Abstract
The World Health Organization (WHO) has embarked on a consultation process to refine the 2030 goals for priority neglected tropical diseases (NTDs), onchocerciasis among them. Current goals include elimination of transmission (EOT) by 2020 in Latin America, Yemen and selected African countries. The new goals propose that, by 2030, EOT be verified in 10 countries; mass drug administration (MDA) with ivermectin be stopped in at least one focus in 34 countries; and that the proportion of the population no longer in need of MDA be equal or greater than 25%, 50%, 75% and 100% in at least 16, 14, 12, and 10 countries, respectively. The NTD Modelling Consortium onchocerciasis teams have used EPIONCHO and ONCHOSIM to provide modelling insights into these goals. EOT appears feasible in low-moderate endemic areas with long-term MDA at high coverage (≥75%), but uncertain in areas of higher endemicity, poor coverage and adherence, and where MDA has not yet, or only recently, started. Countries will have different proportions of their endemic areas classified according to these categories, and this distribution of pre-intervention prevalence and MDA duration and programmatic success will determine the feasibility of achieving the proposed MDA cessation goals. Highly endemic areas would benefit from switching to biannual or quarterly MDA and implementing vector control where possible (determining optimal frequency and duration of anti-vectorial interventions requires more research). Areas without loiasis that have not yet initiated MDA should implement biannual (preferably with moxidectin) or quarterly MDA from the start. Areas with loiasis not previously treated would benefit from implementing test-and(not)-treat-based interventions, vector control, and anti- Wolbachia therapies, but their success will depend on the levels of screening and coverage achieved and sustained. The diagnostic performance of IgG4 Ov16 serology for assessing EOT is currently uncertain. Verification of EOT requires novel diagnostics at the individual- and population-levels.
Keywords: onchocerciasis, elimination of transmission, mass drug administration, ivermectin, alternative treatment strategies, EPIONCHO, ONCHOSIM, NTD Modelling Consortium
Abbreviations
ABR, annual biting rate; APOC, African Programme for Onchocerciasis Control; ATS, alternative treatment strategies; DALY, disability-adjusted life-year; EMC, Erasmus Medical Center; EOT, elimination of transmission; EPHP, elimination as a public health problem; ICL, Imperial College London; IU, implementation unit; MAP, maximum a posteriori (parameter set); MDA, mass drug administration; NTD, neglected tropical disease; OCP, Onchocerciasis Control Programme in West Africa; PCR, polymerase chain reaction; pOTTIS, provisional operational thresholds for treatment interruption and initiation of surveillance; PPV, positive predictive value; SSA, sub-Saharan Africa; Ta(N)T, test-and-not-treat; TaT, test-and-treat; WHO, World Health Organization; YLD, years of life lost to disability.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of the World Health Organization. Publication in Gates Open Research does not imply endorsement by the Gates Foundation.
Background
Onchocerciasis is a filarial disease caused by infection with Onchocerca volvulus, a vector-borne parasitic nematode transmitted via the bites of several Simulium blackfly species. In sub-Saharan Africa (SSA), where 99% of the cases occur, the main vectors belong to the Simulium damnosum sensu lato species complex. Modelling of data from the 2013 Global Burden of Disease Study suggests that at least 17 million people are currently infected with O. volvulus 1. An estimated 198 million people live in areas where there is potential for transmission of the parasite, although this number may increase as the mapping of areas of low transmission is finalized 2. The disease is also known as river blindness because blackflies breed in and bite near fast-flowing rivers, and because the most devastating sequela is irreversible loss of vision. High infection load, measured by the density of microfilariae (the larval progeny of adult worms) in the skin, is associated both with blindness incidence 3 and excess human mortality, the latter over and above that due to blindness 4, 5. In addition to ocular sequelae, onchocerciasis is also responsible for skin disease and troublesome itching (which disturbs sleep and work patterns). The Global Burden of Disease Study 2015 estimated the disability-adjusted life years (DALYs) due to onchocerciasis as 1,136,000 in 2015 (a 21% decrease since 2005) 6. More recently, the term river epilepsy has been used to highlight the association between onchocerciasis and nodding syndrome (a condition in the epilepsy spectrum) 7. The burden of onchocerciasis-associated epilepsy for 2015 was estimated as circa 13% of the total years lost due to disability (YLDs) attributable to onchocerciasis and 10% of those attributable to epilepsy 8.
Currently, the mainstay of onchocerciasis control is through mass drug administration (MDA) of the microfilaricidal drug ivermectin (Mectizan®), donated by Merck & Co. Inc. to endemic countries through the Mectizan Donation Program 9. Ivermectin MDA (mostly of annual frequency in SSA) started in the late 1980’s, and great strides in reducing morbidity have been made 10. Although questions were raised concerning the feasibility of eradicability based on ivermectin MDA alone 11– 13, studies in some foci of Mali and Senegal (in the western extension of the Onchocerciasis Control Programme in West Africa, OCP), indicated that local elimination may be achieved after 15–17 years of ivermectin MDA 14, 15. Based on this, in 2010, the African Programme for Onchocerciasis Control (APOC) shifted its goals from elimination of the disease as a public health problem (EPHP), to elimination of transmission (EOT) 16. This shift necessitates a drastic geographical extension of treatment. Whilst for EPHP, mesoendemic and hyperendemic areas had been prioritized for ivermectin MDA, EOT at a Pan-African scale requires that treatment be distributed also in hypoendemic areas. This poses challenges, particularly where loiasis (a filariasis caused by Loa loa) is co-endemic due to severe adverse events, including fatalities, that may result from microfilaricidal treatment of individuals heavily infected with L. loa 17. In onchocerciasis, the original definition of endemicity levels comprises three categories: i) hypoendemic (microfilarial prevalence <30–35%), ii) mesoendemic (prevalence between 30–35% and 60%), and iii) hyperendemic (≥60%) 18; EOT should be achieved in all three. A microfilarial prevalence ≥80% has also been used to indicate holoendemicity 19.
EOT is formally defined as a reduction to zero in the incidence of infection (rate at which new cases arise) 20. However, since in macroparasite epidemiology the number of parasites in the population is more relevant than the number of cases, the definition of EOT used here is the absence of adult worms and larval stages (in humans and vectors) after cessation of interventions 21. Onchocerciasis is in the fortunate position that ivermectin reduces: i) morbidity (mainly caused by microfilariae), ii) microfilarial prevalent cases and microfilarial load, and iii) transmission to vectors (microfilariae are the stages infective to blackflies). This is achieved by a combination of ivermectin’s microfilaricidal effect, temporary embryostatic effect on adult female worms (macrofilariae) 22, and irreversible effects on female worm fecundity and/or survival 23, 24. Given the disease- and transmission-curtailing benefits of ivermectin MDA, the 2012 World Health Organization (WHO) roadmap to accelerate progress in combating neglected tropical diseases (NTDs) proposed that onchocerciasis be eliminated in selected African countries by 2020 25. The Joint Action Forum of APOC reformulated this goal to EOT in 80% of endemic African countries by 2025 26.
To help evaluate progress towards these goals, the developers of two mathematical models of onchocerciasis transmission dynamics and control (EPIONCHO and ONCHOSIM) have joined forces under the umbrella of the Bill & Melinda Gates Foundation-funded NTD Modelling Consortium. The modelling groups, based at Imperial College London (ICL), UK, and Erasmus Medical Center (EMC), The Netherlands, along with multi-disciplinary collaborators have led the recent onchocerciasis work. Firstly, a comparison of modelling assumptions and resulting outputs was conducted 27, highlighting the need to present and discuss the models jointly 28, and modify some key assumptions crucial to fitting the microfilarial prevalence trends observed in the elimination studies of Mali and Senegal 29. A policy paper followed, discussing the role of intervention strategies other than annual ivermectin MDA to achieve elimination in SSA 30. A further understanding of critical uncertainties led to a paper discussing these in light of data needs 31. Due to knowledge gaps surrounding the processes regulating parasite establishment and fecundity of O. volvulus 32– 34, the magnitude of exposure heterogeneity and its interaction with such regulatory processes 31, and the impact of treatment on population biology parameters, including the development of acquired immunity 35, the models have contrasting underlying assumptions leading to differences in model predictions 29, 30. Despite these differences, the models generally agree on the treatment strategies required to achieve EOT.
Moving towards the post-2020 goals, new NTD goals have been proposed by the WHO for 2030 within the framework of a consultation process. Table 1 summarizes the current (2020) and proposed (2030) goals for onchocerciasis, the scenarios in which both models agree that EOT is technically feasible with current interventions, the alternative treatment strategies (ATS 36, 37) required when current ones are not sufficient, the suitability of available tools for evaluating EOT, the principal uncertainties driving differences in model outputs, and the biggest risks associated with the proposed 2030 goals.
Table 1. Summary of modelling insights and challenges for reaching the World Health Organization (WHO) 2030 goals for onchocerciasis.
| Current WHO Goal (2020 Goal) | Elimination of transmission (EOT) by 2020 in Latin America, Yemen, selected African countries. |
| Proposed New WHO Goal (2030
Goal) |
Verified EOT in 10 countries; stopped mass drug administration (MDA) in at least one focus in 34
countries; stopped MDA in ≥ 25%, ≥50%, ≥75% and 100% of population in >16, >14, >12, >10 countries, respectively. |
| Is the new target technically
feasible under the current intervention strategy? |
EOT feasible in areas with low or moderate endemicity, where MDA has been ongoing for several
years; uncertain for hyper- and holoendemic areas or where MDA has not yet started (distribution of pre-intervention prevalence in different countries will determine feasibility of stopping MDA goals). |
| If not, what is required to achieve
the target? (updated strategy, use of new tools, etc.) |
Hyper- and holoendemic areas: switch to biannual or quarterly MDA, complementary vector control;
areas without loiasis not previously treated: biannual MDA, quarterly MDA, moxidectin. Areas with loiasis not previously treated: Ta(N)T *, vector control, TaT** with anti- Wolbachia therapies. |
| Are current tools able to reliably
measure the target? |
Sensitivity and specificity of standardized IgG4 antibody tests to Ov16 antigen for assessing EOT
are uncertain. Diagnostic tools for detection of reproductively active adult worms are needed. |
| What are the biggest unknowns? | Whether parasite acquisition becomes more efficient with declining transmission intensity; age- and
sex-dependence and individual heterogeneity in exposure; additional impact of vector control; dynamics of Ov16 antibody responses; human/vector movement. |
| What are the biggest risks? | EOT may not be feasible with current tools in hyper- and holoendemic areas; risk of resurgence if
MDA is stopped prematurely; interruption/late commencement of MDA; population movement. |
*Ta(N)T: Test-and-not-treat: test for Loa loa microfilaraemia load (e.g. with LoaScope, and if ≥20,000 microfilariae/ml blood (associated with severe adverse events following microfilaricidal treatment 17), not treat with ivermectin 39. TaT: Test-and-treat: test for O. volvulus (e.g. with skin snips) and if positive treat with anti- Wolbachia (e.g. doxycycline) therapy ( L. loa lacks Wolbachia).
Insights gained from quantitative and mathematical modelling analyses
1) Initial model comparison: Predictions from EPIONCHO and ONCHOSIM were compared over a range of baseline endemicities and treatment scenarios. In particular, comparisons were made on: 1) microfilarial prevalence and intensity during 25 years of (annual or biannual) MDA with ivermectin; 2) required duration of treatment to bring microfilarial prevalence below a provisional operational threshold for treatment interruption (pOTTIS) of 1.4% 16, 38; and 3) required duration to drive the parasite population to local elimination (defined by stochastic fade-out in ONCHOSIM and passing the transmission breakpoint in EPIONCHO). In mesoendemic areas, the models predicted that the provisional operational prevalence threshold could be reached with annual MDA. In highly hyperendemic areas, the models indicated that annual MDA would be insufficient. In lower endemicity settings, ONCHOSIM predicted that the time needed to reach 1.4% microfilarial prevalence would be longer than that required to reach local elimination; the opposite was true for higher endemicity settings. In EPIONCHO, the pOTTIS was reached consistently sooner than the breakpoint 27.
2) Modelling elimination studies: Based on the initial model comparison, technical refinements were implemented (e.g. age-dependent adult worm mortality in EPIONCHO). EPIONCHO and ONCHOSIM projections were tested against microfilarial prevalence data from the two foci in Mali and Senegal where the observed prevalence had been brought to zero in 2007–2009 after 15–17 years of ivermectin MDA only 14, 15. Model projections were trained using longitudinal (microfilarial prevalence) data from 27 communities in two transmission foci, incorporating programmatic information (treatment frequency, duration and coverage), and evaluating whether the projected outcome was elimination (local parasite extinction) or resurgence. The epidemiological trends during MDA were captured by both models but resurgence was predicted by EPIONCHO in some communities of the River Gambia focus in Senegal with the highest (inferred) annual biting rates (no. vector bites/person/year) and associated pre-intervention endemicities ( Figure 1 and Figure 2) 29. Resurgence was never predicted by ONCHOSIM.
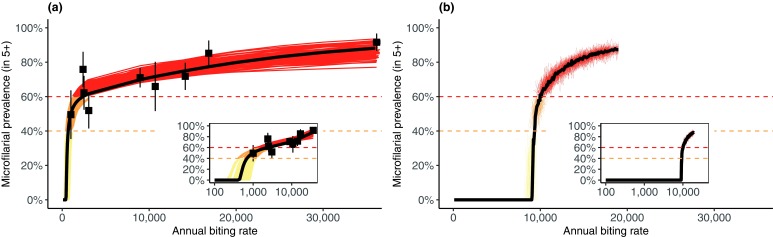
Figure 1. Modelled relationship between annual biting rate (ABR) and endemic microfilarial prevalence.
In EPIONCHO ( a), the coupled ABR-prevalence data are from nine communities in northern Cameroon, with each ABR measured as an average from multiple years and locations within and around each community, weighted by the proportion of time community residents spent at these locations 40. Each thin line corresponds to an EPIONCHO parameter set identified by a sampling importance resampling procedure to account for parametric uncertainty. These are colored sequentially from yellow (hypoendemic) to red (hyperendemic). The thick black line corresponds to the parameter set that achieved the highest likelihood. In ONCHOSIM ( b), the thin lines correspond to stochastic realizations using the default parameter set 21, colored sequentially according to endemicity category; the thick black line is the median of 500 simulations. The coupled ABR-prevalence data are not shown in ( b) because ONCHOSIM has not been re-fitted to these data. ABR = No. blackfly bites/person/year. This figure has been reproduced from 29 under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license.
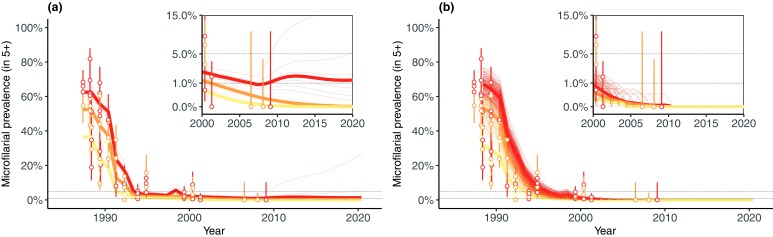
Figure 2. Observed and modelled microfilarial prevalence dynamics in 14 communities from the River Gambia focus, Senegal.
Left ( a) and right ( b) panels show, respectively, EPIONCHO and ONCHOSIM projections. The thin lines correspond to community-specific simulations using maximum likelihood estimates of the community-specific ABRs and either the maximum a posteriori (MAP) parameter set (EPIONCHO) or the default parameter set (ONCHOSIM). The estimated ABRs and MAP parameter sets were derived using the complete longitudinal sequence of microfilarial prevalence for each community. For ONCHOSIM there are many stochastic projections for each community; for EPIONCHO there is a single deterministic projection for each community, corresponding to the MAP parameter set. The thick solid lines show the median dynamics by endemicity category (yellow: hypoendemic; orange: mesoendemic; red: hyperendemic). In the River Bakoye focus of Mali, no resurgence was predicted by either model using the entire longitudinal microfilarial prevalence set. Panel insets show the period between 2000 and 2020 using a transformed y-axis for a better visual appraisal of the model projections compared to the data close to zero. This figure has been reproduced from 29 under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license.
3) Alternative treatment strategies: ONCHOSIM and the refined version of EPIONCHO 29 were used to simulate trends in microfilarial prevalence for 80 different settings. These settings were defined by their baseline endemicity and past programmatic scenario (frequency and coverage of MDA) and future treatment scenarios defined by the frequency (annual, biannual, or quarterly), with or without vector control. Each strategy was assessed whether it eventually led to elimination 30. Both models predicted that in areas with 40%–50% pre-control microfilarial prevalence and ≥10 years of annual MDA, elimination may be achieved within a further 7 years without changing strategy. For areas with 70%–80% pre-control microfilarial prevalence, both annual and biannual MDA were predicted by ONCHOSIM (but not by EPIONCHO) to be sufficient strategies to reach elimination by 2025. The likelihood that elimination will be reached thus depends on pre-control endemicity (i.e. transmission intensity), duration and frequency of past MDA, and the strategy implemented from now until 2025. Biannual or quarterly MDA will accelerate progress toward elimination but it cannot be guaranteed by 2025 in high-endemicity areas. In such areas, MDA plus concomitant vector control would be useful 30.
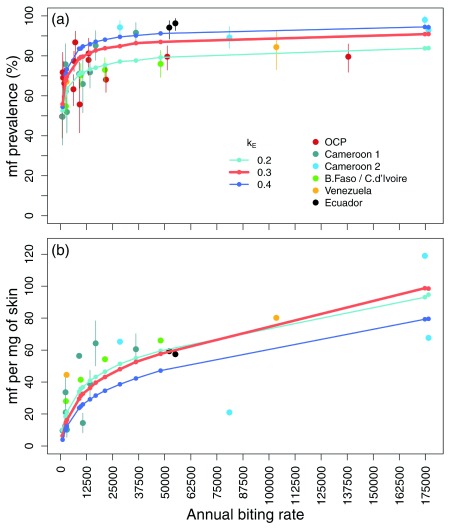
4) Resilience to interventions: Both models incorporate microfilarial density-dependent establishment of O. volvulus L1 larvae within savannah species of S. damnosum sensu lato (i.e. S. damnosum sensu stricto / S. sirbanum) 28. However, only EPIONCHO assumes transmission intensity-dependent parasite establishment within humans 31, 32. An individual-based, stochastic version of EPIONCHO (EPIONCHO-IBM) was developed to explore the interaction between individual heterogeneity in exposure to infection (via vector bites) and parasite establishment within humans (greater at lower transmission intensities and vice versa). Transmission intensity is measured by the annual rate of human exposure to infective (L3) larvae (no. L3 larvae/person/year). EPIONCHO-IBM was fitted (using Latin hypercube sampling) to matched (savannah) pre-intervention microfilarial prevalence, load and annual biting rate (ABR) data (extending Figure 1a range). Density dependence in parasite establishment within humans was estimated for different levels of (fixed) exposure heterogeneity ( Figure 3) 31. The interaction between overdispersion in exposure to blackfly bites (parameter k E) and density-dependent parasite establishment stabilizes low (hypoendemic) prevalence, but accentuates resilience to MDA, explaining the lower EOT probabilities predicted by EPIONCHO.
Figure 3. Observed and predicted pre-intervention microfilarial prevalence and intensity vs. annual biting rates (ABRs).
EPIONCHO-IBM-predicted (solid lines) ( a) microfilarial prevalence (percent) and ( b) intensity (mean no. of microfilariae, mf, per mg of skin) for ABRs in the epidemiological dataset (solid color circles), using the estimated parameters. The overdispersion exposure parameter (of a gamma distribution) k E was varied between 0.2 (stronger aggregation of blackfly bites among humans) and 0.4 (lesser aggregation). A value of k E = 0.3 provided the best overall fit. Error bars are 95% confidence intervals (bootstrapped for intensity when raw data were available). Fitting data are from Cameroon [1] 40, [2] 41, Burkina Faso/Côte d’Ivoire 42; validation data are from the Onchocerciasis Control Programme in West Africa (OCP) 43, Venezuela 44 and Ecuador 45 (the latter two for vectors with similar vector competence to S. damnosum sensu stricto). This figure has been reproduced from 31 under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
In view of (1)–(4) above, Box 1 summarizes the insights gained thus far and their implications for post-2020 goals.
Box 1. Lessons learned from onchocerciasis modelling and implications for the post-2020 goals.
Elimination of transmission (EOT) prospects depend strongly on local transmission conditions. The required duration of interventions increases and the probability of reaching EOT decreases with higher baseline endemicity, higher vector biting rates, and stronger aggregation of infection in the human host population (due to exposure heterogeneity) 27, 29, 31.
Programme effectiveness is important. Program duration increases, and probability of EOT decreases, with lower coverage and higher systematic non-adherence 46, 47.
Current strategies may not be sufficient. Implementation of current strategies (annual mass drug administration [MDA], with at least 65% coverage) would lead to long timelines to EOT in some countries. Even with the more optimistic model (ONCHOSIM), some countries would need to continue their programs until 2045 48.
Alternative treatment strategies (ATS) to accelerate EOT. a) Improve MDA coverage (to 80%); b) minimize systematic non-adherence; c) increase MDA frequency (to biannual or quarterly) 30, 46; d) use more efficacious treatment regimens (moxidectin 49, anti- Wolbachia drugs 50; e) where feasible, implement additional vector control (e.g. ground-based larviciding of vector breeding sites; slash-and-clear of vegetation substrates of vector immature stages; adult blackfly traps) 30, 51, 52.
Key uncertainties. EPIONCHO and ONCHOSIM model outputs diverge concerning the feasibility of EOT in hyperendemic areas (60-80% microfilarial prevalence) with current or thus far modelled ATS strategies 30, reflecting uncertainty about key biological processes (e.g. those regulating parasite establishment within humans and exposure heterogeneity) 31. For holoendemic areas (80% prevalence and above), both models agree that current strategies will be insufficient.
Expansion of interventions into (currently untreated) hypoendemic areas. Work is ongoing to define efficient sampling strategies for delineation of new (hypoendemic) treatment areas, based on serology-based start-MDA thresholds. This is problematic in areas where loiasis is co-endemic, as ivermectin treatment can be fatal in individuals with high Loa loa microfilaraemia 17. A test-(for loiasis)-and-not-treat (if microfilaraemia is above dangerous thresholds) strategy can be used 39. Because the proportion of people with heavy L. loa microfilaraemia is often small (~2–4%), models predict that this strategy could lead to EOT if screening and treatment coverage/adherence are high.
Practical implications of the currently proposed EOT goals
Measuring the target
1) Definition of EOT according to the models.
EOT is modelled as absence of parasites in humans and flies 50 years after treatment cessation 21. The probability of EOT is the proportion of runs out of the total number of simulations in which EOT is achieved. In practice, evaluating whether foci are in track to achieve EOT depends on the ability of current (and novel) diagnostics to measure accurately interruption of transmission. Indicators of exposure in children and fly samples have been suggested for such evaluation.
2) Diagnostics.
The WHO guidelines for verification of EOT require <0.1% IgG4 antibody seropositivity to the Ov-16 O. volvulus antigen in children younger than 10 years, and <0.05% positivity by pool screen PCR in at least 6,000 wild-caught flies (heads only) 53. Models suggest that the magnitude of the serological thresholds will depend on: i) local transmission conditions (e.g., the higher the ABR, the lower the thresholds will need to be and vice versa; this also applies to entomological indicators); ii) age-dependent exposure patterns (e.g. the younger the age at which exposure to transmission starts, and the greater the rate of increase with age, the younger the age group for which serological monitoring will be informative and vice versa; see below for sampling implications); iii) history of previous control interventions; iv) heterogeneity concerning the above within evaluation areas; v) spatial scale at which EOT is assessed; vi) desired positive predictive value (PPV, the probability of elimination below the said threshold) of EOT; vii) assumptions on parasite population regulation; and viii) diagnostic performance of the tests 54.
3) Sources of uncertainty in current threshold estimates.
A key uncertainty is the operation of regulatory processes upon within-human parasite establishment (e.g. due to acquisition of anti-L3 immunity in areas of intense transmission). Omitting such processes (ONCHOSIM) leads to higher (more lenient) thresholds; including them (EPIONCHO) results in lower (more stringent) thresholds. Adjusting modelling results by the diagnostic performance of the tests changes the apparent magnitude of the thresholds. Diagnostic performance (e.g. sensitivity/specificity) of current tests is another key uncertainty 54.
4) Sampling implications.
Modelling indicates that the childhood age group most informative for Ov-16 serology will depend on the age-specific patterns of exposure that prevail in the various foci. In areas where exposure increases quickly from birth, the 0–9yr age group may be suitable. In areas where exposure increases more slowly, the 5–14yr age group will be more informative. Deciding which age group to choose can be aided by inspecting baseline infection age profiles 55.
Timeline to achieve the target
1) Technical feasibility
General considerations. Elimination prospects depend strongly on local transmission conditions ( Box 1). The required strategy intensifies (e.g. the duration and/or frequency of interventions increases, and the need for complementary/ATS increases) with higher baseline endemicity, higher ABRs, and stronger aggregation of infection in the human population (acting as a core group maintaining transmission) 13, 27, 31.
Moderate transmission settings. EOT can be achieved with annual or biannual MDA if coverage and adherence are high (in the models, ‘enhanced’ coverage is defined as 80% therapeutic coverage and 1% systematic non-adherence).
Hyper- and holoendemic settings. The model-predicted feasibility of EOT is more uncertain, even when modelling the ATS considered thus far (increased coverage and frequency, vector control 30). The epidemiological impact of other ATS (e.g. moxidectin 49, macrofilaricides 50, 56) needs to be evaluated.
2) Operational feasibility
General considerations. Multiple intervention strategies will need to be combined in an optimal way to achieve continent-wide EOT. Further research is needed to identify such strategies according to epidemiological features 30.
Role of epidemiology/transmission ecology. The choice of intervention strategies will depend on epidemiological and ecological features. Hyperendemic areas would benefit from biannual/quarterly MDA if feasible to implement (non-loiasis co-endemic 57, 58; no sub-optimal responses to ivermectin 59); highly hyperendemic/holoendemic areas will likely need optimized ATS, including macrofilaricidal interventions 36, 37. The optimal ATS suite (vector control, moxidectin, anti- Wolbachia therapies, macrofilaricides) needs to be determined according to setting. Programs need to expand into currently untreated hypoendemic areas, including untreated loiasis co-endemic areas 58 (see above for test-and(not)treat strategies 39).
Programmatic features. Achieving and maintaining high coverage and adherence over each MDA round is paramount, especially in highly endemic settings. Long-term treatment can suffer from programme, communities and donor fatigue. Weak EOT programs need strengthening 60.
3) Ability to sustain achievement of the goal
Dynamics of resurgence. The dynamics of resurgence according to setting need further investigation 29, as well as the factors leading to resurgence, e.g. residual (endogenous) infections or re-introduction of (allogenous) infections from surrounding areas through migration of humans and/or flies. Investigation of these dynamics will inform post-treatment and post-elimination surveillance protocols 53.
4) Considerations of cost
Health and economic benefits. Achieving EOT in Africa would lead to substantial health and economic benefits, reducing the needs for health workforce and outpatient services 61. ATS needs and cost-effectiveness remain to be captured 37, 49, 62, 63.
Logistic and cost implications. Striving for Pan-African EOT has considerable logistic and cost implications, due to required programme expansion into currently untreated areas, prospects of enhanced or more frequent MDA or ATS, and improved (post-treatment and post-EOT) surveillance. The increased costs should be weighed against shorter programme duration and higher probability of success. Costs per year are higher for biannual than for annual MDA, but costs do not simply double 38, 63. Annual moxidectin may be more cost-effective than biannual ivermectin, should this drug be donated 49. Economic analyses of moxidectin, anti- Wolbachia therapies, macrofilaricides, and vector control (ground larviciding, slash-and-clear, adult fly traps) need to be conducted. The costs of test-and-not-treat for loiasis co-endemic areas were recently assessed in Cameroon 62.
Risks faced by treatment programs that need to be mitigated to achieve EOT
Some programs, particularly those in areas affected by conflict are more likely to under-perform, suffer from interrupted MDA schedules, or be delayed in starting and up-scaling implementation. Internal population displacement as well as mass migration to other countries could jeopardize progress towards EOT. Weak programs/health systems need to be identified, supported and strengthened 60. Movement of infected individuals (between endemic communities within countries, and between-countries) due to traditional cultural and trade patterns may lead to re-introduction of infection in areas achieving EOT. Risk of declining drug efficacy following multiple rounds of MDA/existence of sub-optimal responses to treatment should be monitored 59, 64. Low coverage and systematic non-adherence may lead to persistent transmission, particularly (but not exclusively) in loiasis co-endemic areas 65. Factors associated with non-compliance need further investigation. Reported coverage levels are generally higher than true coverage, possibly because coverage has been a primary indicator of program effectiveness. Population denominators may not be correct due to lack of updated censuses. Reported coverages are aggregated by district, but disaggregate data are needed to evaluate and correct coverage heterogeneity.
Modelling priorities to support goals in the 2030 horizon and beyond
Table 2 outlines the priority modelling questions for further research that were elaborated in discussion with the WHO.
Table 2. Modelling priorities for further work discussed with the World Health Organization (WHO).
| Priority issue / question identified in
discussion with WHO |
How can quantitative and mathematical modelling address this? |
|---|---|
| 1. Assess time to elimination of
transmission (EOT) based on ivermectin mass drug administration (MDA) |
1.1 Generate projections, by implementation unit (IU), of trends in infection since the
start of MDA, using both models. Identify countries that are likely to achieve country- wide EOT by 2030 or beyond. 1.2 Identify IUs that are expected to be able to stop MDA by 2023, 2025, 2030. Estimate the endemic population size by IU and estimate the % of the endemic population no longer requiring MDA by 2023, 2025, 2030 for each country. |
| 2. Estimate elimination thresholds with
uncertainty boundaries for different infection indicators at community level |
2.1 Simulate serological (and potentially entomological) thresholds for elimination.
First estimates have already been published for ONCHOSIM 54, 66. Compare these to estimates for EPIONCHO-IBM to understand their dependency on structural uncertainties and assumptions about local transmission conditions. 2.2. Identify optimal demographic groups for serological monitoring. |
| 3. Assess potential for resurgence when
MDA is stopped based on current/revised decision algorithms and stopping criteria |
3.1 Revise current (serological and entomological) stopping criteria based on (2)
above. 3.2 Investigate dynamics of recrudescence following cessation of MDA with both models. |
| 4. Evaluate the potential of macrofilaricides
as alternative treatment strategies to accelerate EOT |
4.1 Generate projections of the epidemiological impact of macrofilaricides.
4.2 Conduct cost-effectiveness analyses. |
Data availability
No data are associated with this article.
Acknowledgments
Members of the NTD Modelling Consortium Onchocerciasis Group:
Maria-Gloria Basáñez ( m.basanez@imperial.ac.uk; corresponding author) 1, 2, Martin Walker ( mwalker@rvc.ac.uk) 1, 3, Jonathan I D Hamley ( jonathan.hamley11@imperial.ac.uk) 1, 2, Philip Milton ( philip.milton15@imperial.ac.uk) 1, 2, Claudio Fronterre ( c.fronterr@lancaster.ac.uk) 4, Sake J. de Vlas ( s.devlas@erasmusmc.nl) 5, Wilma A. Stolk ( w.stolk@erasmusmc.nl; corresponding author) 5.
1 London Centre for Neglected Tropical Disease Research, Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, Norfolk Place, London W2 1PG, UK.
2 MRC Centre for Global Infectious Disease Analysis, Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, Norfolk Place, London W2 1PG, UK.
3 Department of Pathobiology and Population Sciences and London Centre for Neglected Tropical Disease Research, Royal Veterinary College, Hatfield, UK.
4 Centre for Health Informatics, Computing and Statistics (CHICAS), Lancaster Medical School, Lancaster University, Lancaster LA1 4YW, UK.
5 Department of Public Health, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
We would like to thank Luc E Coffeng, Suzanne Verver, and Hugo C Turner for contributing to the work represented in this article. We thank Torey de Rozario, Simon Brooker and other members of the Gates Foundation NTD team for helpful feedback. We also thank Paul Cantey for valuable comments on the modelling priorities.
Funding Statement
This work was supported by the Bill and Mellinda Gates Foundation through funding to the NTD Modelling Consortium [OPP1184344 to MGB, MW, JIDH, CF, SJdV and WAS]. MGB, JIDH and PM acknowledge joint Centre funding from the UK Medical Research Council and Department for International Development [MR/R015600/1].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Herricks JR, Hotez PJ, Wanga V, et al. : The global burden of disease study 2013: What does it mean for the NTDs? PLoS Negl Trop Dis. 2017;11(8):e0005424. 10.1371/journal.pntd.0005424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization: 681 Progress report on the elimination of human onchocerciasis, 2016–2017. Wkly Epidemiol Rec. 2017;92(45):681–94. [PubMed] [Google Scholar]
- 3. Little MP, Basáñez MG, Breitling LP, et al. : Incidence of blindness during the Onchocerciasis Control Programme in western Africa, 1971-2002. J Infect Dis. 2004;189(10):1932–41. 10.1086/383326 [DOI] [PubMed] [Google Scholar]
- 4. Little MP, Breitling LP, Basáñez MG, et al. : Association between microfilarial load and excess mortality in onchocerciasis: an epidemiological study. Lancet. 2004;363(9420):1514–21. 10.1016/S0140-6736(04)16151-5 [DOI] [PubMed] [Google Scholar]
- 5. Walker M, Little MP, Wagner KS, et al. : Density-dependent mortality of the human host in onchocerciasis: relationships between microfilarial load and excess mortality. PLoS Negl Trop Dis. 2012;6(3):e1578. 10.1371/journal.pntd.0001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GBD 2015 DALYs and HALE Collaborators: Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–58. 10.1016/S0140-6736(16)31460-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chesnais CB, Nana-Djeunga HC, Njamnshi AK, et al. : The temporal relationship between onchocerciasis and epilepsy: a population-based cohort study. Lancet Infect Dis. 2018;18(11):1278–86. 10.1016/S1473-3099(18)30425-0 [DOI] [PubMed] [Google Scholar]
- 8. Vinkeles Melchers NVS, Mollenkopf S, Colebunders R, et al. : Burden of onchocerciasis-associated epilepsy: first estimates and research priorities. Infect Dis Poverty. 2018;7(1):101. 10.1186/s40249-018-0481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colatrella B: The Mectizan Donation Program: 20 years of successful collaboration - a retrospective. Ann Trop Med Parasitol. 2008;102 Suppl 1:7–11. 10.1179/136485908X337418 [DOI] [PubMed] [Google Scholar]
- 10. Coffeng LE, Stolk WA, Zouré HG, et al. : African programme for onchocerciasis control 1995-2015: updated health impact estimates based on new disability weights. PLoS Negl Trop Dis. 2014;8(6):e2759. 10.1371/journal.pntd.0002759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dadzie Y, Neira M, Hopkins D: Final report of the Conference on the Eradicability of Onchocerciasis. Filaria J. 2003;2(1):2. 10.1186/1475-2883-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borsboom GJ, Boatin BA, Nagelkerke NJ, et al. : Impact of ivermectin on onchocerciasis transmission: assessing the empirical evidence that repeated ivermectin mass treatments may lead to elimination/eradication in West-Africa. Filaria J. 2003;2(1):8. 10.1186/1475-2883-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winnen M, Plaisier AP, Alley ES, et al. : Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull World Health Organ. 2002;80(5):384–91. [PMC free article] [PubMed] [Google Scholar]
- 14. Diawara L, Traoré MO, Badji A, et al. : Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis. 2009;3(7):e497. 10.1371/journal.pntd.0000497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Traore MO, Sarr MD, Badji A, et al. : Proof-of-principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: final results of a study in Mali and Senegal. PLoS Negl Trop Dis. 2012;6(9):e1825. 10.1371/journal.pntd.0001825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. African Programme for Onchocerciasis Control: Conceptual and Operational Framework of Onchocerciasis Elimination with Ivermectin Treatment. World Health Organization; WHO/APOC/MG/10.1.2010; Accessed 31 Aug 2019. Reference Source [Google Scholar]
- 17. Gardon J, Gardon-Wendel N, Demanga-Ngangue, et al. : Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350(9070):18–22. 10.1016/S0140-6736(96)11094-1 [DOI] [PubMed] [Google Scholar]
- 18. Prost A, Hervouet JP, Thylefors B: [Epidemiologic status of onchocerciasis]. Bull World Health Organ. 1979;57(4):655–662. [PMC free article] [PubMed] [Google Scholar]
- 19. O'Hanlon SJ, Slater HC, Cheke RA, et al. : Model-Based Geostatistical Mapping of the Prevalence of Onchocerca volvulus in West Africa. PLoS Negl Trop Dis. 2016;10(1):e0004328. 10.1371/journal.pntd.0004328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Molyneux DH, Hopkins DR, Zagaria N: Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol. 2004;20(8):347–51. 10.1016/j.pt.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 21. Coffeng LE, Stolk WA, Hoerauf A, et al. : Elimination of African onchocerciasis: modeling the impact of increasing the frequency of ivermectin mass treatment. PLoS One. 2014;9(12):e115886. 10.1371/journal.pone.0115886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basáñez MG, Pion SD, Boakes E, et al. : Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(5):310–22. 10.1016/S1473-3099(08)70099-9 [DOI] [PubMed] [Google Scholar]
- 23. Plaisier AP, Alley ES, Boatin BA, et al. : Irreversible effects of ivermectin on adult parasites in onchocerciasis patients in the Onchocerciasis Control Programme in West Africa. J Infect Dis. 1995;172(1):204–10. 10.1093/infdis/172.1.204 [DOI] [PubMed] [Google Scholar]
- 24. Walker M, Pion SDS, Fang H, et al. : Macrofilaricidal efficacy of repeated doses of ivermectin for the treatment of river blindness. Clin Infect Dis. 2017;65(12):2026–34. 10.1093/cid/cix616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization: Accelerating work to overcome the global impact of neglected tropical diseases. A roadmap for implementation. Geneva: World Health Organization; WHO/HTM/NTD/PCT/20121:2012; Accessed 31 Aug 2019. Reference Source [Google Scholar]
- 26. African Programme for Onchocerciasis Control (APOC): Eighteenth Session of the Joint Action Forum. Bujumbura, Burundi. Final Communique. 2012. Accessed 31 Aug 2019. Reference Source [Google Scholar]
- 27. Stolk WA, Walker M, Coffeng LE, et al. : Required duration of mass ivermectin treatment for onchocerciasis elimination in Africa: a comparative modelling analysis. Parasit Vectors. 2015;8:552. 10.1186/s13071-015-1159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basáñez MG, Walker M, Turner HC, et al. : River blindness: Mathematical models for control and elimination. Adv Parasitol. 2016;94:247–341. 10.1016/bs.apar.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 29. Walker M, Stolk WA, Dixon MA, et al. : Modelling the elimination of river blindness using long-term epidemiological and programmatic data from Mali and Senegal. Epidemics. 2017;18:4–15. 10.1016/j.epidem.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verver S, Walker M, Kim YE, et al. : How can onchocerciasis elimination in Africa be accelerated? Modeling the impact of increased ivermectin treatment frequency and complementary vector control. Clin Infect Dis. 2018;66(suppl_4):S267–S274. 10.1093/cid/cix1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamley JID, Milton P, Walker M, et al. : Modelling exposure heterogeneity and density dependence in onchocerciasis using a novel individual-based transmission model, EPIONCHO-IBM: implications for elimination and data needs. PLoS Negl Trop Dis.(in press). Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basáñez MG, Collins RC, Porter CH, et al. : Transmission intensity and the patterns of Onchocerca volvulus infection in human communities. Am J Trop Med Hyg. 2002;67(6):669–79. 10.4269/ajtmh.2002.67.669 [DOI] [PubMed] [Google Scholar]
- 33. Duerr HP, Dietz K, Schulz-Key H, et al. : Density-dependent parasite establishment suggests infection-associated immunosuppression as an important mechanism for parasite density regulation in onchocerciasis. Trans R Soc Trop Med Hyg. 2003;97(2):242–50. 10.1016/s0035-9203(03)90132-5 [DOI] [PubMed] [Google Scholar]
- 34. Duerr HP, Dietz K, Schulz-Key H, et al. : The relationships between the burden of adult parasites, host age and the microfilarial density in human onchocerciasis. Int J Parasitol. 2004;34(4):463–73. 10.1016/j.ijpara.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 35. Njongmeta LM, Nfon CK, Gilbert J, et al. : Cattle protected from onchocerciasis by ivermectin are highly susceptible to infection after drug withdrawal. Int J Parasitol. 2004;34(9):1069–74. 10.1016/j.ijpara.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization/African Programme for Onchocerciasis Control: Report of the Consultative Meetings on Strategic Options and Alternative Treatment Strategies for Accelerating Onchocerciasis Elimination in Africa. WHO/MG/15202015. Accessed 31 Aug 2019. Reference Source [Google Scholar]
- 37. Boussinesq M, Fobi G, Kuesel AC: Alternative treatment strategies to accelerate the elimination of onchocerciasis. Int Health. 2018;10(suppl_1):i40–i48. 10.1093/inthealth/ihx054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turner HC, Walker M, Churcher TS, et al. : Reaching the London Declaration on Neglected Tropical Diseases goals for onchocerciasis: an economic evaluation of increasing the frequency of ivermectin treatment in Africa. Clin Infect Dis. 2014;59(7):923–32. 10.1093/cid/ciu467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamgno J, Pion SD, Chesnais CB, et al. : A test-and-not-treat strategy for onchocerciasis in Loa loa-endemic areas. N Engl J Med. 2017;377(21):2044–52. 10.1056/NEJMoa1705026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Renz A, Wenk P: Studies on the dynamics of transmission of onchocerciasis in a Sudan-savanna area of North Cameroon I. Prevailing Simulium vectors, their biting rates and age-composition at different distances from their breeding sites. Ann Trop Med Parasitol. 1987;81(3):215–28. 10.1080/00034983.1987.11812115 [DOI] [PubMed] [Google Scholar]
- 41. Duke BO, Anderson J, Fuglsang H: The Onchocerca volvulus transmission potentials and associated patterns of onchocerciasis at four Cameroon Sudan-savanna villages. Tropenmed Parasitol. 1975;26(2):143–54. [PubMed] [Google Scholar]
- 42. Thylefors B, Philippon B, Prost A: Transmission potentials of Onchocerca volvulus and the associated intensity of onchocerciasis in a Sudan-savanna area. Tropenmed Parasitol. 1978;29(3):346–54. [PubMed] [Google Scholar]
- 43. Duerr HP, Leary CC, Eichner M: High infection rates at low transmission potentials in West African onchocerciasis. Int J Parasitol. 2006;36(13):1367–72. 10.1016/j.ijpara.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 44. Botto C, Basañez MG, Escalona M, et al. : Evidence of suppression of onchocerciasis transmission in the Venezuelan Amazonian focus. Parasit Vectors. 2016;9:40. 10.1186/s13071-016-1313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vieira JC, Brackenboro L, Porter CH, et al. : Spatial and temporal variation in biting rates and parasite transmission potentials of onchocerciasis vectors in Ecuador. Trans R Soc Trop Med Hyg. 2005;99(3):178–95. 10.1016/j.trstmh.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 46. Turner HC, Churcher TS, Walker M, et al. : Uncertainty surrounding projections of the long-term impact of ivermectin treatment on human onchocerciasis. PLoS Negl Trop Dis. 2013;7(4):e2169. 10.1371/journal.pntd.0002169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dyson L, Stolk WA, Farrell SH, et al. : Measuring and modelling the effects of systematic non-adherence to mass drug administration. Epidemics. 2017;18:56–66. 10.1016/j.epidem.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim YE, Remme JH, Steinmann P, et al. : Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl Trop Dis. 2015;9(4):e0003664. Erratum in: PLoS Negl Trop Dis.2015; 9(5): e0003777. 10.1371/journal.pntd.0003664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turner HC, Walker M, Attah SK, et al. : The potential impact of moxidectin on onchocerciasis elimination in Africa: an economic evaluation based on the Phase II clinical trial data. Parasit Vectors. 2015;8:167. 10.1186/s13071-015-0779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walker M, Specht S, Churcher TS, et al. : Therapeutic efficacy and macrofilaricidal activity of doxycycline for the treatment of river blindness. Clin Infect Dis. 2015;60(8):1199–207. 10.1093/cid/ciu1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Routledge I, Walker M, Cheke RA, et al. : Modelling the impact of larviciding on the population dynamics and biting rates of Simulium damnosum (s.l.): implications for vector control as a complementary strategy for onchocerciasis elimination in Africa. Parasit Vectors. 2018;11(1):316. 10.1186/s13071-018-2864-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jacob BG, Loum D, Lakwo TL, et al. : Community-directed vector control to supplement mass drug distribution for onchocerciasis elimination in the Madi mid-North focus of Northern Uganda. PLoS Negl Trop Dis. 2018;12(8):e0006702. 10.1371/journal.pntd.0006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. World Health Organization/Department of Control of Neglected Tropical Diseases: Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis. Criteria and procedures.(Ukety T, Ed.),2016;44, Accessed 31 August 2019. Reference Source [PubMed] [Google Scholar]
- 54. Coffeng LE, Stolk WA, Golden A, et al. : Predictive Value of Ov16 Antibody Prevalence in Different Subpopulations for Elimination of African Onchocerciasis. Am J Epidemiol. 2019;88(9):1723–1732. 10.1093/aje/kwz109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Filipe JAN, Boussinesq M, Renz A, et al. : Human infection patterns and heterogeneous exposure in river blindness. Proc Natl Acad Sci U S A. 2005;102(42):15265–70. 10.1073/pnas.0502659102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alley WS, van Oortmarssen GJ, Boatin BA, et al. : Macrofilaricides and onchocerciasis control, mathematical modelling of the prospects for elimination. BMC Public Health. 2001;1:12. 10.1186/1471-2458-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cano J, Basáñez MG, O'Hanlon SJ, et al. : Identifying co-endemic areas for major filarial infections in sub-Saharan Africa: seeking synergies and preventing severe adverse events during mass drug administration campaigns. Parasit Vectors. 2018;11(1):70. 10.1186/s13071-018-2655-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vinkeles Melchers NVS, Coffeng LE, Boussinesq M, et al. : Projected number of people with onchocerciasis-loiasis co-infection in Africa, 1995 to 2025. Clin Infect Dis. 2019; pii: ciz647. 10.1093/cid/ciz647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frempong KK, Walker M, Cheke RA, et al. : Does Increasing Treatment Frequency Address Suboptimal Responses to Ivermectin for the Control and Elimination of River Blindness? Clin Infect Dis. 2016;62(11):1338–47. 10.1093/cid/ciw144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Colebunders R, Basáñez MG, Siling K, et al. : From river blindness control to elimination: bridge over troubled water. Infect Dis Poverty. 2018;7(1):21. 10.1186/s40249-018-0406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim YE, Stolk WA, Tanner M, et al. : Modelling the health and economic impacts of the elimination of river blindness (onchocerciasis) in Africa. BMJ Glob Health. 2017;2(2):e000158. 10.1136/bmjgh-2016-000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lenk EJ, Moungui HC, Boussinesq M, et al. : A test-and-not-treat strategy for onchocerciasis elimination in Loa loa co-endemic areas: cost analysis of a pilot in the Soa health district, Cameroon. Clin Infect Dis. 2019; pii: ciz461. 10.1093/cid/ciz461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Turner HC, Osei-Atweneboana MY, Walker M, et al. : The cost of annual versus biannual community-directed treatment of onchocerciasis with ivermectin: Ghana as a case study. PLoS Negl Trop Dis. 2013;7(9):e2452. 10.1371/journal.pntd.0002452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Doyle SR, Bourguinat C, Nana-Djeunga HC, et al. : Genome-wide analysis of ivermectin response by Onchocerca volvulus reveals that genetic drift and soft selective sweeps contribute to loss of drug sensitivity. PLoS Negl Trop Dis. 2017;11(7):e0005816. 10.1371/journal.pntd.0005816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wanji S, Kengne-Ouafo JA, Esum ME, et al. : Relationship between oral declaration on adherence to ivermectin treatment and parasitological indicators of onchocerciasis in an area of persistent transmission despite a decade of mass drug administration in Cameroon. Parasit Vectors. 2015;8:667. 10.1186/s13071-015-1283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lont YL, Coffeng LE, de Vlas SJ, et al. : Modelling Anti-Ov16 IgG4 Antibody Prevalence as an Indicator for Evaluation and Decision Making in Onchocerciasis Elimination Programmes. PLoS Negl Trop Dis. 2017;11(1):e0005314. 10.1371/journal.pntd.0005314 [DOI] [PMC free article] [PubMed] [Google Scholar]