Summary
Introduction
Heat shock protein 90 (Hsp90) has been studied as a therapeutic target in many cancers. In pre-clinical trials, the Hsp90 ATPase inhibitor ganetespib demonstrated potent inhibition of solid tumor growth, with superior potency than prior Hsp90 inhibitors. Given the promising pre-clinical outcome and favorable pharmacologic properties of ganetespib, we conducted a phase II trial of single-agent ganetespib in patients with metastatic, castrate-resistant prostate cancer (mCRPC). The primary objective of the study was to determine the 6-month progression-free survival (PFS) rate.
Methods
Patients with mCRPC who had been previously treated with docetaxel were enrolled after meeting eligibility criteria. All patients received ganetespib at 200 mg/m2 on days 1, 8, and 15 of every 28 days (one cycle). Subjects who tolerated therapy were continued on ganetespib until disease progression. Considering that Hsp90 acetylation may confer insensitivity to Hsp90 inhibitors and maspin inhibits protein deacetylation, maspin-associated molecular markers were evaluated.
Results
Eighteen patients were recruited into the trial; most were Caucasian, had performance status 1, had received prior docetaxel, and were heavily pretreated. Of the 17 patients who were treated, none attained 6-month PFS. Only 2 patients achieved PFS > 4 months. The median PFS was 1.9 months. As per the study design, the trial was terminated after the interim analysis. The most frequent types of Grade 3 toxicity were dehydration, diarrhea, and fatigue. Molecular markers provided little additional insight regarding drug activity.
Conclusions
Ganetespib demonstrated minimal clinical activity in men with mCRPC. The true 6-month PFS rate was, at most, 0.20. Possible reasons for this include selection of a heavily pretreated patient population and lack of agent potency in patients with mCRPC.
Keywords: Prostate cancer, Heat shock protein, Ganetespib
Introduction
Advancements in the mechanistic studies of cancer progression and treatment resistance have fueled the search for novel therapeutic agents for metastatic castrate-resistant prostate cancer (mCRPC) [1]. Some of the mechanisms of progression involve dysregulation of the androgen receptor (AR) and changes in AR function, including mutations, increased phosphorylation, or increased transcription of AR [2, 3]. These changes allow the AR to respond to lower levels of androgens and steroids. Thus, inhibiting the AR and reducing the level of androgen are both desired in order to block AR-dependent prostate tumor progression. Heat shock protein (Hsp)-based chaperone mechanisms have been shown to regulate the AR by affecting the levels of Hsp stability and activity [4-6]. Although several heat shock proteins in the chaperone complex, such as Hsp90, Hsp70, and Hsp56, contribute to the overall regulation of AR, Hsp90, the most abundant Hsp in mammalian cells, is upregulated in prostate cancer [7]. Hsp90 regulates AR by forming a complex with unliganded AR along with co-chaperones to create a foldosome complex [8]. This AR-Hsp90 interaction complex then regulates the activation, maturation, and stability of AR and maintains AR in a conformation that potentiates high-affinity ligand binding but can also result in proteasome-mediated AR turnover if the ligand is not available [5].
Although AR is a bona fide drug target for human prostate tumors, prostate cancer metastasis is typically characterized by a variety of genetic alterations that collectively contribute to the transformed state. A subset of prostate cancer appears to depend on a single oncoprotein for its genesis, sustained proliferation, and survival. This phenomenon has been termed “oncogene addiction” [9]. Thus, we reasoned that inhibiting Hsp90-mediated chaperone activity, which is propelled by Hsp90 ATPase may have extensive anti-tumor effects due to Hsp90 regulation of the folding, stability, and function of many oncogenic clients, such as AKT, B-Raf, c-KIT, c-MET, and EGFR [10].
Although preclinical studies showed that several pharmacologic inhibitors of Hsp90 ATPase have anti-tumor activities [11-14], clinical trials of single-agent Hsp90 inhibitors in prostate cancer have not shown promising results. We were the first to report the results of a Phase II study of 17-allylamino-17-demethoxygeldanamycin (17-AAG) in men with mCRPC in which 17-AAG showed negligible activity with regard to PSA response [15]. Another Hsp90 inhibitor, IPI-504 (retaspimycin hydrochloride), had minimal effect on PSA or tumor burden and was associated with unacceptable toxicities in several patients [16]. It is thought that the previous pharmacological Hsp90 inhibitors are ineffective, at least in part, because they have insufficient potency.
However, ganetespib (formerly known as STA-9090 [5-[2,4-Dihydroxy-5-(1-methylethyl)phenyl]-4-(1-methyl-1 H-indol-5-yl)-2,4-dihydro-[1, 2, 4]triazol-3-one], a novel, synthetic small molecule with a unique triazolone-containing chemical structure [17] acts as a potent Hsp90 inhibitor. By inhibiting Hsp90, it in turn downregulates Hsp90 client protein levels. It binds to the ATP-binding pocket at the N-terminus of Hsp90 and was more potent than 17-AAG [18, 19] and other first-generation agents. In initial Phase 1 clinical trials, ganetespib was well tolerated and safe [20, 21]. Given the scientific rationale for Hsp90 inhibition in advanced prostate cancer and ganetespib’s favorable pharmacologic properties with significant preclinical activity, we conducted a phase II trial of single-agent ganetespib in patients with mCRPC.
In the current study, in addition to monitoring cancer growth using prostate-specific antigen (PSA) and other clinical parameters, we examined whether the tumor suppressor maspin and maspin-associated molecules could serve as surrogate predictors of prostate cancer response to ganetespib. We have shown previously that maspin acts as a deacetylase inhibitor and binds to Hsp90 [22]. Maspin also sensitizes prostate tumor cells to drug-induced apoptosis [23-25] and controls the expression of a set of genes that might regulate tumor cell differentiation and drug sensitivity [26].
Materials and methods
Study design
This was a single-arm, multicenter Phase 2 study of ganetespib in patients with mCRPC who previously were treated with docetaxel. The primary objective of the study was to determine the 6-month progression-free survival (PFS) rate. The secondary objectives were to assess overall safety and tolerability of ganetespib, to evaluate the overall survival, and to investigate the association of PFS with primary and secondary target markers, including maspin, PSA, cytokeratin 18 (CK18), interleukin (IL)-6, urokinase plasminogen activator (uPA) and receptor activator of nuclear factor κB (RANK) that could evaluate the effect of Hsp90 inhibition. The study was approved by the institutional review board at each clinical center, and written informed consent was obtained from all patients before registration.
Patient selection
Patients who had metastatic adenocarcinoma of the prostate and who received at least one prior docetaxel-based regimen for metastatic disease were included. All patients had to be castrate with a testosterone level ≤ 50 ng/dl, and Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy must be continued. Patients had to discontinue antiandrogens, including flutamide for a minimum of 4 weeks and bicalutamide or nilutamide for 6 weeks, before ganetespib treatment. Patients must have had adequate performance status of 0–2 and a life expectancy of at least 3 months. Patients were required to have functioning bone marrow as defined by an absolute neutrophil count ≥1500 cell/μL, platelets ≥100,000/μL, hemoglobin ≥9.0 g/dL, serum creatinine ≤1.5 x Upper Limit of Normal (ULN) or calculated creatinine clearance ≥50 mL/min, and total bilirubin ≤1. 5 x ULN. Patients without documented bone me-tastases or patients with liver metastases had to have aspartate transaminase (SGOT) and/or alanine transaminase (SGPT) ≤ 2.5 x ULN if alkaline phosphatase was ≤ ULN; alternatively, alkaline phosphatase could have been up to 4 x ULN if SGOT and/or SGPT were ≤ ULN. For patients with documented bone metastases, SGOT and/or SGPT had to be <2.5 x ULN, without regard to the alkaline phosphatase level.
Patients must have had adequate cardiac function, defined as baseline QTc < 450 msec, ejection fraction >50 % at baseline, and no history of, or current, coronary artery disease, myocardial infarction, angina pectoris, angioplasty or coronary bypass surgery; history of, or current, uncontrolled dysrhythmias, or requirement for antiarrhythmic medications, or Grade 2 or greater left bundle branch block that occurred previously. Any other significant comorbidities, which in the investigator’s judgment would rendered the subject inappropriate for entry into this study, were considered exclusion criteria.
Treatment plan
Ganetespib (200 mg/m2 on days 1, 8, and 15 every 28 days) was administered to all subjects. Every 28 days was considered one cycle. Subjects who tolerated this therapeutic regimen were continued on ganetespib until disease progression. A medical history, physical examination, performance status assessment, measurement of PSA, hematologic and chemistry laboratory tests, and 12-lead electrocardiogram (ECG) were repeated before each cycle. Survival information was collected every 12 weeks (± 1 week) from the date of last dose of study drug until the subject’s death or until the subject was lost to follow-up, or until study closure (approximately 6 months after the last subject terminated treatment).
Correlative markers
Blood specimens were collected from patients at baseline and at the conclusion of treatment. The level of CK18, maspin, PSA, IL-6, uPA, and RANK mRNA was determined by the RNA extraction from peripheral blood mononuclear cells (PBMCs) using the RNeasy Mini kit (Qiagen, Valencia, CA) and reverse-transcribed by iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), according to the manufacturer’s instructions. The q-RT-PCR analysis was performed as described where all data were normalized to the housekeeping gene GAPDH and baseline values [15]. List of all primers is provided in Table 1.
Table 1.
List of q-RT-PCR primers
| Gene | Primer Sequence |
|---|---|
| Maspin | 5′-cta ctt tgt tgg caa gtg gat gaa-3’ 5′-act ggt ttg gtg tct gtc ttg ttg |
| GAPDH | 5′-atc acc atc ttc cag gag cga-3’ 5′-gcc agt gag ctt ccc gtt ca-3’ |
| CK-18 | 5′-atc ttg gtg atg cct tgg aca-3’ 5′-ctt tgc cat cca cta tcc gg-3’ |
| uPA | 5′-ggg ggc tct gtc acc tac g-3’ 5′- ccc cag ctc aca att cca gtc-3’ |
| IL-6 | 5′- ggt aca tcc tcg acg gca tc-3’ 5′- cct tct ttg ctg ctt tca caa c-3’ |
| RANK | 5′- aag atg atg gca gcc ac-3’ 5′- taa atg ctt gct gca taa ag-3’ |
| PSA | 5′-cct gag gaa tcg att ctt cag –3’ 5′- gca tca gga aca aaa gcg tga-3 |
Statistical methods
Our primary objective was to determine the 6-month PFS rate by using a binary (yes/no) endpoint of 6 months of PFS. Treatment success was defined as achievement of at least 6 months of PFS. Patients who did not complete 6 months of ganetespib therapy for any reason (including death from any cause) were considered treatment failures and were recorded as not achieving the primary endpoint. Progression after docetaxel treatment was determined by radiography or rising PSA level consistent with the Prostate Cancer Working Group 2 guidelines.
This multi-institution phase 2 trial was planned with a Simon two-stage near-optimal design [27] resulting from the Simon algorithm modifications of Hintze [28]. The statistical goal was to determine whether the true 6-month PFS rate was >20 % or not. The study was powered for an alternative rate of 35 %. We assumed alpha =0.15 and power = 0.85. Under those assumptions, the design called for a maximum of 51 patients, 18 in Stage 1 and 33 in Stage 2 (if needed). If, among the first 18 patients, 3 or fewer successes are observed, the study was to be stopped, with the conclusion that ganetespib was not effective enough for further evaluation. The probability of early termination with this design was 0.501 when the true success rate was 20 %, and the average total sample size for this design was 34.47 patients.
Ninety percent confidence intervals (CIs) for response and toxicity rates were calculated using Wilson’s method as implemented in Stata 12 software. PFS was measured from treatment start date to the first date of documented PSA progression, discontinuation of ganetespib therapy, or death from any cause, whichever occurred first. Patients not experiencing any of those 3 terminating events were censored for PFS as of the date of their last PSA determination. Overall survival (OS) was measured from treatment start date to the date of death from any cause. Patients still alive were censored for OS as of the last date on which they were confirmed to be still alive. Standard Kaplan-Meier (K-M) estimates of the censored PFS and OS distributions were computed. Due to the small sample size, survival statistics (e.g., median) were estimated more conservatively using linear interpolation among successive event times on the K-M curves [29].
For statistical analysis of the molecular marker measurements, 1-tailed paired Student’s t-tests were used. P values <0.05 are considered significant.
Results
Between January 2011 and September 2012, 18 eligible patients were recruited into Stage 1 of the trial. One patient never started ganetespib treatment due to rapid disease progression. Patient follow-up continued through April 2013.
Patient characteristics
The baseline patient characteristics are summarized in Table 2. Seventeen patients completed the trial. Thirteen patients had received 1 prior (docetaxel-based) chemotherapy regimen. Fifty percent of the patients were treated with at least 4 prior regimens. Seventy two percent of the patients were Caucasian and 72 % had performance status 1.
Table 2.
Patient Characteristics (N = 18)
| Age, median (range) | 68 years (range 51–82 years) |
| Race | Patient number (%) |
| Caucasian | 13 (72) |
| African American | 4 (22) |
| Asian | 1 (6) |
| Pretherapy PSA, median (range) | 211 ng/ml (25.9–3489.2 ng/ml) |
| Performance status | Patient number (%) |
| 0 | 2 (11) |
| 1 | 13 (72) |
| 2 | 3 (17) |
| Prior surgery | Patient number (%) |
| No | 11 (61) |
| Yes | 7 (39) |
| Prior radiation | Patient number (%) |
| No | 5 (28) |
| Yes | 13 (72) |
| Disease sites | Patient number (%) |
| Visceral and bone metastasis | 4 (22) |
| Lymph node and bone metastasis | 4 (22) |
| Bone metastasis only | 10 (55) |
| Number of prior (medical) therapiesa | Patient number (%) |
| 1 | 4 (22) |
| 2 | 3 (17) |
| 3 | 2 (11) |
| 4 | 6 (33) |
| 5 | 3 (17) |
PSA = prostate-specific antigen
From among the following: docetaxel, cabazitaxel, abiraterone, Provenge, other. No patient received prior enzalutamide
Treatment and adverse events
Five patients received 1 cycle of ganetespib, 5 received 2 cycles, 6 received 3 cycles, and 1 received 5 cycles. There were 22 unique types of Grade 3 toxicity observed (Table 3). The 4 most frequent types of Grade 3 toxicity were dehydration, diarrhea, and fatigue. Hypocalcemia in one patient was the only Grade 4 toxicity observed.
Table 3.
Grade 3 Toxicities a (N = 17 treated patients)
| Type of toxicity | N | Rate (%) | Lower CL (%)b | Upper CL (%)b |
|---|---|---|---|---|
| Dehydration | 3 | 18 | 7 | 37 |
| Diarrhea | 3 | 18 | 7 | 37 |
| Fatigue | 3 | 18 | 7 | 37 |
| Abdominal pain | 2 | 12 | 4 | 30 |
| Aspartate aminotransferase increased | 2 | 12 | 4 | 30 |
| Nausea | 2 | 12 | 4 | 30 |
| Vomiting | 2 | 12 | 4 | 30 |
| Each of 15 different types of toxicitiesc | 1 | 6 | 1 | 23 |
Toxicity types are listed in decreasing order of frequency
90 % Wilson type confidence limit (CL)
Anemia, hyperkalemia, hypoalbuminemia, lymphocyte count decreased, generalized muscle weakness, constipation, hyponatremia, hip fracture, blood bilirubin increased, dyspnea, enterocolitis, hypophosphatemia, platelet count decreased, non-cardiac chest pain, and elevated LDH
Clinical efficacy
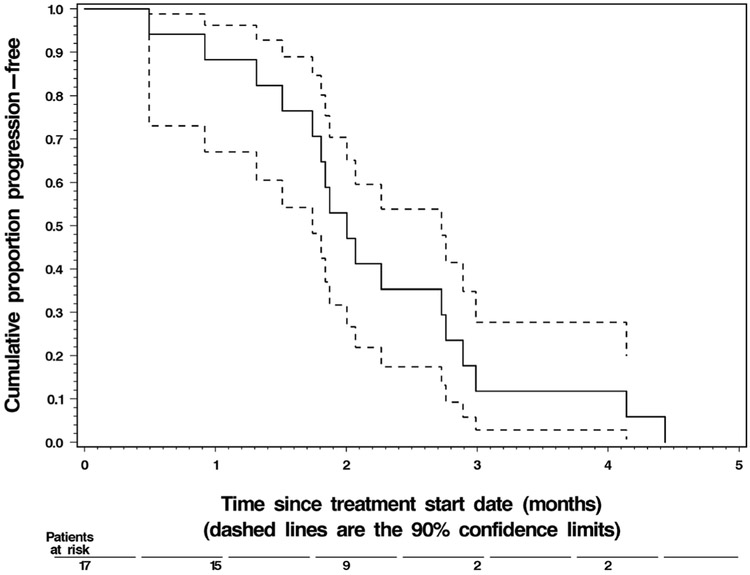
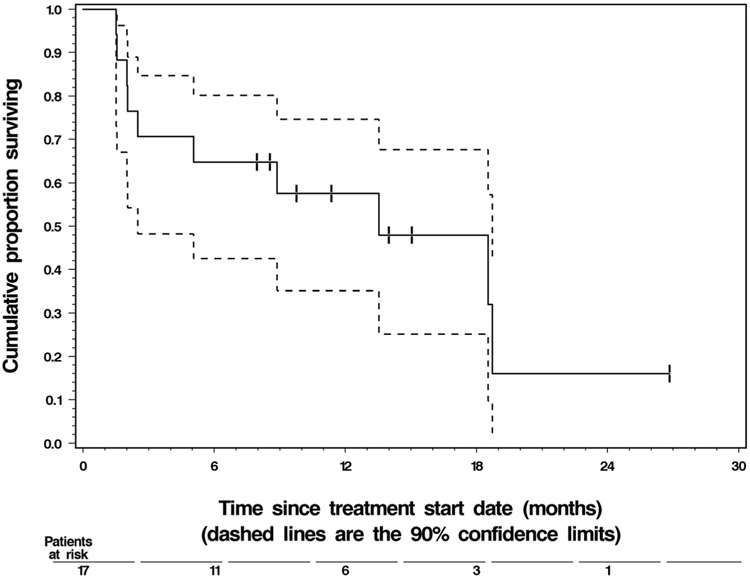
None of the 17 treated patients achieved the primary endpoint of remaining on ganetespib and progression free for at least 6 months (90 % CI: 0.00–0.14). However, the true 6-month PFS rate was at most 0.20, which was not considered adequate. Thus, as per the statistical design, the trial was terminated early after the interim analysis at the end of Stage 1. Only 2 patients achieved PFS > 4 months: one progressed at 4.1 months, and the other at 4.4 months after start of treatment with ganetespib. The median PFS was 1.9 months. The median OS was 12.5 months, and 7 patients were still alive as of the data cutoff in April 2013. Detailed summary statistics of PFS and OS are given in Table 4. Their Kaplan-Meier graphs are shown in Figs. 1 and 2, respectively.
Table 4.
Summary Statistics of Time-to-Event Endpoints (N = 17 treated patients)
| Time-to-event endpoint | N | Events | Point estimate | 90 % confidence interval | |
|---|---|---|---|---|---|
| Progression-free survival | 17 | 17 | |||
| Median | 1.9 months | 1.7 months | 2.7 months | ||
| 1-month rate | 87 % | 72 % | 100 % | ||
| 2-month rate | 47 % | 27 % | 67 % | ||
| 3 -month rate | 12 % | 0 % | 25 % | ||
| Overall survival | 17 | 10 | |||
| Median | 12.5 months | 1.5 months | 17.7 months | ||
| 3-month rate | 69 % | 50 % | 88 % | ||
| 6-month rate | 63 % | 43 % | 83 % | ||
| 9-month rate | 57 % | 35 % | 79 % | ||
| 12-month rate | 51 % | 29 % | 73 % | ||
Fig. 1.
Progression-free survival of the 17 treated patients. Prostate cancer progressed in all patients by the data cutoff date of April 2013
Fig. 2.
Overall survival of the 17 treated patients. Vertical tick marks on the Kaplan-Meier curve represent the 7 patients still alive at those respective points of follow-up
Correlative biomarkers
To evaluate the expression of tumor suppressor maspin and its associated proteins at the baseline and at the end of the treatment we analyzed the mRNA levels of these markers in patients’ PBMCs. As shown in Fig. 3, there was no statistically significant difference in mRNA expression before and after treatment of any of the markers studied.
Fig. 3.
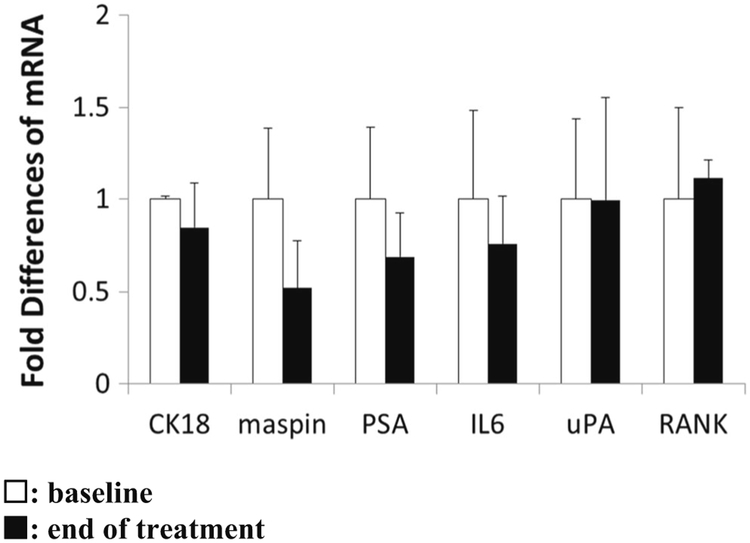
Detection of CK18, maspin, PSA, IL-6, uPA, and RANK mRNA by q-RTPCR in the patients’ PBMC before (n = 12) and at the end of treatment (n = 6). The threshold cycle (CT) numbers obtained from q-RT-PCR were first normalized by the internal control of GAPDH, then by the baseline values and presented as the fold change in mRNA expression
Discussion
Despite the potent inhibition of Hsp90 in preclinical studies, ganetespib did not show clinical efficacy in men with mCRPC. The primary objective of this clinical trial was to evaluate the impact of ganetespib on the 6-month PFS rate. No patients achieved 6 months of PFS, and only 2 achieved PFS > 4 months. This group of patients had fairly advanced disease with half of the patients receiving at least 4 prior regimens, and their results are comparable to those of patients who were treated with 17-AAG in our previous trial [15]. Although these results are disappointing, they seem to support an emerging consensus that the ineffectiveness of Hsp90 inhibition may not be due to failure of the Hsp90 inhibition, but rather that Hsp90 inhibition alone may not be mechanistically sufficient to yield cytotoxic effects in men with advanced prostate cancer. Centenera et al. reported evidence of efficacy of new Hsp90 inhibitors, NVP-AUY922 and NVP-HSP990, used to treat ex vivo culture of human prostate tumors [30]. They showed higher antiproliferative and proapoptotic activity than 17-AAG. Although similar results were seen with ganetespib in animal models, it is possible that in humans, treatment targeted at Hsp90 may lead to stasis or autophagy. Hsp90-dependent stability and the activity of Hsp90 client molecules, such as AR and other signaling molecules, might further depend on co-chaperone molecules, which may help preserve tumor cell viability by essential Hsp90-dependent chaperone mechanism even when Hsp90 ATPase is significantly inhibited.
The correlative markers, which were available both before and after treatment in only 6 patients, did not show any appreciable changes, suggesting that the current dose and schedule of treatment did not impact the expression of these specific markers. It remains unclear at this time of the significance of these results as the trial did not yield any patients with PFS ≥ 6 months.
Ganetespib is currently evaluated in a large Phase 3 study in patients with metastatic lung cancer. The role of Hsp90 is also being investigated in a variety of other tumor types with Hsp90 inhibitor as a single agent or in combination with cytotoxic therapy. Since Hsp90 not only chaperones oncogenic proteins but also tumor suppressors, such as p53 [31], inhibition of Hsp90 could be a double-edged sword. Results from this study underscores the need for a better understanding of the underlying molecular mechanisms of Hsp90 in prostate cancer.
Acknowledgments
This study was partially supported by NIH Cancer Center Support Grant CA022453 (to Bepler, G), NIH grants (CA127735 and CA084176 to Sheng, S), Fund for Cancer Research (to Sheng, S and Heath, E), and the Ruth Sager Memorial Fund (to Sheng, S).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Grossmann ME, Huang H, Tindall DJ (2001) Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst 93(22):1687–1697 [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD (2004) Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer 11(3):459–476 [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Sawyers CL (2005) Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol Off J Am Soc Clin Oncol 23 (32):8253–8261. doi: 10.1200/jco.2005.03.4777 [DOI] [PubMed] [Google Scholar]
- 4.Smith DF, Toft DO (2008) Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol (Baltimore, Md) 22(10):2229–2240. doi: 10.1210/me.2008-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10(8):537–549. doi: 10.1038/nrc2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5(10):761–772. doi: 10.1038/nrc1716 [DOI] [PubMed] [Google Scholar]
- 7.Cardillo MR, Ippoliti F (2006) IL-6, IL-10 and HSP-90 expression in tissue microarrays from human prostate cancer assessed by computer-assisted image analysis. Anticancer Res 26(5a):3409–3416 [PubMed] [Google Scholar]
- 8.Reebye V, Querol Cano L, Lavery DN, Brooke GN, Powell SM, Chotai D, Walker MM, Whitaker HC, Wait R, Hurst HC, Bevan CL (2012) Role of the HSP90-associated cochaperone p23 in enhancing activity of the androgen receptor and significance for prostate cancer. Mol Endocrinol (Baltimore, Md) 26(10):1694–1706. doi: 10.1210/me.2012-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein IB (2002) Cancer. Addiction to oncogenes–the achilles heal of cancer. Science (New York, NY) 297(5578):63–64. doi: 10.1126/science.1073096 [DOI] [PubMed] [Google Scholar]
- 10.Caplan AJ (1999) Hsp90’s secrets unfold: new insights from structural and functional studies. Trends Cell Biol 9(7):262–268 [DOI] [PubMed] [Google Scholar]
- 11.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, Scher HI, Rosen N (2002) 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res 8 (5):986–993 [PubMed] [Google Scholar]
- 12.Eskew JD, Sadikot T, Morales P, Duren A, Dunwiddie I, Swink M, Zhang X, Hembruff S, Donnelly A, Rajewski RA, Blagg BS, Manjarrez JR, Matts RL, Holzbeierlein JM, Vielhauer GA (2011) Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer 11:468. doi: 10.1186/1471-2407-11-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamoureux F, Thomas C, Yin MJ, Kuruma H, Fazli L, Gleave ME, Zoubeidi A (2011) A novel HSP90 inhibitor delays castrate-resistant prostate cancer without altering serum PSA levels and inhibits osteoclastogenesis. Clin Cancer Res 17(8):2301–2313. doi: 10.1158/1078-0432.ccr-10-3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Malley KJ, Langmann G, Ai J, Ramos-Garcia R, Vessella RL, Wang Z (2012) Hsp90 inhibitor 17-AAG inhibits progression of LuCaP35 xenograft prostate tumors to castration resistance. Prostate 72(10):1117–1123. doi: 10.1002/pros.22458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath EI, Hillman DW, Vaishampayan U, Sheng S, Sarkar F, Harper F, Gaskins M, Pitot HC, Tan W, Ivy SP, Pili R, Carducci MA, Erlichman C, Liu G (2008) A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin Cancer Res 14(23):7940–7946. doi: 10.1158/1078-0432.ccr-08-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh WK, Galsky MD, Stadler WM, Srinivas S, Chu F, Bubley G, Goddard J, Dunbar J, Ross RW (2011) Multicenter phase II trial of the heat shock protein 90 inhibitor, retaspimycin hydrochloride (IPI-504), in patients with castration-resistant prostate cancer. Urology 78(3):626–630. doi: 10.1016/j.urology.2011.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Trepel JB, Neckers LM, Giaccone G (2010) STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs (London, England : 2000) 11 (12) :1466–1476 [PubMed] [Google Scholar]
- 18.Ying W, Du Z, Sun L, Foley KP, Proia DA, Blackman RK, Zhou D, Inoue T, Tatsuta N, Sang J, Ye S, Acquaviva J, Ogawa LS, Wada Y, Barsoum J, Koya K (2012) Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther 11(2): 475–484. doi: 10.1158/1535-7163.mct-11-0755 [DOI] [PubMed] [Google Scholar]
- 19.Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, Chen L, Li D, Carretero J, Li YC, Sinha P, Carey CD, Borgman CL, Jimenez JP, Meyerson M, Ying W, Barsoum J, Wong KK, Shapiro GI (2012) Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res 18 (18):4973–4985. doi: 10.1158/1078-0432.ccr-11-2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho DC HE, Cleary JM, Kwak EL, Gandhi L, Lawrence DP, Zack C, Teofilovici F, Bradley R, Karol MD, Shapiro G, LoRusso P A phase I dose-escalation study of the Hsp90 inhibitor ganetespib (STA-9090) administered twice weekly in patients with solid tumors: Updated report. AM SOC CLIN ONCOL ANN MEET 2011. 29 June 3–7 Abs 3051 [Google Scholar]
- 21.Goldman JW RR, Gordon GA, Vukovic VM, Bradley R, Rosen LS A phase I dose-escalation study of the Hsp90 inhibitor STA-9090 administered once weekly in patients with solid tumors. AM SOC CLIN ONCOL ANN MEET 2010. 46 June 5 Abs 2529 [Google Scholar]
- 22.Li X, Yin S, Meng Y, Sakr W, Sheng S (2006) Endogenous inhibition of histone deacetylase 1 by tumor-suppressive maspin. Cancer Res 66(18):9323–9329. doi: 10.1158/0008-5472.can-06-1578 [DOI] [PubMed] [Google Scholar]
- 23.Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S (2002) Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene 21(26):4089–4098. doi: 10.1038/sj.onc.1205507 [DOI] [PubMed] [Google Scholar]
- 24.Li X, Chen D, Yin S, Meng Y, Yang H, Landis-Piwowar KR, Li Y, Sarkar FH, Reddy GP, Dou QP, Sheng S (2007) Maspin augments proteasome inhibitor-induced apoptosis in prostate cancer cells. J Cell Physiol 212(2):298–306. doi: 10.1002/jcp.21102 [DOI] [PubMed] [Google Scholar]
- 25.Tahmatzopoulos A, Sheng S, Kyprianou N (2005) Maspin sensitizes prostate cancer cells to doxazosin-induced apoptosis. Oncogene 24(34):5375–5383. doi: 10.1038/sj.onc.1208684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernardo MM, Meng Y, Lockett J, Dyson G, Dombkowski A, Kaplun A, Li X, Yin S, Dzinic S, Olive M, Dean I, Krass D, Moin K, Bonfil RD, Cher M, Sakr W, Sheng S (2011) Maspin reprograms the gene expression profile of prostate carcinoma cells for differentiation. Genes & Cancer 2(11):1009–1022. doi: 10.1177/1947601912440170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10 [DOI] [PubMed] [Google Scholar]
- 28.Hintze J (2008) Power and sample size (PASS). NCSS, Kaysville [Google Scholar]
- 29.Lee E, Wang JW (2003) Statistical methods for survival data analysis 3edn. Wiley, New York [Google Scholar]
- 30.Centenera MM, Gillis JL, Hanson AR, Jindal S, Taylor RA, Risbridger GP, Sutherland PD, Scher HI, Raj GV, Knudsen KE, Yeadon T, Tilley WD, Butler LM (2012) Evidence for efficacy of new Hsp90 inhibitors revealed by ex vivo culture of human prostate tumors. Clin Cancer Res 18(13):3562–3570. doi: 10.1158/1078-0432.ccr-12-0782 [DOI] [PubMed] [Google Scholar]
- 31.Blacklock K, Verkhivker GM (2013) Experimentally guided structural modeling and dynamics analysis of Hsp90-p53 interactions: allosteric regulation of the Hsp90 chaperone by a client protein. J Chem Inf Model 53(11):2962–2978. doi: 10.1021/ci400434g [DOI] [PubMed] [Google Scholar]