Abstract
Purpose. Age related macular degeneration (ARMD) remains a serious cause of vision loss in elderly people worldwide. The purpose of the study is to investigate the fellow eye of the patients with exudative ARMD using optical coherence tomography angiography (OCTA).
Methods. We conducted a retrospective study from the data of patients with exudative ARMD. Patients undergoing intravitreal anti-VEGF treatment for one eye were selected. OCTA images of both eyes with choroidal neovascularization (CNV) and the fellow eyes were evaluated retrospectively. Midchoroid, choriocapillaris (CC), retina pigment epithelium (RPE), and outer retina levels were evaluated using RTVue XR AVANTI OCTA.
Results. There were 5 male and 5 female patients included in this study. We detected drusen and pigment epithelial detachments (PED) in fellow eyes. Six fellow eyes had vascularized PED. Four of them were acute flat irregular type; two of them were chronic dome shaped type. Four patients had soft drusen that had shown signal loss on choriocapillaris level.
Conclusion. The follow-up of both eyes of the patients with ARMD is very important. OCTA seems a promising non-invasive method in order to detect subclinical early stage CNV and distinguish among types of PEDs.
Keywords: choroidal neovascularization, drusen, optical coherence tomography angiography, pigment epithelial detachment
Introduction
Age related macular degeneration (ARMD) is one of the significant reason of irreversible vision loss in adults over the age of 50 years in developed countries [1,2]. The ARMD has been divided into two subtypes as neovascular or non-neovascular, according to the presence of choroidal neovascularization (CNV) [3]. Three types of CNV have been identified. Type 1 is typically placed under retinal pigment epithelium (RPE) and related with a pigment epithelial detachment (PED). Type 2 is present above the RPE in the subretinal layer. Initial location of type 3 is intraretinal [4]. Type 1 and 3 constitute the majority of neovascular ARMD cases [5]. In a recent study, the authors reported that most fellow eyes had signs of early ARMD, scarring was started with 34.6% of fellow eyes. [6]. Therefore, the situation and the follow-up of these eyes became very serious.
Indocyanine green angiography (ICGA) and fluorescein angiography (FA) are effective methods for detecting CNV, however, these techniques require using intravenous contrast agent and side effects including anaphylaxis can be seen with these agents [7,8]. Although, CNV associated subretinal, intraretinal fluid and/ or PED are effectively seen with optical coherence tomography (OCT); these devices do not allow a direct visualization of neovascular network [9]. Optical coherence tomography angiography (OCTA) is a novel method to evaluate the retinal and choroidal vascular layers without the need for contrast agent [10,11].
The efficacy of the OCTA on diagnosis of CNV was shown in previous studies [5,9,12]. The early detection of the CNV on fellow eyes may become more important. In this study, we aimed to evaluate the fellow eyes of patients with CNV using OCTA.
Methods
This retrospective study was performed on fellow eyes of patients with CNV who were under intravitreal anti-VEGF treatment were evaluated using OCTA, retrospectively. This study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from each patients. Due to the retrospective nature, the IRB/ Ethics Committee ruled that an approval was not required for the study.
Patients, whose have exudative neovascular ARMD in one eye and non-exudative ARMD in the fellow eye documented by drusen and/ or RPE changes. All patients underwent total ophthalmic examination including: best corrected visual acuity assessment, anterior segment evaluation with slit lamp, intraocular pressure measurement by goldmann applanation tonometry, fundoscopy with dilatation, FA, OCT, and OCTA.
RTVue XR AVANTI; Optovue Inc, Fremont, CA were used to obtain images. The AngioVue OCTA system was operated at 70,000 A scans per second. The system uses a light source centralized on 840 nm and a bandwidth of 50 nm. The OCTA volumes included 304 · 304 Ascans with 2 consecutive Bscans. Split-spectrum amplitude-decorrelation angiography (SSADA) was used to obtain the OCTA data [13]. Every OCTA volume was achieved over 3 seconds, and 2 orthogonal OCTA volumes were achieved to make motion correction to decrease the motion artifacts. [14]. The modifications in reflectivity are directly related to blood flow.
OCTA was also capable of visualizing PED through semi-automated segmentation of the outer retina and subretinal or sub-RPE space by using a volumetric SD-OCT data set. OCTA software was used to automatically split the retinal layers and was able to remove retinal vessel shadowing by subtracting vessels seen above the outer plexiform layer from the outer retina OCTA image. Additionally, OCTA images were manually segmented into four layers as superficial capillary plexus, deep capillary plexus, outer retina (to analyze CNV), and choriocapillaris. The outer border of each segment was then individually setted to align with Bruch membrane. This time-intensive method of segmentation ensured accurate determination of the vessel depth within the OCTA scan, thus allowing a more precise differentiation of CNV from choroidal vasculature and a more detailed analysis of CNV characteristics. Qualitative analysis of the OCTA images were conducted by two masked clinicians (M.O. Ulusoy and S. Emre), at different times.
Results
Twenty eyes of 10 patients were evaluated (mean age was 72±9.33 years; 5 males and 5 females) in this study. Each patient’s eye had CNV shown with FA and the fellow eye did not. Seven of the CNV were type 1 and 3 of them were type 2. There were 4 sea fan type CNV, 2 medusa type and 1 indistinct type in eyes with CNV (Table 1). In two fellow eyes, more than one type pigment epithelial detachment (PED) was found. We detected vascularized PED in 6 fellow eyes. Four of them were acute flat irregular type (Fig. 1), two of them were chronic dome shaped type (Fig. 2). Four of the eyes had drusenoid PED (Fig. 3) and 2 of them had serous PED (Fig. 4). In addition, 4 patients had soft drusen that had shown signal loss on choriocapillaris level.
Table 1.
OCTA characteristics of CNV and fellow eyes of the patients
| Patients | Gender | Age | CNV Eye | CNV type | Fellow Eye |
| 1 | M | 67 | Sea fan | 1 | Drusenoid PED |
| 2 | M | 67 | Sea fan | 1 | Drusenoid PED |
| 3 | F | 72 | Sea fan | 1 | Acute Flat Irregular Vascularized PED |
| 4 | F | 77 | Medusa | 1 | Serous PED |
| 5 | M | 52 | Indistinct | 1 | Chronic Dome Shaped Vascularized PED, Drusenoid PED, Serous PED |
| 6 | F | 69 | 2 | Acute Flat Irregular Vascularized PED | |
| 7 | F | 79 | 2 | Serous PED | |
| 8 | F | 73 | Sea fan | 1 | Acute Flat Irregular Vascularized PED |
| 9 | M | 87 | Medusa | 1 | Acute Flat Irregular Vascularized PED |
| 10 | M | 77 | 2 | Chronic Dome Shaped Vascularized PED | |
| OCTA = Optical coherence tomography angiography, CNV = choroidal neovascularization, PED = pigment epithelial detachment, |
Fig. 1.
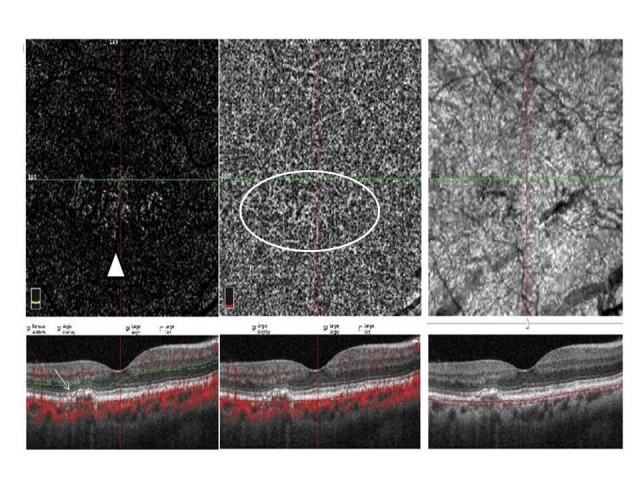
This image shows outer retina level and choriocapillaris level of OCTA (Optical coherence tomography angiography) scan and an OCT (Optical coherence tomography) scan. Acute flat irregular vascularized PED (pigment epithelial detachment) is seen on OCT image (white arrow) and neovascular network is seen at outer retina (white arrowhead) and choriocapillaris level (white circle) on OCTA image
Fig. 2.
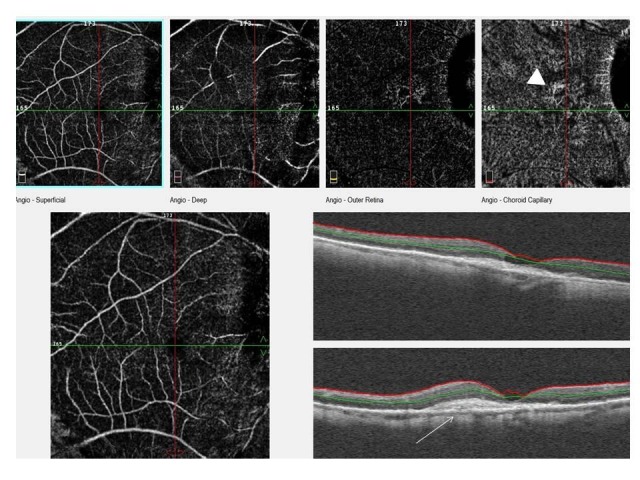
This image shows the superficial and deep capillary level, outer retina level and choriocapillaris level of OCTA scan and an OCT scan. Chronic dome shaped vascularized PED is seen on OCT image (white arrow) and neovascularization is seen at choriocapillaris level on OCTA image (white arrowhead)
Fig. 3.
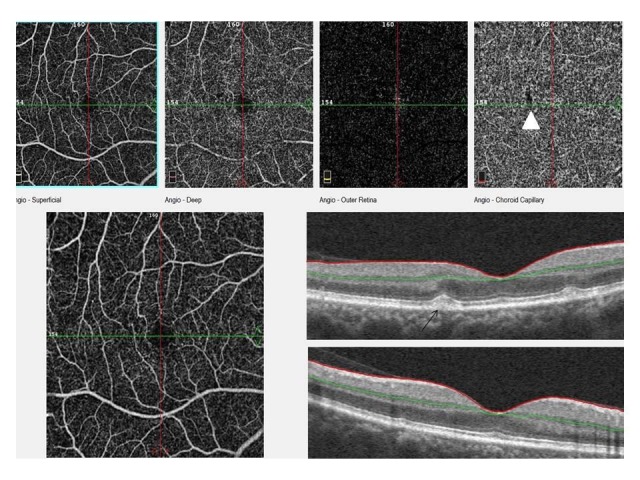
This image shows the superficial and deep capillary level, outer retina level and choriocapillaris level of OCTA scan and an OCT scan. Drusenoid PED is seen on OCT image (black arrow) and signal loss of drusen area is seen at choriocapillaris level on OCTA (white arrowhead)
Fig. 4.
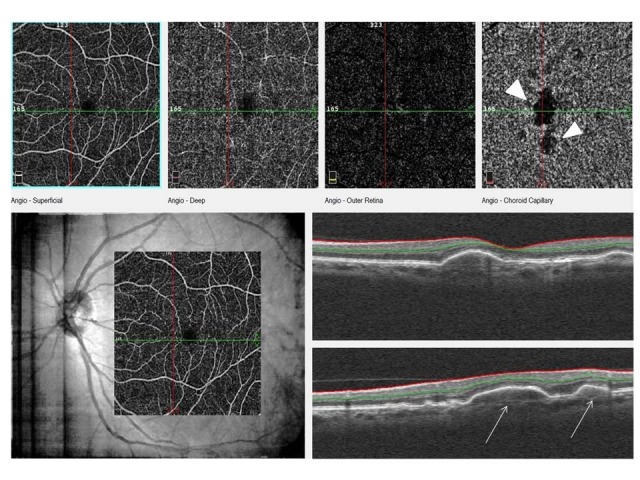
This image shows the superficial and deep capillary level, outer retina level and choriocapillaris level of OCTA scan and an OCT scan. Serous PED on OCT image (white arrows) and large signal loss area is seen at choriocapillaris level on OCTA image (white arrowheads)
Discussion
Early diagnosis and treatment of CNV is crucial to ensure superior visual outcomes. Therefore, it is noteworthy to evaluate and differentiate early changes of ARMD. Drusen and PED are often seen in early and intermediate ARMD [15,16]. However, FA and OCT are effective methods for evaluating drusen and PED, having some limitations [17]. OCTA is a noninvasive technique for rapidly acquiring images of the vascular layers of the retina and choroid without any contrast agent injection [18].
A previous study revealed that type 1 CNV is characterized with a minor demarcation from choroidal vessels and visible on the slab mid-choroid and choriocapillaris (CC); however, type 2 has good demarcation from choroidal vessels and visible on the slab outer retina [18]. According to these classifications, 7 of the CNV were type 1 and 3 of them were type 2 in our study. Kuehlewein et al. reported two different type 1 membrane morphologies as medusa and sea fan [4]. In our study, 4 of the type 1 CNV were sea fan type and 3 of them were medusa type.
Extracellular deposits which accumulate between the RPE and the inner collagenous layer of Bruch’s membrane are named drusen[19]. The drusen and pigmentary changes on the retina are characteristic findings of early and intermediate ARMD [20]. The increase of drusen size is as a sign for progression toward advanced stages of ARMD [21]. In previous studies, it has been suggested that the site of drusen formation is not random and they are presumably to form at sites of deficiant choroidal perfusion [16,22]. Therefore, visualization of CC under drusen is noteworthy for probable CNV or geographic atrophy development. In OCTA, false signal loss can be seen on CC slab under drusen. Lane et al. reported that swept-source OCTA is less prone to producing areas of false-positive flow impairment under drusen than in spectral domain OCTA [17]. In our study, drusen were seen in 4 of the patients’ fundus examinations. These patients’ fellow eyes needed careful evaluation in regular examinations with OCTA to avoid false positive signal loss.
PED is a pathological process in which the RPE separates from the underlying Bruch’s membrane [23]. In ARMD, PED can be classified into drusenoid, serous, vascularized, or mixed types. Drusenoid and serous PEDs are characteristics of nonneovascular ARMD. In contrast, vascularized PEDs are associated with Type 1 CNV [24].
It is noteworthy to differentiate the vascularized PED from other types, using non-invasive methods especially. In a previous study, authors reported that OCTA could differentiate the PEDs from each other [24]. Vascularized PEDs were classified according to OCT images as acute flat irregular and chronic dome shaped. Acute flat irregular vascularized PEDs were associated with monolayered CNV on OCTA, however chronic dome-shaped vascularized PEDs were shown as multilayered CNV on OCTA. In a previous study, it was noted that CNV of chronic vascularized PED appeared multilayered in OCT [25]. In our study, 6 of the fellow eyes had vascularized PED. 4 of them were acute flat irregular and 2 of them were chronic dome shaped PED. These vascularized PEDs did not show any sign of CNV with FA that was applied on the same day. In a recent case series, the authors reported subclinical CNV in three asymptomatic fellow eyes of patients [26]. A previous similar study reported that they have identified a 6.25% rate of CNV with no clinical signs of exudative ARMD using OCTA [27]. In a recent study, the authors detected subclinical CNV 14.4% (23/ 160) of asymptomatic patients with SS-OCTA and 13 of them turned to exudative ARMD [28]. Also in another study that evaluated the vessel densities, choroidal thickness and vascular tortuosity of the CNV eye, asymptomatic fellow eye and controls, it was reported that vessel densities of CNV and fellow eyes were significantly different from controls; however, they did not differ among themselves [29]. In addition, they did not evaluate subclinical CNV in the fellow eyes.
In conclusion, in this study we evaluated fellow eyes of patients with CNV using with OCTA. OCTA can differentiate vascularized PEDs from serous and drusenoid PEDs. The most important point is that non-exudative CNVs can be revealed with OCTA. We suggested that the follow-up of the fellow eye of patients with ARMD should be done carefully and in short intervals using OCTA, which is a novel non-invasive method.
Competing/ conflicts of interest
No conflict of interest stated.
Funding sources
No funding sources stated.
References
- 1.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 2.The Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC, The Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study Research Group. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehlewein L, Bansal M, Lenis TL, Iafe NA, Sadda SR, Bonini Filho MA, De Carlo TE, Waheed NK, Duker JS, Sarraf D. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. Am J Ophthalmol. 2015;160(4):739–748. doi: 10.1016/j.ajo.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Jung JJ, Chen CY, Mrejen S, Gallego-Pinazo R, Xu L, Marsiglia M, Boddu S, Freund KB. The incidence of neovascular subtypes in newly diagnosed neovascular age-related macular degeneration. Am J Ophthalmol. 2014;158(4):769–779. doi: 10.1016/j.ajo.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Wolf S, Bandello F, Loewenstein A, Slakter J, Katz T, Sowade O, Korobelnik JF. Baseline Characteristics of the Fellow Eye in Patients with Neovascular Age-Related Macular Degeneration: Post Hoc Analysis of the VIEW Studies. Ophthalmologica. 2016;236(2):95–99. doi: 10.1159/000447725. [DOI] [PubMed] [Google Scholar]
- 7.Bressler N, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32(6):375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- 8.Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, Zang E. Fluorescein angiography complication Survey. Ophthalmology. 1986;93(5):611–617. doi: 10.1016/s0161-6420(86)33697-2. [DOI] [PubMed] [Google Scholar]
- 9.Lindner M, Fang PP, Steinberg JS, Domdei N, Pfau M, Krohne TU, Schmitz-Valckenberg S, Holz FG, Fleckenstein M. OCT Angiography-Based Detection and Quantification of the Neovascular Network in Exudative AMD. Invest Ophthalmol Vis Sci. 2016;57(14):6342–6348. doi: 10.1167/iovs.16-19741. [DOI] [PubMed] [Google Scholar]
- 10.Spaide RF, Klancnik JM Jr., Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 11.Matsunaga D, Yi J, Puliafito CA, Kashani AH. OCT angiography in healthy human subjects. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):510–515. doi: 10.3928/23258160-20141118-04. [DOI] [PubMed] [Google Scholar]
- 12.Moult E, Choi W, Waheed NK, Adhi M, Lee B, Lu CD, Jayaraman V, Potsaid B, Rosenfeld PJ, Duker JS, Fujimoto JG. Ultrahigh-speed sweptsource OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D. Split-spectrum amplitude decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraus MF, Potsaid B, Mayer MA, Bock R, Baumann B, Liu JJ, Hornegger J, Fujimoto JG. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express. 2012;3(6):1182–1199. doi: 10.1364/BOE.3.001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] [Google Scholar]
- 16.Lengyel I, Tufail A, Hosaini HA, Luthert P, Bird AC, Jeffery G. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci. 2004;45(9):2886–2892. doi: 10.1167/iovs.03-1083. [DOI] [PubMed] [Google Scholar]
- 17.Lane M, Moult EM, Novais EA, Louzada RN, Cole ED, Lee B, Husvogt L, Keane PA, Denniston AK, Witkin AJ, Baumal CR, Fujimoto JG, Duker JS, Waheed NK. Visualizing the Choriocapillaris Under Drusen: Comparing 1050-nm Swept-Source Versus 840-nm Spectral-Domain Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT585–OCT590. doi: 10.1167/iovs.15-18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farecki ML, Gutfleisch M, Faatz H, Rothaus K, Heimes B, Spital G, Lommatzsch A, Pauleikhoff D. Characteristics of type 1 and 2 CNV in exudative AMD in OCT-Angiography. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):913–921. doi: 10.1007/s00417-017-3588-y. [DOI] [PubMed] [Google Scholar]
- 19.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44(1):1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Klein B, Knudtson M, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 21.Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, Milton RC, Bressler SB, Klein R. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(3):1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gass JD. Serous retinal pigment epithelial detachment with a notch: a sign of occult choroidal neovascularization. Retina. 1984;4(4):205–220. doi: 10.1097/00006982-198400440-00001. [DOI] [PubMed] [Google Scholar]
- 24.Veronese C, Maiolo C, Morara M, Armstrong GW, Ciardella AP. Optical coherence tomography angiography to assess pigment epithelial detachment. Retina. 2016;36(3):645–650. doi: 10.1097/IAE.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 25.Rahimy E, Freund KB, Larsen M, Spaide RF, Costa RA, Hoang Q, Christakopoulos C, Munch IC, Sarraf D. Multilayered pigment epithelial detachment in neovascular age-related macular degeneration. Retina. 2014;34(7):1289–1295. doi: 10.1097/IAE.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 26.Roisman L, Zhang Q, Wang RK, Gregori G, Zhang A, Chen CL, Durbin MK, An L, Stetson PF, Robbins G, Miller A, Zheng F, Rosenfeld PJ. Optical Coherence Tomography Angiography of Asymptomatic Neovascularization in Intermediate Age-Related Macular Degeneration. Ophthalmology. 2016 Jun;123(6):1309–1319. doi: 10.1016/j.ophtha.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palejwala NV, Jia Y, Gao SS, Liu L, Flaxel CJ, Hwang TS, Lauer AK, Wilson DJ, Huang D, Bailey ST. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina. 2015;35(11):2204–2211. doi: 10.1097/IAE.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira Dias JR, Zhang Q, Garcia JMB, Zheng F, Motulsky EH, Roisman L, Miller A, Chen CL, Kubach S, de Sisternes L, Durbin MK, Feuer W, Wang RK, Gregori G, Rosenfeld PJ. Natural History of Subclinical Neovascularization in Nonexudative Age-Related Macular Degeneration Using Swept-Source OCT Angiography. Ophthalmology. 2018 Feb;125(2):255–266. doi: 10.1016/j.ophtha.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arrigo A, Aragona E, Capone L, Pierro L, Romano F, Bandello F, Parodi MB. Advanced optical coherence tomography angiography analysis of age-related macular degeneration complicated by onset of unilateral choroidal neovascularization. Am J Ophthalmol. 2018 Aug 8; doi: 10.1016/j.ajo.2018.08.001. pii: S0002-9394(18)30441-0. doi: 10.1016/j.ajo.2018.08.001. [DOI] [PubMed] [Google Scholar]


