ABSTRACT
Denosumab has been used successfully to treat disease‐associated osteoclast overactivity, including giant cell tumor of bone. Given its mechanism of action, denosumab is a potent potential treatment of other osteoclast bone dysplasias including central giant cell granuloma (CGCG), aneurysmal bone cyst (ABC), and cherubism. Relatively little is known about the safety and efficacy of denosumab in patients with these conditions, especially in children. We report on 3 pediatric patients treated with denosumab over a 3‐year period at UCLA Medical Center (Los Angeles and Santa Monica, CA, USA): a 12‐year‐old with recurrent ABC of the pelvis, a 14‐year‐old with CGCG of the mandible, and a 12‐year‐old with cherubism. All were started on a 1‐year course of 15 doses 120 mg s.c., given monthly with two loading doses on day 8 and 15. All patients demonstrated rapid and pronounced clinical improvement while on denosumab, including a significant reduction in pain and sclerosis of lytic lesions on radiographs. Within 1 month of initiating therapy, 2 patients experienced hypocalcemia (Common Terminology Criteria for Adverse Events [CTCAE] grade 2) and hypophosphatemia, with 1 patient experiencing symptoms. One patient went on to experience symptomatic rebound hypercalcemia (CTCAE grade 4) 5 months after completing therapy, requiring bisphosphonates and calcitonin. For the second patient, we developed a schedule to wean denosumab involving the progressive lengthening of time between doses from 1 to 4 months in 1‐month increments before cessation. We found that denosumab therapy results in significant clinical and radiographic improvement for pediatric patients with nonresectable ABC, CGCG, and cherubism. Problems with serum calcium may be more common in younger patients, with symptomatic and protracted rebound hypercalcemia after cessation of therapy the most significant. We present a potential solution to this problem with progressive spacing of doses. Potential serious adverse events from alterations in calcium homeostasis should be explored in prospective clinical trials. © 2019 The Authors. JBMR Plus published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
Keywords: Osteoclasts, Denosumab, RANKL, Hypercalcemia, Oncology
Introduction
Denosumab, a monoclonal antibody against RANKL, has been used successfully to treat diseases associated with osteoclast overactivity, including giant cell tumor of bone (GCTB), osteoporosis, and lytic lesions associated with bony metastases.1, 2, 3 Increasingly, denosumab has been used off‐label for other disorders of bone thought to result, at least in part, from similar osteoclastic pathology. These include central giant cell granuloma (CGCG), aneurysmal bone cyst (ABC), cherubism, and fibrous dysplasia (FD).
Cherubism is characterized by focal resorption and replacement with proliferative fibro‐osseous masses of the maxillary and mandibular bones. A gain‐of‐function mutation in SH3BP2 has been identified as a driver of cherubism; this mutation leads to osteoclastogenesis in the presence of RANKL and TNF‐α through interactions with a variety of proteins in hemopoietic progenitor cells.4 Similarly, osteoclast multinucleated giant cells have been identified in CGCG.5 These same cells have been found in pathological specimens of ABCs, though it is hypothesized they are reactive in nature.6, 7 Finally, excess osteoclastogenesis has been consistently identified in patients with FD.8 The potential role of multinucleated giant cells in these conditions has led to the limited application of denosumab in their treatment.
No clinical trials of denosumab have been published, and there are no ongoing clinical trials listed on Clinicaltrials.gov for these groups of patients. Cases have been reported for the use of denosumab in ABC,7, 9, 10, 11, 12, 13 CGCG,14, 15, 16, 17, 18 cherubism,19 and FD20, 21 for 8, 10, 1, and 3 patients, respectively. Of these, 5 patients were <16 years old when they began treatment. Although this therapy appears to be equally effective in younger patients based on this small sample, problems with calcium regulation may be more prevalent.
Though the safety of denosumab in adults and skeletally mature populations has been studied in patients with GCTB, little information exists on possible side‐effects in skeletally immature patients or patients with other pathology. These known side‐effects in adult populations include hypocalcemia (5%), hypophosphatemia (3%), back and or extremity pain (1%), and osteonecrosis of the jaw (1%).22 In pediatric patients, there have been two reports of hypercalcemia. In one 9‐year‐old patient with CGCG of the mandible, hypercalcemia was asymptomatic and resolved without intervention.17 In another 9‐year‐old patient with FD of the femur, severe symptomatic hypercalcemia developed after cessation of treatment requiring i.v. calcitonin and bisphosphonate therapy, returning to normal serum calcium levels after approximately 5 months.21
The use of denosumab in pediatric patients remains poorly studied; consequently clinicians are understandably reluctant to use this therapy in this population. Here we present our experience treating 3 pediatric patients off‐label with denosumab for a diagnosis of CGCG, ABC, or cherubism. We report a similar case of rebound hypercalcemia as reported previously,21 as well as our strategy for preventing this in our most recently treated patient.
Patients and Methods
All patients were treated at UCLA Medical Centers in Los Angeles and Santa Monica, California, USA. Diagnosis was established using tissue histopathology, radiographic appearance, and clinical history. All patients weighed over 40 kg when therapy was initiated, and all were started on an adult dose of 120 mg s.c. every 4 weeks, with additional loading doses on day 8 and 15 in accordance with the dosing schedule established for adult patients.2, 3 The first patient, with recurrent ABC of the pelvis, started treatment when she was 12 years old, receiving 14 doses. The second patient, with CGCG of mandible, began therapy when he was 14 years old and received 15 doses. The third patient, with cherubism, began therapy when she was 12 years old and has received 13 doses to date. No patient had clinical or radiological signs of a wider syndrome.
All patients received daily calcium carbonate and vitamin D supplementation, initially with 500 mg calcium carbonate and 1000 units of vitamin D3 daily and increased as necessary. No restrictions were placed on diet or activity for patients with these pathologies. For the patients with extremity tumors, full weight‐bearing is allowed, but moderate‐to‐high impact exercise and sports is discouraged. Patient follow‐up schedules, laboratory studies, and imaging studies were not standardized and were established and administered at the discretion of the providers managing treatment. Plain film X‐rays were the standard imaging modality; CT and MRI were utilized where appropriate.
Results
Patient 1: recurrent aneurysmal bone cyst of the pelvis
This patient was a 10‐year‐old female who was diagnosed with an extensive ABC of the pelvis. She underwent curettage and bone grafting; she tolerated the procedure well. However, 2 years later she presented with pain following a fall; it was found that her pelvic ABC had recurred, hence she underwent a second curettage and bone grafting. Histology revealed a cystic bone lesion with connective tissue, reactive woven bone, and hemorrhage, with fibroblasts and scattered osteoclast‐type giant cells noted. Her case was reviewed by a multidisciplinary musculoskeletal tumor board: The consensus was that an additional ABC was present, and that the lesion was not amenable to repeat curettage without significant potential morbidity. At a weight of 40 kg, she initiated denosumab therapy 1 month prior to her 13th birthday. She received denosumab on a monthly schedule with a day 8 loading dose (patient missed a day 15 loading dose) of 120 mg over the course of a year.
She achieved excellent clinical response and showed sclerosis of lesions at most recent imaging (Fig. 2). Plain film radiography showed impressive sclerosis of her cysts, with no increase in size (Fig. 2C). Hip pain that was present prior to denosumab initiation resolved rapidly over the course of the first month of treatment with denosumab. She continues to be pain‐free in her hip and has had no issues ambulating in the 13 months since discontinuation of denosumab.
Figure 2.
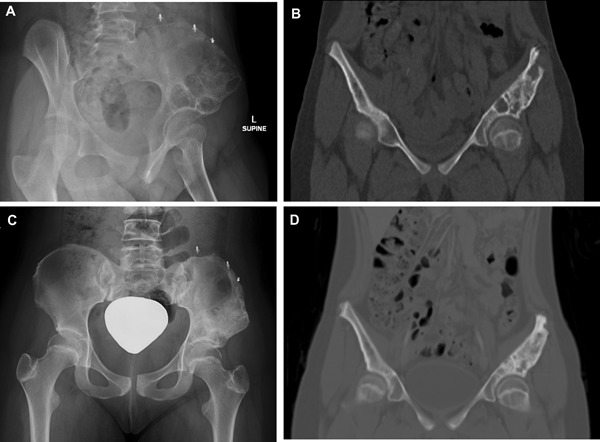
Patient with aneurysmal bone cyst of pelvis. (A, B) Judet plain film and sagittal CT before denosumab therapy and after two prior surgical interventions (C, D) and posteroanterior pelvis plain film and sagittal CT after denosumab therapy.
Toxicities associated with treatment included Common Terminology Criteria for Adverse Events (CTCAE v4.0) grade 2 hypocalcemia at the start of therapy and rebound grade 3 to 4 hypercalcemia upon cessation of denosumab, which are described here. In the first month of therapy, the patient experienced asymptomatic hypocalcemia (Fig. 1 A, C) with associated hypophosphatemia (Fig. 1 D). Her serum calcium normalized within 2 months without any intervention. Five months after her final dose, she was admitted with serum calcium of 15.5 mg/dL (Fig. 1 A, C) and symptoms of hypercalcemia, including severe diffuse abdominal pain, nausea, and vomiting. She had no laboratory or radiological evidence of nephrocalcinosis. She required bisphosphonates, furosemide, and calcitonin to lower her serum calcium levels. After discharge, her serum calcium had returned to normal at 9.6. She experienced two episodes of asymptomatic hypercalcemia the following month to 11.6 and 11.5mg/dL, respectively, that corrected with 2 weeks of furosemide therapy. She has subsequently had normal serum calcium levels.
Figure 1.
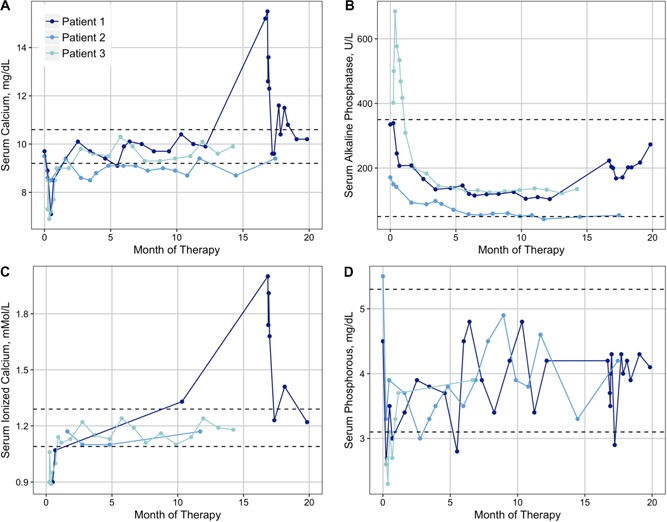
Patient laboratory studies over the duration of denosumab therapy for serum calcium (A), alkaline phosphate (B), ionized calcium (C), and phosphate (D). Dashed lines represent laboratory reference ranges at the University of California Los Angeles.
Patient 2: central giant cell granuloma of the mandible
This patient presented with a progressive mandibular mass over the course of 4 years that was diagnosed as CGCG at 14 years of age. He experienced a recurrence of CGCG with free‐floating lower teeth and severe pain with mastication. Given the progressive severe pain, failure of intralesional steroids and calcitonin, and significant functional limitation and progressive growth of the mass, he was considered for a partial mandibulectomy. Because of the potential morbidity from this surgical procedure, denosumab was discussed as a potential way to avoid or delay the need for mandibulectomy. He initiated denosumab therapy when he was 14 years old, and received monthly denosumab with loading doses at day 8 and day 15 for a total of 15 doses of 120 mg over the course of a year. He weighed 56 kg at the start of therapy. He did not experience any complications while on denosumab or after the completion of therapy, and his laboratory studies did not demonstrate any marked abnormalities in serum calcium levels (Fig. 1A), and serum alkaline phosphatase was appropriately suppressed to below the normal range while on therapy (Fig. 1 B).
He had an excellent clinical and radiological response (Fig. 3). Within 1 month of starting denosumab, he had complete resolution of severe mandibular pain; achieved fully functional mastication; and dentition, which was loose and painful, became fixed in the mandible. As with patient 1, serum alkaline phosphatase decreased appropriately with therapy.
Figure 3.
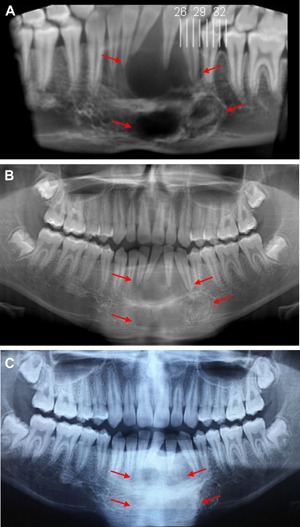
Patient with central giant cell granuloma of the mandible (A) before denosumab, (B) after 6 months of therapy, and (C) after 10 months of therapy.
Patient 3: cherubism
This patient was diagnosed with cherubism at 5 years of age, and she experienced refractory progressive disease. She presented with severe pain in her bilateral mandibles with uncontrolled oral–gingival bleeding, resulting in numerous emergency room visits, opioid use, and oral procoagulants (aminocaproic acid). She started denosumab therapy as a 12 year old. She weighed 42 kg at the start of therapy.
Within 1 month of starting denosumab, her pain had completely resolved as did her oral bleeding. After 6 months of therapy, she showed an excellent radiographic response with increased sclerosis in the severely osteolytic mandibular and maxillary lesions (Fig. 4).
Figure 4.
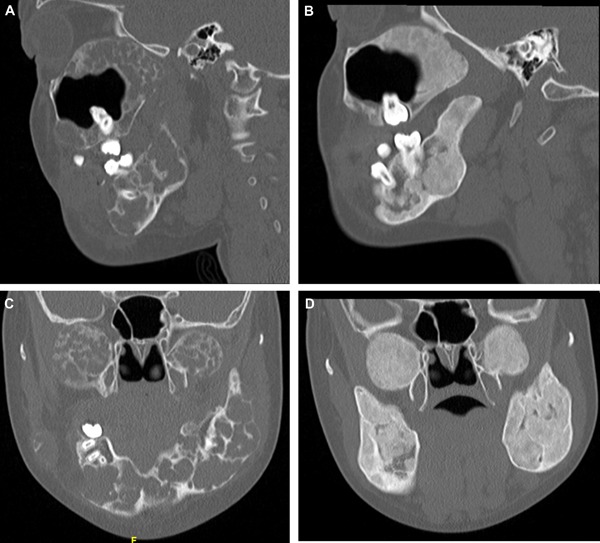
Patient with cherubism (A, C). Representative sagittal and coronal images before initiation of denosumab (B, D) and after 6 months of therapy.
Toxicity associated with treatment included serious adverse event CTCAE grade 3 hypocalcemia requiring hospitalization (Fig. 1 A, C); symptoms included tingling and numbness in her hands, as well as generalized weakness. She had concomitant hypophosphatemia to 2.6 mg/dL (Fig. 1 D) with a normal serum PTH of 48 pg/mL (range 11 to 51 pg/mL). In contrast to patients 1 and 2, serum alkaline phosphatase initially increased in response to denosumab therapy before subsequently decreasing (Fig. 1 B).
After this episode, denosumab was dose‐reduced by 50% to 60 mg monthly. After 10 months of therapy, she was started on a weaning schedule to gradually discontinue denosumab. She received her 11th dose 2 months after her 10th, and her 12th dose 2 months after that. Her 13th dose is planned for 3 months after her 12th; her final dose will be 4 months after the previous dose.
Discussion
In this case series, we describe the off‐label use of denosumab in 3 pediatric patients with recurrent ABC, CGCG, and cherubism. All patients demonstrated a robust and rapid clinical and radiographic response to denosumab. However, 2 patients experienced hypocalcemia following initiation of therapy, with 1 patient progressing to symptomatic hypercalcemia requiring inpatient hospitalization, as well as i.v. bisphosphonate and calcitonin therapy to normalize serum calcium levels. This is similar to a pediatric case that has been described previously.21 Our proposed method of avoiding hypercalcemia in pediatric patients is by gradually increasing the time between doses before completely terminating therapy.
Previous studies have used different dosing schedules for pediatric patients. Examples include a starting dose of 1.2 mg/kg, increasing by 0.1 kg/mg weekly, to a target dose of 1.6 mg/kg given monthly after the loading dose.12 Others have used a starting dose of 1 mg/kg, increasing by 0.25 mg/kg every 3 months to a final dose 1.75 mg/kg.21 Others have opted for a surface area‐based adjustment of 70 mg/m2 in children.7 One 9‐year‐old pediatric patient was treated with a full adult dose of 120 mg for 18 months.17 Recently at our institution, we have eliminated loading doses of denosumab to avoid hypocalcemia with the initiation of therapy.
In the 3 patients described here, hypocalcemia and hypercalcemia were the primary complications noted. In the 22 other published cases of off‐label denosumab use in pediatric and adult patients (Table 1),7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 complications described include symptomatic and asymptomatic hypercalcemia (2 patients, 1 with FD, 1 with CGCG), asymptomatic hypocalcemia (3 patients, 2 with FD, 1 with ABC), asymptomatic hypophosphatemia (3 patients with FD), extremity/skeletal pain (1 patient with CGCG), and delayed wound healing (1 patient with CGCG). In the only other case of symptomatic hypercalcemia described previously, also in a pediatric patient, the hypercalcemia was preceded by hypophosphatemia and increased serum PTH upon initiation of therapy. Our patient who experienced rebound hypercalcemia initially experienced asymptomatic hypocalcemia and hypophosphatemia upon initiation of therapy.
Table 1.
Summary of complications from previous studies regarding the use of Denosumab in ABC, CGCG, Cherubism and FD
| Study | Year | # Patientsts | Age (years) | Tumor location | Complications | |
|---|---|---|---|---|---|---|
| Aneurysmal bone cyst | ||||||
| Lange et al.7 | 2013 | 2 | 8 and 11 | Cervical spine | Asymptomatic hypocalcemia in 8‐year‐old | |
| Pauli et al.13 | 2014 | 1 | 21 | Forearm | None | |
| Pelle et al.12 | 2014 | 1 | 5 | Sacrum | None | |
| Skubitz et al.11 | 2015 | 1 | 27 | Sacrum | None | |
| Dubory et al.10 | 2016 | 1 | 26 | Cervical spine | None | |
| Ghermandi et al.9 | 2016 | 2 | 16 and 42 | Lumbar spine | None | |
| Central giant cell granuloma | ||||||
| Schreuder et al.18 | 2014 | 1 | 25 | Maxilla | Fatigue, myalgia, dizziness, constipation, and cramps | |
| Naidu et al.17 | 2014 | 2 | 9 and 42 | Mandible | Asymptomatic hypercalcemia in 9‐year‐old | |
| Gupta et al.16 | 2015 | 1 | 33 | Mandible | None | |
| O'Connell et al.15 | 2015 | 1 | 18 | Mandible | Shoulder, sternal and neck pain | |
| Bredell et al.14 | 2018 | 5 | 4, 18, 19, 22, 26 | Mandible (3 patients), maxilla (1), mandible, and maxilla (1) | Delayed wound healing following surgery while on drug in 18‐year‐old | |
| Cherubism | ||||||
| Eller‐Vainicher et al.19 | 2016 | 1 | 20 | Mandible | None | |
| Fibrous dysplasia | ||||||
| Boyce et al.21 | 2012 | 1 | 9 | Femur | Asymptomatic hypophosphatemia with initiation, severe hypercalcemia (vomiting) on discontinuation, required inpatient admission | |
| Ganda and Seibel20 | 2013 | 2 | 44 and 48 | Polyostotic | Asymptomatic hypocalcemia and hypophosphatemia | |
Rebound hypercalcemia may be related to elevated PTH and PTH receptors in people with recently closed growth plates or certain pathologies. Unfortunately, we cannot correlate this theory with the cases described here as serum PTH levels were not routinely measured. In addition, hypophosphatemia and/or hypocalcemia upon initiation of therapy may signal potential problems with rebound hypercalcemia upon cessation of therapy. Moreover, in the patient with cherubism, hypocalcemia was associated with an inappropriately normal serum PTH concentration. This particular patient also displayed higher serum alkaline phosphatase levels at baseline and an initial increase—rather than the typically observed decrease—in alkaline phosphatase activity in response to denosumab. Although further studies are warranted to determine whether these findings are consistent in patients with SH3BP2 mutations, previous reports suggest that cherubism may be associated with impaired osteoblast function and altered PTHrP‐PTHrP receptor interactions,23, 24 suggesting that an underlying disease mutation may contribute to accentuated changes in mineral ion homeostasis in some patients.
Conclusions
In the cases described here, denosumab therapy results in significant clinical and radiographic improvement for pediatric patients with ABC, CGCG, and cherubism. In younger patients, problems with serum calcium levels both at the initiation of treatment and at discontinuation may be more common, with symptomatic and protracted rebound hypercalcemia after cessation of therapy the most significant. We present a potential solution to this problem involving the progressive lengthening of time between doses from 1 to 4 months in 1‐month increments before final cessation of denosumab. We also recommend potentially reducing or eliminating loading doses to avoid hypocalcemia with the initiation of therapy. Denosumab may be a viable therapeutic option for children with nonresectable osteoclastic bone dysplasias such as ABC, CGCG, cherubism, and FD. However, because of potential serious adverse events from alterations in calcium homeostasis, this option should be explored carefully in prospective clinical trials of denosumab in children with the aforementioned diseases. The incidence of treatment‐related and treatment‐emergent adverse events with rebound hypercalcemia and hypocalcemia in our patients warrants careful oversight and frequent serial monitoring when administering denosumab and discontinuing denosumab in children and adolescents.
Disclosures
None of the authors has an actual or perceived conflict of interest with this work.
Acknowledgments
Support for this work came in part from St. Baldrick's Foundation Career Development Award.
Authors' roles: Study design: AUB and NF. Study conduct and data collection: SB, NB, AF, SN, AS, KWP, FE, NF. Data summarization: AUB and NF. Data interpretation: AUB, SB, NB, AF, SN, AS, KWP, FE, NF. Drafting manuscript: AUB, KWP, NF. Approving final version of manuscript: AUB, SB, NB, AF, SN, AS, KWP, FE, NF. AUB and NF take responsibility for the integrity of the data.
References
References
- 1. Federman N, Brien EW, Narasimhan V, Dry SM, Sodhi M, Chawla SP. Giant cell tumor of bone in childhood: clinical aspects and novel therapeutic targets. Pediatr Drugs. 2013;16(1):21–8. [DOI] [PubMed] [Google Scholar]
- 2. Rutkowski P, Ferrari S, Grimer RJ, et al. Surgical downstaging in an open‐label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22(9):2860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant‐cell tumour of bone: an open‐label, phase 2 study. Lancet Oncol. 2010;11(3):275–80. [DOI] [PubMed] [Google Scholar]
- 4. Mukai T, Ishida S, Ishikawa R, et al. SH3BP2 Cherubism mutation potentiates TNF‐α‐induced osteoclastogenesis via NFATc1 and TNF‐α‐mediated inflammatory bone loss. J Bone Miner Res. 2014;29(12):2618–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu B, Yu S‐F, Li T‐J. Multinucleated giant cells in various forms of giant cell containing lesions of the jaws express features of osteoclasts. J Oral Pathol Med. 2003;32:367–75. [DOI] [PubMed] [Google Scholar]
- 6. Oliveira AM, Perez‐Atayde AR, Inwards CY, et al. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so‐called secondary aneurysmal bone cysts. Amer J Pathol. 2004;165(5):1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lange T, Stehling C, Fröhlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22(6):1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riminucci M, Kuznetsov SA, Cherman N, Corsi A, Bianco P, Robey PG. Osteoclastogenesis in fibrous dysplasia of bone: in situ and in vitro analysis of IL‐6 expression. Bone. 2003;33(3):434–42. [DOI] [PubMed] [Google Scholar]
- 9. Ghermandi R, Terzi S, Gasbarrini A, Boriani S. Denosumab: non‐surgical treatment option for selective arterial embolization resistant aneurysmal bone cyst of the spine and sacrum. Case report. Eur Rev Med Pharmacol Sci. 2016;20:3692–5. [PubMed] [Google Scholar]
- 10. Dubory A, Missenard G, Domont J, Court C. Interest of denosumab for the treatment of giant‐cells tumors and aneurysmal bone cysts of the spine. About nine cases. Spine. 2016;41(11):E654–60. [DOI] [PubMed] [Google Scholar]
- 11. Skubitz KM, Peltola JC, Santos ER, Cheng EY. Response of aneurysmal bone cyst to denosumab. Spine. 2015;40(22):E1201–4. [DOI] [PubMed] [Google Scholar]
- 12. Pelle DW, Ringler JW, Peacock JD, et al. Targeting receptor‐activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res. 2014;164(2):139–48. [DOI] [PubMed] [Google Scholar]
- 13. Pauli C, Fuchs B, Pfirrmann C, Bridge JA, Hofer S, Bode B. Response of an aggressive periosteal aneurysmal bone cyst (ABC) of the radius to denosumab therapy. World J Surg Oncol. 2014;12(17):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bredell M, Rordorf T, Kroiss S, Rücker M, Zweifel DF, Rostetter C. Denosumab as a treatment alternative for central giant cell granuloma: a long‐term retrospective cohort study. J Oral Maxillofac Surg. 2018;76(4):775–84. [DOI] [PubMed] [Google Scholar]
- 15. O'Connell JE, Bowe C, Murphy C, Toner M, Kearns GJ. Aggressive giant cell lesion of the jaws: a review of management options and report of a mandibular lesion treated with denosumab. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(5):e191–8. [DOI] [PubMed] [Google Scholar]
- 16. Gupta B, Stanton N, Coleman H, White C, Singh J. A novel approach to the management of a central giant cell granuloma with denosumab: a case report and review of current treatments. J Cranio‐Maxillofac Surg. 2015;43(7):1127–32. [DOI] [PubMed] [Google Scholar]
- 17. Naidu A, Malmquist MP, Denham CA, Schow SR. Management of central giant cell granuloma with subcutaneous denosumab therapy. J Oral Maxillofac Surg. 2014;72(12):2469–84. [DOI] [PubMed] [Google Scholar]
- 18. Schreuder WH, Coumou AW, Kessler PAHW, de Lange J. Alternative pharmacologic therapy for aggressive central giant cell granuloma: denosumab. J Oral Maxillofac Surg. 2014;72(7):1301–9. [DOI] [PubMed] [Google Scholar]
- 19. Eller‐Vainicher C, Rossi DS, Guglielmi G, et al. Prompt clinical and biochemical response to denosumab in a young adult patient with craniofacial fibrous dysplasia. Clin Cases Miner Bone Metabol. 2016;13(3):253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganda K, Seibel MJ. Rapid biochemical response to denosumab in fibrous dysplasia of bone: report of two cases. Osteoporos Int. 2013;25(2):777–82. [DOI] [PubMed] [Google Scholar]
- 21. Boyce AM, Chong WH, Yao J, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012;27(7):1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chawla S, Henshaw R, Seeger LL, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open‐label, parallel‐group, phase 2 study. Lancet Oncol. 2013;14(9):901–8. [DOI] [PubMed] [Google Scholar]
- 23. Levaot N, Simoncic PD, Dimitriou ID, et al. 3BP2‐deficient mice are osteoporotic with impaired osteoblast and osteoclast functions. J Clin Invest. 2011;121(8):3244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hyckel P, Berndt A, Schleier P, et al. Cherubism—new hypotheses on pathogenesis and therapeutic consequences. J Cranio‐Maxillofac Surg. 2005;33(1):61–8. [DOI] [PubMed] [Google Scholar]


