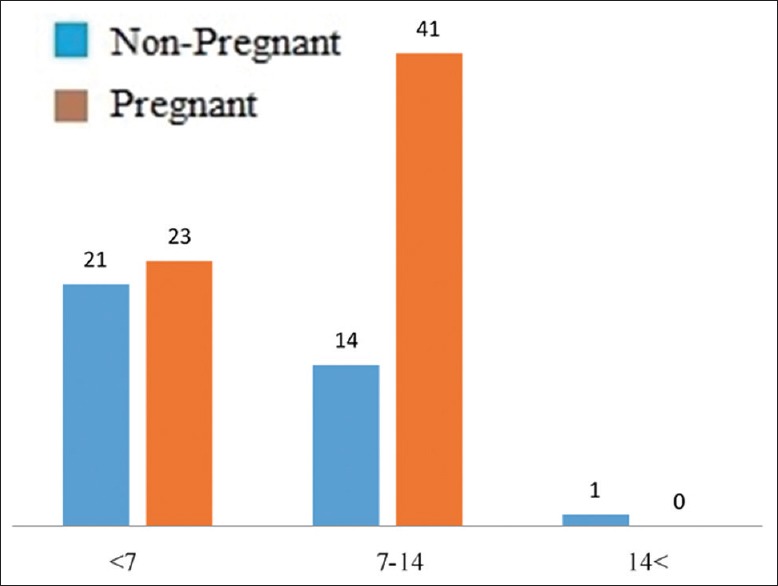
Endometrial thickness in pregnant and non-pregnant patients (blue: pregnant; red: non- pregnant)
The endometrial blood flow was measured using the method of Doppler ultrasonography. On this subject, in the pregnant women group, 21 patients (58/3%) were diagnosed with a meaningful blood flow and 15 subjects (41/7%) with a mild blood flow; in the non-pregnant women group, 17 patients (26/2%) were diagnosed with no flow, 36 patients (56/2%) with a mild flow, and 11 of them (17/2%) with a meaningful flow [Figure 4]. Data analysis using the Chi-square test demonstrated a meaningful difference between the two groups as regards the endometrial blood flow (P = 0/00)
Figure 4.
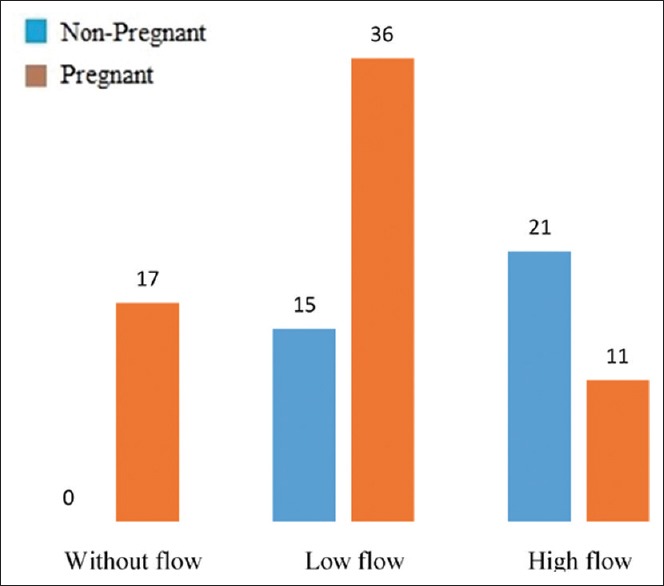
Vascular endometrium flow in pregnant and non-pregnant patients
The endometrial ultrasound pattern was represented in brief as A, B, and C. In the pregnant women group, 20 individuals (55/5%) were described as pattern A, 13 individuals (36/1%) as pattern B, and 3 individuals (8/4%) as pattern C. Data analysis using the Chi-square test also indicated no meaningful differences between the groups in terms of the endometrial ultrasound pattern (P = 0/198).
Discussion
In accordance with the results of the current study, we evaluated endometrial thickness within 3 ranges of ≤7, 7<ET≤14 and >14 mm. We did not find correlation between endometrial thickness and pregnancy. In this stydy endometrial blood flow in the patients who got pregnant using IUI cycles is considerably different from that of the ones who did not become pregnant. Women who had a more favorable cycle showed a higher endometrial vascular flow rate as against the non-pregnant ones; nevertheless, endometrial thickness was higher in the majority of non-pregnant subjects compared with pregnant ones; the non-pregnant women's endometrial thickness was between 7-14 mm, whereas in pregnant women it was less than 7 mm.
In accordance with the current study, older ages are not connected with negative pregnancy outcomes; such findings are not in conformity with the results of the research done by Esmaeilzade who assessed the pregnancy outcome and endometrial thickness and reported that age was adversely connected with the pregnancy outcome in women experiencing IUI.[1] Secretory and proliferative changes are required to carry out successful IUI.[1,2] The results of the study carried out by Esmaielzadehzadeh et al., being consistent with the results of the current paper, indicated a considerable difference in the mean of the endometrial thickness and the pregnancy outcome in the IUI cycles.[1] As regards the results of the study carried out by Yaman et al., through measuring the endometrial volume using the three-dimensional ultrasound, pregnancy can be predicted in assisted reproductive technology (ART) cycles.[2] Tsai et al. realized that women with the ultrasound demonstrating a triple-line pattern got pregnant more commonly compared with women without this pattern.[3] Kovaces et al. reported that at least a 10-mm increase in the endometrial thickness can be directly connected with the increased pregnancy rate.[4] Nevertheless, all researches have not achieved homogenous results, and a handful of them do not confirm any meaningful relationship between the pregnancy outcome and endometrial thickness.[5,6,7] As regards the results of the study carried out by Yaman et al., Sonographic measurement of endometrium thickness on the day of administring human chorionic gonadotropin is not useful in predicting IVF outcome.[8] Weissman et al. reported that women who had less than or equal to 14 Endometrial (≤ 14) mm of endometrial thickness on the hCG injection day[9] had a lower implantation and pregnancy rate,[9] while Dietterich et al. did not identify any negative effect produced by endometrial thickening (<14 millimeters) on the rates of pregnancy, implantation, and abortion.[10]
Reuter et al. reached the conclusion that the higher number of follicles (up to three) and the endometrial thickness of at least 8 mm were connected with the higher rates of pregnancy.[11] In accordance with the results of the study carried out by Tomlinson et al., endometrial thickness is considered as one of the major variables for the prediction of the pregnancy outcome.[12]
In another study carried out by Habibzadeh et al., the results indicated no connection between the number of follicles, the age, gonadotropin injections, and endometrial thickness; nevertheless, the pregnancy probability is higher in the endometrial thicknesses of 6--10 mm, with age not taken into account.[13] On the basis of the results of the studies carried out by Kamath et al. although the pregnancy rate in the endometrial thickness of over 6 mm reported a higher pregnancy probability, the difference was regarded as insignificant.[14]
In the study carried out by Ghosh et al., the probability of pregnancy in subjects being over 30 years old was reported to be half of the ones being less than 30 years old.[15] Nevertheless, another study carried out by Iberico et al. on 1,010 IUI cycles demonstrated that age cannot significantly predict the pregnancy outcome.[16] This finding is in line with the findings of the current study, also verified by Geyter et al.[17]
Thus endometrial thickness is independent of these factors: (age, number of gonadotropin ampule and FSH level.[18] There are still controversial results about the role of endometrial thickness, pattern, volume, and vascularity in endometrial receptivity.[19,20] The patients with detectable endometrial blood flow had higher clinical pregnancy rates and implantation rates.[21] Aghahoseini M, et al. concluded that Endometrial-subendometrial blood flow (according the findings of Doppler ultrasonography) in women undergoing ART could not predict endometrial receptivity and IVF outcome.[22] Riad et al. reported that The presence of subendometrial flow is associated with successful IUI in women under stimulated cycles undergoing IUI. HMG seems a superior option for induction of ovulation regarding success of implantation.[23] Hock et al. assessed endometrium making use of ultrasound; they realized that a homogeneous pattern on the hCG injection day is connected with the reduced pregnancy rate, yet the case is not relevant to the triple-line pattern.[24] Javedani masroor et al. reported that endometrial thickness does not have the potential for the prediction of the success of the IUI cycles.[25]
The small sample size of the women included in this study can be regarded as one of its limitations; furthermore, the sperm quality should have already been evaluated.
Conclusion
No conclusive cut-off value of endometrial thickness has been established in order to help clinicians in counseling the couple about pregnancy outcome. Using ultrasound is a suitable and efficacious method of assessing the endometrial and uterine blood flow; in general, it appears that the vascular endometrium flow could have the potential for the predicting of the results of IUI cycles. However, wider studies are required to obtain applicable results.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study has been supported only by the current authors. Authors are of no financial interest in the materials presented in the manuscript.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
There is no contributor to this study apart from the authors referred to on the first page of this paper. Also, nobody has supported this article financially or by material. The manuscript is the original work of the authors. All data, figures, tables, etc., used in the manuscript are originally provided by the current authors, otherwise the sources have been mentioned and a reprint permission can be attached.
References
- 1.Esmailzadeh S, Faramarzi M. Endometrial thickness and pregnancy outcome after intrauterine insemination. Fert Steril. 2007;88:432–7. doi: 10.1016/j.fertnstert.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Yaman C, Ebner T, Sommergruber M, Polz W, Tews G. Role of three-dimensional ultrasonographic measurement of endometrium volume as a predictor of pregnancy outcome in an IVF-ET program: A preliminary study. Fertil Steril. 2000;74:797–801. doi: 10.1016/s0015-0282(00)01493-x. [DOI] [PubMed] [Google Scholar]
- 3.Tsai HD, Chang CC, Hsieh YY, Lee CC, Lo HY. Artificial insemination. Role of endometrial thickness and pattern, of vascular impedance of the spiral and uterine arteries, and of the dominant follicle. J Reprod Med. 2000;45:195–200. [PubMed] [Google Scholar]
- 4.Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18:2337–41. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 5.Rieger L, Honig A, Sutterlin M, Kapp M, Dietl J, Ruck P, et al. Antigen-presenting cells in human endometrium during the menstrual cycle compared to early pregnancy. J Soc Gynecol Investig. 2004;11:488–93. doi: 10.1016/j.jsgi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Kolibianakis EM, Zikopoulos KA, Fatemi HM, Osmanagaoglu K, Evenpoel J, Van Steirteghem A, et al. Endometrial thickness cannot predict ongoing pregnancy achievement in cycles stimulated with clomiphene citrate for intrauterine insemination. Reprod Biomed Online. 2004;8:115–8. doi: 10.1016/s1472-6483(10)60505-6. [DOI] [PubMed] [Google Scholar]
- 7.Vlaisavljevic V, Reljic M, Gavric-Lovrec V, Kovacic B. Subendometrial contractility is not predictive for in vitro fertilization (IVF) outcome. Ultrasound Obstet Gynecol. 2001;17:239–44. doi: 10.1046/j.1469-0705.2001.00316.x. [DOI] [PubMed] [Google Scholar]
- 8.Yaman C, Ebner T, Jesacher K, Sommergruber M, Radner G, Tews G. [Sonographic measurement of endometrium thickness as a predictive value for pregnancy through IVF] Ultraschall Med. 2002;23:256–9. doi: 10.1055/s-2002-34053. [DOI] [PubMed] [Google Scholar]
- 9.Weissman A, Gotlieb L, Casper RF. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertil Steril. 1999;71:147–9. doi: 10.1016/s0015-0282(98)00413-0. [DOI] [PubMed] [Google Scholar]
- 10.Dietterich C, Check JH, Choe JK, Nazari A, Lurie D. Increased endometrial thickness on the day of human chorionic gonadotropin injection does not adversely affect pregnancy or implantation rates following in vitro fertilization-embryo transfer. Fertil Steril. 2002;77:781–6. doi: 10.1016/s0015-0282(01)03276-9. [DOI] [PubMed] [Google Scholar]
- 11.Reuter KL, Cohen S, Furey L, Baker S. Sonographic appearance of the endometrium and ovaries during cycles stimulated with human menopausal gonadotropin. J Reprod Med. 1996;41:509–14. [PubMed] [Google Scholar]
- 12.Tomlinson MJ, Amissah-Arthur JB, Thompson KA, Kasraie JL, Bentick B. Prognostic indicators for intrauterine insemination (IUI): Statistical model for IUI success. Hum Reprod. 1996;11:1892–6. doi: 10.1093/oxfordjournals.humrep.a019513. [DOI] [PubMed] [Google Scholar]
- 13.Habibzadeh V, Nematolahi Mahani SN, Kamyab H. The correlation of factors affecting the endometrial thickness with pregnancy outcome in the IUI cycles. Iran J Reprod Med. 2011;9:41–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath MS, Bhave P, Aleyamma T, Nair R, Chandy A, Mangalaraj AM, et al. Predictive factors for pregnancy after intrauterine insemination: A prospective study of factors affecting outcome. J Hum Reprod Sci. 2010;3:129–34. doi: 10.4103/0974-1208.74154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh C, Buck G, Priore R, Wacktawski-Wende J, Severino M. Follicular response and pregnancy among infertile women undergoing ovulation induction and intrauterine insemination. Fertil Steril. 2003;80:328–35. doi: 10.1016/s0015-0282(03)00601-0. [DOI] [PubMed] [Google Scholar]
- 16.Iberico G, Vioque J, Ariza N, Lozano JM, Roca M, Llacer J, et al. Analysis of factors influencing pregnancy rates in homologous intrauterine insemination. Fertil Steril. 2004;81:1308–13. doi: 10.1016/j.fertnstert.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 17.De Geyter C, Schmitter M, De Geyter M, Nieschlag E, Holzgreve W, Schneider HP. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1,186 infertile women. Fertil Steril. 2000;73:106–13. doi: 10.1016/s0015-0282(99)00484-7. [DOI] [PubMed] [Google Scholar]
- 18.Berek JS, Novak E. Lippincott Williams & Wilkins; 2007. Berek and Novak's Gynecology. [Google Scholar]
- 19.Yilmaz N, Kiliç S. Endometrial parameters in IVF and IUI administration on elderly women. Turk J Med Sci. 2010;40:343–8. [Google Scholar]
- 20.Kim A, Han JE, Yoon TK, Lyu SW, Seok HH, Won HJ. Relationship between endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound and pregnancy after intrauterine insemination. Fertil Steril. 2010;94:747–52. doi: 10.1016/j.fertnstert.2009.03.084. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Qiao J, Li R, Zhen X, Liu Z. Role of endometrial blood flow assessment with color Doppler energy in predicting pregnancy outcome of IVF-ET cycles. Reprod Biol Endocrinol. 2010;8:122. doi: 10.1186/1477-7827-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghahoseini M, Tuba K, Marsousi V, Aleyasin A. Assessment of endometrial-subendometrial blood flow detected by color Doppler sonography and uterine receptivity in infertile women. Acta Med Iran. 2008;46:461–6. [Google Scholar]
- 23.Riad ON, Hak AA. Assessment of endometrial receptivity using Doppler ultrasonography in infertile women undergoing intrauterine insemination. Gynecol Endocrinol. 2014;30:70–3. doi: 10.3109/09513590.2013.859668. [DOI] [PubMed] [Google Scholar]
- 24.Hock DL, Bohrer MK, Ananth CV, Kemmann E. Sonographic assessment of endometrial pattern and thickness in patients treated with clomiphene citrate, human menopausal gonadotropins, and intrauterine insemination. Fertil Steril. 1997;68:242–5. doi: 10.1016/s0015-0282(97)81509-9. [DOI] [PubMed] [Google Scholar]
- 25.Javedani Masrour M, Shafaie A, Yoonesi L, Aerabsheibani H, Javedani masrour S. Evaluating endometrial thickness and vascular ultrasound pattern and pregnancy outcomes in intrauterine insemination cycle. Asian journal of pharmaceutical research and health care. 2016;8:24–2. [Google Scholar]


