Abstract
One of the fundamental traits of immune cells in rheumatoid arthritis (RA) is their ability to proliferate, a property shared with the joint-resident cells that form the synovial pannus. The building of biomass imposes high demands for energy and biosynthetic precursors, implicating metabolic control as a basic disease mechanism. During preclinical RA, when autoreactive T cells expand and immunological tolerance is broken, the main sites of disease are the secondary lymphoid tissues. A metabolic signature has been identified for naïve CD4 T-cells in RA patients, characterized by dampened glycolysis, low ATP levels and enhanced shunting of glucose into the pentose phosphate pathway. Equipped with high levels of NADPH and depleted of intracellular reactive oxygen species, such T cells hyperproliferate and acquire proinflammatory effector functions. During clinical RA, immune cells co-exist with stromal cells in the acidic milieu of the inflamed joint. This microenvironment is rich in metabolic intermediates, such as ATP, glutamate and succinate, which are released into the extracellular space to shape cell-cell communication and the functional activity of tissue-resident cells. Increasing awareness of how metabolites regulate signalling pathways, guide posttranslational modifications, change the epigenetic landscape and condition the tissue microenvironment will help in connecting environmental factors to pathogenic behaviour of T cells in RA.
Subject ontology terms: Health sciences / Rheumatology / Rheumatic diseases / Rheumatoid arthritis, [URI /692/4023/1670/498], Health sciences / Pathogenesis / Immunopathogenesis, [URI /692/420/2780], Biological sciences / Chemical biology / Metabolic pathways, [URI /631/92/1643]
Observations made over 30 years ago, introduced the concept of preclinical autoimmunity, which is characterized by the presence of autoantibodies long before the appearance of disease symptoms, thereby fundamentally changing the way we understand autoimmune disease. This concept, which created a clear separation in time and space between disease onset and clinical manifestations, is now well established in several autoimmune diseases, including rheumatoid arthritis (RA)1, 2, systemic lupus erythematosus (SLE)3 and type 1 diabetes mellitus4, 5. The idea of preclinical autoimmunity has influenced mechanistic studies and has given rise to the emerging field of preventative immunotherapy to re-induce immune tolerance6, 7.
Immune dysregulation in patients with RA occurs many years before joint inflammation begins8–10 and is easily detectable by the presence of antibodies against selected autoantigens. The decisive initial insult is the loss of self-tolerance, a host-protective function guarded by the adaptive immune system. Accordingly, disease-associated genetic polymorphisms identify T cells as key drivers of immune abnormalities in RA11, 12. Aberrant proliferation, commitment to proinflammatory effector functions, help to autoreactive B cells and tissue invasive properties are all phenotypic traits shared by T cells in RA and other chronic inflammatory conditions. These traits impose substantial metabolic demands on T cells; and metabolic reprogramming could have hallmark status in explaining the convergence of phenotypic traits that ultimately result in autoimmune inflammation.
Emerging metabolic patterns in T cells from patients with RA contrast those in chronically activated healthy T cells, fostering the hope that metabolic programmes delineated in patient-derived cells represent vulnerabilities that can be therapeutically exploited. The inflammatory milieu of the inflamed joint has attracted attention as a site of hypermetabolic activity and high energy needs; however, molecular features that distinguish inflammation in rheumatoid joints from other similarly active tissue lesions have not yet emerged. Possible features include molecular signatures of chronically stimulated innate and adaptive immune cells and metabolic profiles derived from stromal components of the joint. Reversing metabolic phenotypes could provide strategies for modulating immune responses with the ultimate aim of reconstituting immune health and intercepting tolerance defects long before joint inflammation occurs.
Major challenges to an integrated view of immunometabolism in RA derive from the fact that the disease process stretches over decades, involves several stages and occurs in multiple tissue environments, including lymphoid and non-lymphoid organ sites. Although information on immune cell-conditioning by different tissue environments is still scant, studies of naive T cell populations not entrapped in the inflamed joints provide insights into primary immune responses and the early stages of RA. The joint lesion in the late stages of RA provides an opportunity to explore how cellular metabolism can condition the tissue milieu and how metabolites can ‘moonlight’ as intracellular and extracellular signalling molecules. In this Review, we examine emerging data on metabolism in immune cells in seropositive RA and look at how metabolic programmes affect the disease process, focusing on T cells as a key driver of tolerance breakdown.
T-cell metabolism in early RA
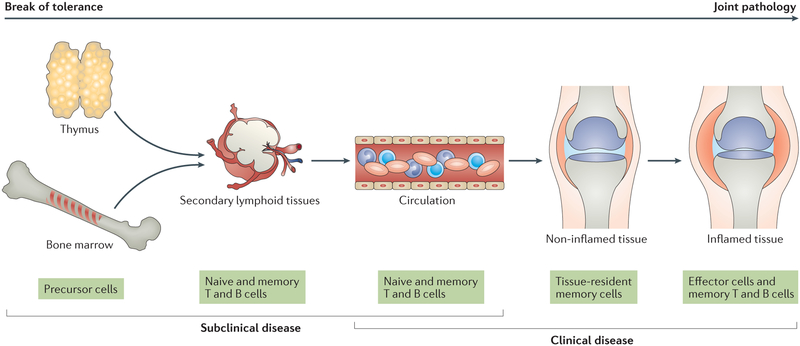
The early steps of the disease process in RA occur in lymphoid organs, where lymphocytes are primed and differentiate into effector and memory cells. Subsequently, self-reactive T cells and B cells become perpetually activated and expand, releasing cytokines and autoantibodies. In some, but not all, individuals who reach this stage, another protective hurdle is broken; autoreactive T cells and B cells invade the synovium, functioning as immunopathologic agents by forming organized lymphoid structures and eliciting defective repair mechanisms, supported by myeloid cells, endothelial cells, fibroblasts, chondrocytes and bone cells. Inflammation-induced neoangiogenesis provides easy access for immune cells into the synovial lesion13, 14. The joint is the most visible battleground, but by far not the only tissue affected by RA. Throughout all stages of RA, secondary lymphoid tissues supply T cells and B cells to peripheral tissues. Eventually, the spectrum of nonlymphoid organs targeted during RA widens, creating extra-articular manifestations. In all these tissue environments, communication between lymphoid and nonlymphoid cells ultimately determines the activation state, longevity and functional behaviour of the cells involved, and subsequently the tissue damage that is clinically associated with RA. Thus, the pathologic process leading to RA stretches over decades and involves multiple, fundamentally different tissue microenvironments (FIG.1). Although more information is needed for a contextual analysis of the metabolic environment in lymphoid tissues, progress has been made in understanding intracellular metabolic conditions in naive T cells, thus shedding light on early events in RA pathogenesis.
Figure 1. The disease process of rheumatoid arthritis in different tissue environments.
The disease process of rheumatoid arthritis (RA) involves multiple tissue environments and extends over several decades. An early event is the breakdown of immunologic tolerance, which occurs in lymphoid tissues. After expansion and maturation of autoreactive lymphocytes, autoantigens are encountered in peripheral tissues, eventually leading to the formation of tertiary lymphoid microstructures and chronic destructive inflammation. The inflamed synovial membrane and the disrupted tissue repair response represent the end stage of RA.
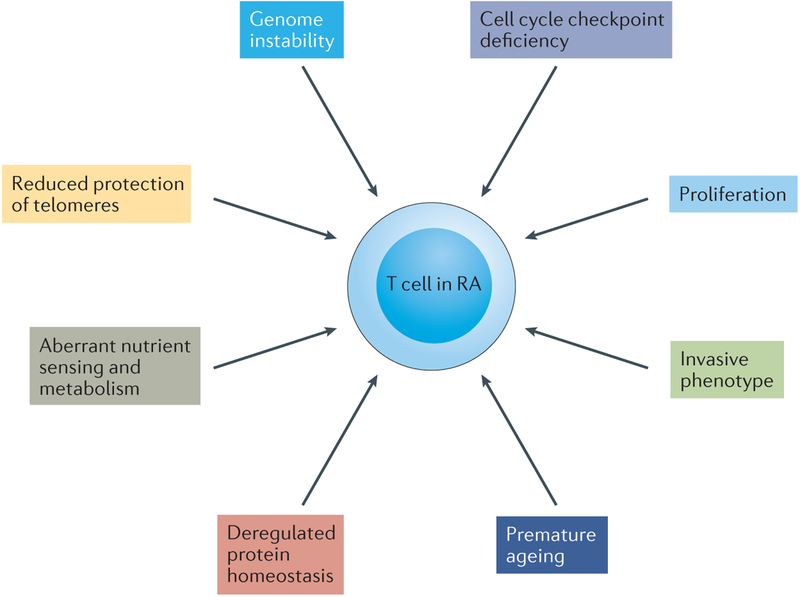
Over the past two decades, a series of fundamental characteristics have emerged that distinguish T cells from patients with RA from those in age-matched healthy individuals (FIG. 2). Notably, such characteristics extend beyond antigen specificity and include basic biological pathways that enable T cells to transition from protective to auto-aggressive modalities. Central to their role in adaptive immunity, T cells undergo profuse expansion and contraction15, 16. T cells are able to adapt to severely restrictive bioenergetic conditions17, continuing to produce cytokines and proliferate as long as glucose remains available18. Creating large amounts of biomass imposes a high demand for energy and biosynthetic precursors19–21.
Figure 2. Emerging hallmarks of T cells in rheumatoid arthritis.
Core hallmarks of T cells in rheumatoid arthritis (RA) are their ability to massively proliferate and to differentiate into proinflammatory effector cells. Several changes in the basic biologic pathways listed here distinguish T cells in healthy individuals from those in patients with RA and enable such T cells to deviate from their protective role to an autoinflammatory one. The molecular defects underlying pathogenic T-cell behaviour are currently being discovered; among them is the reprogramming of cellular metabolism, which fuels the functional capabilities of arthritogenic T cells.
Metabolic control of T cell function in early RA.
Like other proliferative cells, T cells in patients with RA utilize all possible energy sources (sugars, fats and proteins), but glucose remains their major life-sustaining nutrient22.
Glucose acts as an electron donor. Electron acceptor molecules capture some of the energy released by the stepwise oxidation of glucose and convert it into energy-rich ATP and NADH. One molecule of glucose is broken down into two molecules of pyruvate by the process of glycolysis, with a net gain of two molecules of ATP and two molecules of NADH. The glycolytic pathway is an ancient ATP-producing pathway that is extremely adaptable23; under acute energy requirements, glycolytic enzymes can be regulated within minutes. The drawback of this quick regulation is the incomplete oxidation of glucose and the build-up of lactate, which acidifies the cellular and extracellular microenvironments. Under oxygen-rich conditions, pyruvate is transported into the mitochondria and converted into acetyl-CoA to enter the tricarboxylic acid (TCA) cycle24, 25. This 8-step cycle generates NADH, FADH2 and GTP, which function as electron donors for the electron transport chain. During oxidative phosphorylation, protein complexes in the mitochondrial inner membrane transfer electrons, forming a transmembrane proton gradient and ultimately creating water. T cells, like all cells, harness the energy from this proton gradient to generate ATP, with oxidative phosphorylation yielding 15 times as much energy from each molecule of glucose than anaerobic glycolysis26.
Glycolysis and the Pentose Phosphate Pathway in T cells in RA.
Studies using naive CD4+CD45RA+ T cells from RA patients have delivered surprising results. Although naive T cells from healthy individuals meet activation-imposed energy demands by upregulating the glycolysis-activating enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), induction of this enzyme is reduced in T cells from patients with RA27, 28. Through its kinase activity, PFKFB3 produces large amounts of fructose-2,6-bisphosphate, which activates phosphofructokinase 1, a glycolytic rate-limiting enzyme. PFKFB3 thus determines the intensity of glycolytic flux and is considered a preferred pharmacologic target for inhibiting the growth of cancers29. PFKFB3 deficiency occurs at an early point in the life cycle of T cells of patients with RA and has profound metabolic and functional consequences (FIG. 3). As a result, lactate production is reduced and ATP levels are lower than in T cells from healthy individuals27, 28, identifying glycolytic ATP production as a major energy source in CD4+ T cells. In addition to US studies, low ATP production by circulating CD4+ T cells was also seen in patients with RA in a Japanese cohort attesting to the cross-ethnicity of this feature in RA30. A low ATP signature might help to identify naive T cells in secondary lymphoid tissues that are prone to autoreactivity. Moreover, T cells from patients with RA have an increased susceptibility to apoptosis and fail to upregulate autophagy as a compensatory pathway to energy generation27, 31, 32Impaired glycolysis has profound implications for such T cells and their immediate neighbourhood, as low levels of pyruvate production reduces the amount of substrate available for oxidative phosphorylation and thus reduces the generation of reactive oxygen species (ROS). At the same time, low levels of lactate production keeps the extracellular pH relatively high and reduced oxygen utilization counteracts hypoxia and its regulatory effects, meaning that these T cells might fail to adapt to tissue hypoxia.
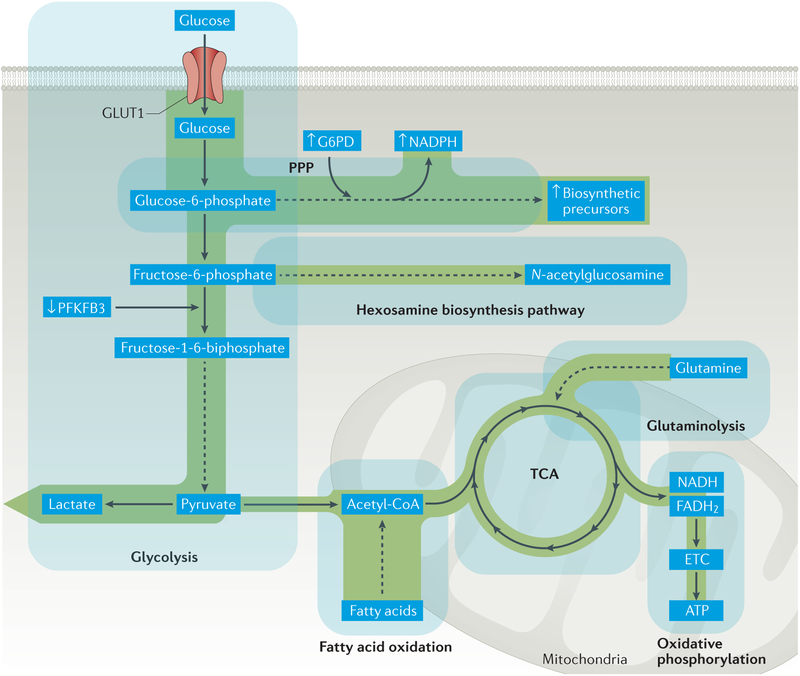
Figure 3. Glucose shunting into the pentose phosphate pathway in T cells in RA.
Glycolytic breakdown of glucose in T cells in rheumatoid arthritis (RA) is reduced as a result of diminished activity of the regulatory enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphophastase 3 (PFKFB3), which curbs ATP production. With increased activity of glucose-6-phosphate dehydrogenase (G6PD), glucose is shunted to the pentose phosphate pathway (PPP), yielding high amounts of the electron donor NADPH and generation of biosynthetic precursors. Cellular stores of glutathione are shifted towards the reduced form, cellular levels of reactive oxygen species (ROS) are depleted and cellular oxidant signalling is impaired. ETC, electron transport chain; GLUT1, glucose transporter type 1; TCA, tricarboxylic acid cycle.
If naive T cells from patients with RA reduce flux through the glycolytic pathway, what is the fate of glucose in these cells? Two side branches of glycolysis exist, which have a critical role in cellular homeostasis: the hexosamine biosynthesis pathway and the pentose phosphate pathway (PPP). Using glucose, glutamine, acetyl-CoA and uridine, cells can form N-acetylglucosamine for protein O-glycosylation and N-glycosylation through the hexosamine biosynthesis pathway33, 34. Post-translational modification of proteins by the addition of a single residue of O‐linked N‐acetylglucosamine has been implicated in regulating the activation of T cells and B cells35–37. Whether the post-translational modification of key molecules by the addition of O‐linked N‐acetylglucosamine differs in T cells in patients with RA from those in healthy individuals is currently unknown.
The PPP enables cells to generate products that are crucial for T-cell function, as they fuel biomass generation and thus enable T-cell expansion. Glucose is converted to glucose-6-phosphate, which enters the PPP to supply the pentose sugars required for nucleotide and nucleic acid synthesis. Glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme of the PPP, responsible for the generation of NADPH and ribulose-5-phosphate. NADPH is the cell’s most abundant reductive molecule, which, by its electron donor function, holds an important position in regulating the cellular redox status and providing reductive elements for the production of biomolecules. Naive T cells from patients with RA can be distinguished from those in healthy individuals by their distinctive shunting of glucose into the PPP (FIG. 3). They express increased amounts of G6PD mRNA and protein, with a level of G6PD enzyme activity twice as high compared to healthy28. Consequently, glucose-6-phosphate is shunted into the PPP, generating high amounts of NADPH and reduced glutathione. NADPH is important in controlling lipid synthesis, but the effect of increased PPP utilization in patients’ T cells on lipid homeostasis is unknown.
Metabolic reprogramming in arthritogenic T-cell function.
By reducing cellular levels of ROS, PPP shunting affects several aspects of essential T-cell functions (BOX 1). Short-lived and highly reactive ROS act as secondary messengers, swiftly altering cellular signalling pathways38, 39. Basic cellular functions, such as proliferation, differentiation, migration and cell death are now considered to be under the control of ROS40. Only if the equilibrium with antioxidant defence systems is disrupted will ROS damage proteins, nucleic acids, lipids, membranes and organelles, eventually triggering cell death. Studies of purified naive T cells from patients with RA and age-matched healthy individuals show that patient-derived T cells are hyperproliferative27, 28, a state inducible in cells from healthy individuals by ROS scavenging28. The underlying molecular defect is a shortening of the G2/M phase of the cell cycle. G2/M bypassing occurs when there is insufficient activation of the cell cycle kinase ATM, part of a redox-dependent signalling pathway41. T cells from patients with RA that have low levels of ATM commit to the type 1 helper T (TH1) cell and type 17 helper T (TH17) cell lineages, rather than differentiating into regulatory T (Treg) cells (BOX 1). In a cohort of patients with RA, the ratio of G6PD to PFKFB3 correlated with clinical disease activity (as measured by DAS28), indicating a clinically relevant outcome of diverting glucose into the PPP28.
Box 1 Functional consequences of metabolic reprogramming in T cells in RA.
Alterations in how T cells in patients with rheumatoid arthritis (RA) utilize glucose and generate biosynthetic precursors has profound implications on their intracellular signalling pathways and, eventually, on their differentiation into immune effector cells. Definition of the underlying molecular defects in such T cells has revealed novel therapeutic opportunities in potentially rewiring metabolic networks.
Metabolic reprogramming of T cells in RA
CD4 T cells from patients with RA have markedly reduced glycolytic activity27, resulting in low levels of ATP and lactate.
These T cells shunt glucose to the pentose phosphate pathway28, generating high levels of NADPH and an abundance of biosynthetic precursors.
Excess NADPH leads to accumulation of reduced glutathione and the depletion of cellular ROS, impairing oxidation-dependent signalling pathways.
Effect of metabolic constraints on cellular signalling pathways27, 28, 136, 137
Functional consequences of intracellular signalling abnormalities
G2/M cell cycle phase bypass, leading to hyperproliferation27, 28
Therapeutic opportunities
Improve T-cell redox signalling by using, for example, a γ-glutamylcysteine synthetase inhibitor or a redox-cycling agent
Restore ATM activation
Enhance AMPK activity
Implicating reductive stress, as opposed to oxidative stress, in conferring risk for autoimmune arthritis might be considered contrary to the prevailing dogma, but fits well with groundbreaking work by Holmdahl et al.42, 43. This research group identified neutrophil cytosolic factor 1 (NCF1) and its associated ROS production as major genetic elements in autoimmune arthritis. Previous studies had identified NCF1 as a protective gene in rat arthritis44. Subsequent work provided detailed mechanistic information on how ROS can suppress inflammatory responses. One of these mechanisms connected macrophage-generated ROS to the suppression of T-cell reactivity and to reduced arthritis severity45. ROS inhibited pro-arthritogenic T cells by altering thiol groups on the T-cell membrane (and possibly in relevant signalling molecules), effectively modulating T-cell activation and expansion46. ROS deficiency seems to facilitate spontaneous autoimmunity by promoting a type I interferon signature47. Notably, sufficient availability of ROS was also required for the induction of Treg cells48, emphasizing the role of redox signalling in balancing proinflammatory and anti-inflammatory immune responses. Thus, redox signalling is critically involved in multiple aspects of T-cell biology, with an emerging theme of ROS preventing inappropriate T-cell activation49, 50.
Besides activating the cell cycle kinase ATM, ROS serve as secondary messengers in many signalling pathways, including networks that sense the energetic status of a cell and regulate metabolism such as the mechanistic target of rapamycin (mTOR) and AMP-activated kinase (AMPK) pathways. Coincidentally, mTOR and AMPK also function as master regulators of T-cell differentiation and cell fate decisions. As AMPK and mTOR monitor the availability of nutrients, they guide T cells into clonal expansion or reduction and into committed functional lineages and effector functions. By adjusting the activity of AMPK and mTOR, lymphocytes not only match energy supply with demand, they also make decisions about entry into the cell cycle and conversion from naive to memory and terminally-differentiated effector cells.
AMPK acts as a redox sensor51, being activated by increasing AMP:ATP ratios, which result in the switching on of catabolic pathways and the switching off of anabolic pathways. Redox conditions predict that patient-derived T cells will not have high levels of activated AMPK (BOX 1). The downstream consequences of a lack of activated AMPK would be profound, as AMPK activation affects several basic cellular functions52, including glucose uptake, glycolytic flux, mitochondrial biogenesis, fatty acid oxidation, transcriptional activity and cell cycle control. Little is known about the status of AMPK activation in T cells, but ROS deficiency is predicted to paralyze this master integrator of metabolism, proliferation and differentiation. In line with this model, therapeutic AMPK activation reportedly suppresses experimental arthritis53, 54 and methotrexate-mediated activation of an AMPK-dependent pathway is implicated in protecting the vasculature against inflammation55.
Protein synthesis, cell growth, survival and proliferation, as well as cell fate decisions in differentiating T cells, are under the control of mTOR. Together with AMPK, mTOR is a central communicator, integrating environmental signals with cellular function and differentiation. Aberrant mTOR activation is associated with cellular senescence and the mTOR complex 1 inhibitor, rapamycin, has been investigated as a therapeutic agent to counteractchronic cellular stimulation. mTOR is one of the anchor molecules that interlinks nutrient availability, energy generation and utilization, mitochondrial activity and T-cell differentiation. Energy deprivation (as indicated by AMPK activation) and the suppression of mTOR activity are supposed to drive T cells to differentiate into Treg cells19, 56. However, the precise contribution of different components of the mTOR pathway is not entirely understood, and somewhat contradictory findings regarding mTOR’s role in T-cell biology have fuelled discussions within the scientific community57. The ability of mTOR to integrate the regulation of nutrient supply, bioenergetics and T-cell differentiation makes it an excellent target for therapeutic intervention to suppress abnormal T-cell differentiation during the early stages of RA58–61.
A common factor of the crosstalk between extracellular and intracellular cues seems to be ROS, which swiftly adapt cellular functions to the immediate tissue environment and the needs of the host. T-cell reliance on ROS-dependent signals is likely to be particularly important in microenvironments with low levels of ROS, e.g. the well oxygenized tissue sites of secondary lymphoid organs. In the arthritic joint, ROS are abundant and participate in the feed-forward amplification of tissue damage62. Although ROS are needed to prevent emerging autoimmunity, ROS scavenging might be beneficial in lowering the inflammatory burden in the arthritic synovium. The multifunctional roles of ROS represent excellent opportunities to target different stages of RA therapeutically.
Immunometabolism in late stage RA
In the later stages of RA, the disease process migrates from the secondary lymphoid tissues to peripheral tissue environments, in particular the synovial lining of diarthrodial joints. Here, T cells, B cells and plasma cells, together with specialized antigen-presenting cells create organized lymphoid structures63, 64 and interact with tissue-resident cells such as synovial fibroblasts, endothelial cells, macrophages, neuronal cells, chondrocytes and bone cells65. The synovial pannus has tissue-destructive and invasive properties66, 67, displaying features of a non-healing tissue wound. Fundamental biologic processes of the synovial pannus such as sustained proliferative signalling, angiogenesis, cellular de-differentiation and unbalanced bone turnover are associated with high metabolic demands in all cell types involved. Overall, the inflamed joint in RA patients is a hypermetabolic lesion.
Hypermetabolic activity of immune and stromal cells creates a tissue environment that by itself regulates cellular behaviour. Tissue-infiltrating immune cells, specifically T cells entrapped in the joints of patients with RA, should encounter chronic stimulatory conditions, which in turn should lead to exhaustion and cellular senescence68–71. This aspect of T cell biology in RA is not understood. Of similar importance is the recent recognition that hypermetabolic cells release into their environment metabolic intermediates, which are being sensed by neighbouring cells and thus shape the intensity, duration and type of inflammation. Obviously, better understanding of such metabolic intermediates and their pro-inflammatory functions could broaden the therapeutic targets in established RA, extending beyond the highly successful blockade of end-stage inflammatory mediators, particularly proinflammatory cytokines such as TNF and IL-672, 73.
Tissue Oxygen.
By engulfing aerobic prokaryotes, which eventually became mitochondria, eukaryotic cells acquired the ability to utilize oxygen for energy production, and tissue oxygen levels became a major regulator of bioenergetics. First investigated in cancer cells, which have high energy needs, hypoxia-inducible factor 1α (HIF-1α) senses and connects oxygen availability to metabolic activity and ATP production, holding a key position in both aerobic and anaerobic glycolysis74, 75. In tumours, HIF-1α is responsible for excessive angiogenesis, clonal selection of tumour cells and the enforcement of metabolic adaptations. Tumours often have anoxic regions and extreme hypoxic gradients throughout the tumour tissue (ranging from 0.1–6% pO2)76. Although there does not seem to be a single hypoxic threshold that can be applied generally, oxygen levels required for hypoxia-induced gene expression are estimated to be in the range of 1–15 mmHg77. The inflammatory lesion of the rheumatic joint is considered to be a hypoxic site, in which HIF-1α functions as a metabolic inducer78, 79. Low tissue oxygen levels are implicated in inducing mitochondrial dysfunction and in promoting a switch to glycolysis62, 80. Biniecka and colleagues arthroscopically measured tissue oxygen tensions in the synovium of patients with inflammatory arthritis and correlated tissue hypoxia with the induction of glycolytic activity80. In a group of 6 patients, synovial oxygen partial pressures increased from below 20 mmHg to above 20 mmHg upon response to treatment with TNF inhibitors. A group of 13 patients who did not respond to TNF inhibitor treatment had median tissue oxygen levels above 20 mmHg before and after TNF blockade. The data indicate that the inflamed synovial lining is not as hypoxic as tumour tissues, in line with the clinical observation that in the inflamed synovium, tissue expansion outweighs tissue death. Hypoxia, as well as excessive angiogenesis, are both considered proinflammatory81, raising the question whether therapeutic targeting should imply increasing or reducing tissue oxygen supply.
Glycolytic intermediates.
The acidification of the rheumatoid joint (and other RA-associated exudates) has fascinated the scientific community for more than 50 years82, 83, giving rise to the idea that low glucose levels and high lactate levels could have diagnostic value. Work from the early 1970s showed higher mean oxygen uptake rates and higher mean rates of lactate appearance in saline deposited in the joints of patients with RA compared with the joints of patients with degenerative joint disease84. Studies from the past few years have confirmed the decline in glucose and the appearance of lactate in synovial fluid85, which are compatible with the requirement of the tissue lesion to utilize glucose for fast access to energy. RA synovial fibroblasts shift their metabolism towards anaerobic glycolysis86 and are especially efficient in exporting lactate into the extracellular space87, where it acidifies the microenvironment and also participates in regulating the function of surrounding cells (FIG.4). Local effects of lactate depend upon its concentration, as well as the ability of tissue-resident cells to sense and take-up acids. Acid-sensing ion channels (cation channels activated by extracellular acid) are implicated in acid-induced cell injury, such as during chondrocyte apoptosis88. Tissue-residing T cells are also affected by an environment that contains lactate and might actively contribute by exporting lactate themselves68. When exposed to sodium lactate or lactic acid, the motility of CD4+ and CD8+ T cells is inhibited, possibly prolonging the retention of tissue-infiltrating T cells89. Additionally, lactate promoted IL-17 production by CD4+ T cells and induced a loss of cytolytic function in CD8+ T cells89. Taken together, these results suggest that the switch towards anaerobic glycolysis in synovial cells might sustain proinflammatory amplification loops and contribute directly to cellular injury (FIG.4).
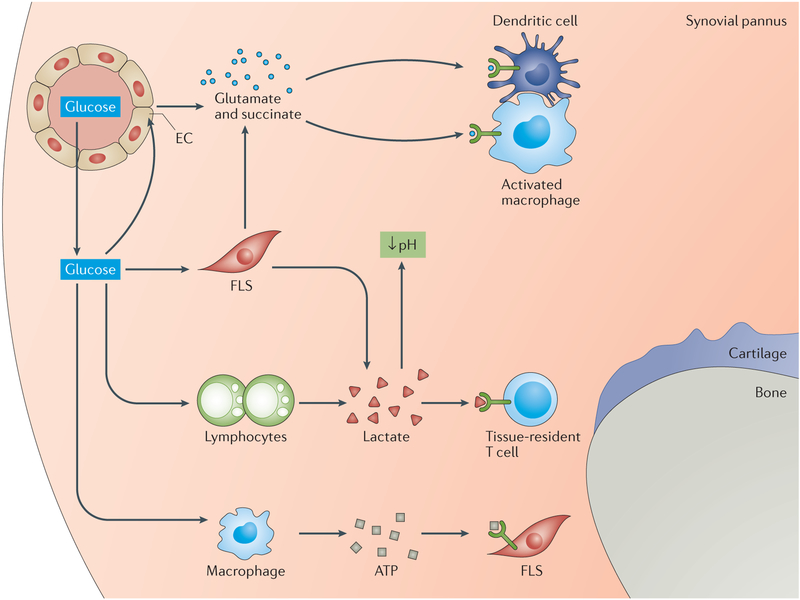
Figure 4. Metabolic intermediates in the RA joint.
The synovial pannus in the joints of patients with rheumatoid arthritis (RA) is a hypermetabolic lesion that demands high amounts of nutrients and oxygen to fulfil the energy and biosynthetic needs of its proliferative cells. The presence of glucose enables rapid and adaptive production of ATP, even under hypoxic conditions. Metabolic products such as lactate and ATP are released into the extracellular space, where they promote cell–cell communication and regulatory control. Lactate acidifies the tissue microenvironment and might directly contribute to cellular injury. With high levels of mitochondrial activity in tissue-resident and invasive cells, intermediates of the tricarboxylic acid cycle such as succinate and glutamate are secreted into the extracellular space. Signals transduced through specialized receptors (such as hydroxycarboxylic acid receptor 1 for lactate and succinate receptor 1 for succinate) regulate the functions of cells that sense extracellular metabolites. Here, metabolites serve as signalling molecules in cell–cell communication and in microenvironmental surveillance.
In contrast to lactate, which seems to have mainly proinflammatory functions, another intermediate of the glycolytic pathway, fructose 1,6-bisphosphate, exhibits strong anti-inflammatory action. A single treatment with fructose 1,6-bisphosphate markedly suppressed arthritis in two animal models90. The protective mechanism has been linked to improved generation of ATP, which is then hydrolyzed by the nucleotidases CD39 and CD73 to produce adenosine. These results support the observation that the low level of ATP in T cells in patients with RA is ultimately proinflammatory27, 28.
Mitochondrial intermediates.
Increased oxygen uptake rates in the RA joint84, helped by the excessive angiogenesis91, 92 known to occur at this chronically stimulated tissue site, indicate that mitochondrial metabolism remains intact and contributes to ATP generation. Mitochondria not only consume oxygen to generate ATP, but also produce metabolic intermediates of the TCA cycle that participate in a multitude of metabolic pathways93. The concentrations of several of these TCA cycle intermediates, including succinate, citrate, glutamate, fumarate and aspartate are altered in continuously proliferating cancer cells94. The same mitochondrial intermediates are also enriched in synovial fluid95 and, like lactate, have functions beyond their role as anabolic metabolites96–98. The presence of such TCA cycle intermediates in synovial fluid suggests that the synovial pannus represents an oxygenated environment with ample access to sources of carbohydrates, amino acids and lipids.
Several TCA cycle intermediates have been identified as potential amplifiers of inflammatory activity and as possible therapeutic targets99–102 (FIG.4). Glutamate concentrations are elevated in arthritic joints; the triggering of glutamate receptors increases the release of IL-6 and induces arthritic pain103. Synovial fluid from patients with RA also contains abundant amounts of succinate, a product released into the extracellular milieu by activated macrophages. Interestingly, such macrophages express succinate receptor 1, through which they sense succinate levels and induce inflammasome activation104. Previously, succinate has been demonstrated to modulate dendritic cell function by triggering succinate receptor 1105 and to induce IL-1β production in lipopolysaccharide-treated macrophages by stabilizing HIF-1α106. Another important product of the mitochondria of activated tissue macrophages are ROS, which remodel inflammatory signalling networks and sustain IL-1β and IL-6 production by enhancing the phosphorylation of signal transducer and activator of transcription 3107.
Alarmins.
Cells under stress actively or passively release endogenous danger signals (known as alarmins), including high mobility group protein B1 (HMGB1), S100A proteins, heat shock proteins and purine metabolites (such as uric acid and ATP). Innate and adaptive immune cells sense such extracellular alarmins through specific receptors, which trigger and fine-tune inflammatory and repair responses108, 109. ATP, produced by glycolysis or oxidative phosphorylation in the mitochondria, is exported into the extracellular space, where it has been associated with anti-inflammatory activity (FIG.4). Lymphocytes from patients with RA have high levels of CD39 activity, which could lead to insufficient preservation of extracellular ATP110. Conversely, ATP-dependent activation of the P2X7 purine receptor on mast cells111 upregulates protein arginine deiminase, an enzyme involved in the conversion of arginine residues into citrulline, a post-translational modification considered to be arthritogenic in RA. P2X7 is found on human fibroblast-like synoviocytes112, enabling them to closely monitor ATP levels in the extracellular milieu. In general, by monitoring ATP levels cells can closely assess the metabolic activity and the metabolic pathways preferred by their neighbouring cells. Expression of CD39 on T cells seems to be particularly important for memory T cells and is a marker of T-cell aging113.
DNA, both of nuclear and mitochondrial origin, can acquire alarmin function when released into the extracellular space, where nucleic acids can engage pattern-recognition receptors on surrounding cells114. This mechanism could enable the communication of metabolic stress, as synovial cells sense not only mitochondrial intermediates but also mitochondrial DNA115.
Other inflammatory networks.
A number of inflammatory processes, ultimately dependent on energy supply and the metabolic environment contribute to the pathogenesis of RA as permissive factors and aggravators of tissue injury. Abnormalities in autophagy are suspected to determine cellular hyperplasia as well as cell loss116. PFKFB3, the glycolytic enzyme implicated in metabolic reprogramming of T cells in the early stages of RA27, 31, is also linked to the control of autophagy32. The identification of genetic mutations in inflammasome activation pathways in patients with autoinflammatory syndromes has also been instructive in connecting molecular networks to inflammatory outcomes, and has highlighted the multiple roles of mitochondria in regulating inflammation117, 118.
Metabolomics in RA
Progress in technologies such as mass spectrometry and chromatography that enable increased mass precision and metabolite identification have driven interest in the measurement of hundreds of metabolites in cells, tissues and fluids. In patients with RA, plasma or serum, synovial fluid and synovial tissue have all been utilized for metabolomics studies119–122. No unifying metabolic marker(s) have been discovered, but some common signatures have emerged. Using metabolomics, patients can be differentiated from healthy controls, anti-inflammatory treatments can be seen to affect metabolite patterns, and patients in therapy-induced remission can still be distinguished from healthy individuals, showing that current therapies improve but do not cure underlying pathologies. Importantly, these studies show that inflammation in patients with RA is a complex process, extending far beyond lactate production.
Although less efficient than oxidative phosphorylation, the evolutionarily ancient process of anaerobic glycolysis can be upregulated within minutes. Metabolomic studies in patients with RA have revealed that a switch to aerobic glycolysis, the so-called ‘Warburg effect’, is not enough to capture all metabolic adaptations associated with the disease process. In a 2010 study by Lauridsen et al.123, increased amounts of cholesterol and unsaturated lipids and a decreased amount of HDL distinguished patients with RA from healthy individuals. Decreased lipid signals were also the major discriminators between patients and healthy individuals in a 2013 study by Young et al.124. These authors also identified high levels of 3-hydroxybutyrate as a marker for RA, suggesting increased lipolytic activity124. A 2016 study highlighted increased levels of fatty acids and cholesterol and decreased levels of amino acids and glucose in RA125. Another signature reported for metabolites in the blood of patients with RA includes low levels of valine, isoleucine, alanine, creatinine, histidine and lactate and high levels of 3-hydroxyisobutyrate, acetate, N-acetylcysteine, acetoacetate and acetone126. Fatigue in patients with RA is associated with downregulation of metabolites from the urea cycle (such as fatty acids, tocopherols, aromatic amino acids and hypoxanthine)127.
Collectively, metabolomic studies provide many opportunities both clinically and conceptually, yet the data have not yet been used to their full effect. Part of the problem lies in the heterogeneity of cells and tissues that actively or passively release metabolites that accumulate in body fluids. Functional effects of such metabolites are dictated by their abundance in tissue microenvironments, the activity of transport mechanisms and the diversity of cell types that generate and use them. Increased levels of a metabolite might indicate a lack of utilization or compensatory production, whereas decreased levels might result from disproportionate utilization or insufficient generation. To define evidence-based pathways for potential clinical translation, researchers will need to use functional metabolomic studies to evaluate how defined metabolic products affect cellular and multi-cellular homeostasis.
An obvious question is whether the analysis of metabolites in the inflammatory environment, as captured in synovial fluid or tissue, yields novel insights into the nature of the inflammation. An abundance of succinate, aspartate, glutamate and citrulline in synovial fluid from patients with RA attests to the intense levels of mitochondrial metabolic activity95. Conversely, levels of isopalmitic acid, glycerol, myristic acid, palmitoleic acid, hydroxylamine, and ethanolamine are low in patients with RA100, 104. Likely, these shifts reflect metabolic adaptations and end-stage disease. Capturing early metabolic abnormalities, during early stages of the RA disease process, may be more amendable to metabolic interference.
Conclusions
As a prototypic autoimmune disease, RA begins with the immune system making a fundamental mistake — not being able to distinguish self from non-self — with the end result of relentless inflammation in the synovial microenvironment. Although the location, intensity and specific pathways of disease vary over the lifetime of the patient, tolerance breakdown, subclinical RA and clinical RA share the need for cellular expansion and biomass generation. How cells fulfil these demands for cellular energy and biosynthetic precursors has emerged as a critical domain in autoimmune inflammation.
The early stages of RA, in which T cells break the state of tolerance and provide help to autoantibody-producing B cells, unfold in lymphoid tissues and involve cells that have not yet made a lineage commitment. Metabolically, naive T cells from patients with RA are deregulated and respond to activation by shunting glucose into the PPP. Such T cells generate low levels of ATP and lactate, but have excess NADPH and biosynthetic precursors. Consequently, these T cells have reduced levels of intracellular ROS, impaired redox signalling and insufficiently activate the cell cycle kinase ATM. The result is a hyperproliferative T cell that is biased towards TH1 and TH17 effector cell functions. While the underlying defects leading to metabolic programming are not fully understood, divergence of glucose utilization towards synthetic and proliferative functions is now recognized as part of the DNA repair and T-cell aging program128. Premature aging in T cells from RA patients has been connected to telomere instability. Reduced activity of the DNA repair nuclease MRE11A induces a cellular senescence module defined by the gain in the cell cycle regulators p16 and p21 and the cell surface receptor CD57129. Both, PPP shunting of glucose and telomeric uncapping have been directly implicated in rendering T cells from patients with RA tissue invasive and pro-inflammatory, pointing towards shared upstream abnormalities27, 129.
The metabolic environment of the inflamed joint is demonstrably altered, following predictions made for chronically active lymphoid and stromal cells. Proliferating stromal cells use glucose as an energy source and acidify the tissue microenvironment by releasing lactate. Excess TCA cycle intermediates suggest high levels of mitochondrial activity, possibly fuelled by angiogenesis delivering oxygen to the tissue. Extracellular glutamate and succinate have been linked to proinflammatory functions, exemplifying the growing role of such metabolites as signalling molecules, above and beyond their anabolic contributions130, 131.
An obvious motivation to better understand the metabolism of inflammatory immune cells is the promise that tailored exercise programmes, dietary habits and selected nutrients might find an application in the management of RA. Omega-3 fatty acids, moderate alcohol consumption and strict adherence to a Mediterranean diet reportedly have beneficial effects on RA disease activity132, supporting the notion that dietary interventions could be developed as additional immunomodulatory treatment for patients with RA. Molecular studies and well-designed clinical trials will help with the design of disease-specific recommendations. Conversely, clarifying how immunosuppressive drugs that successfully treat RA such as methotrexate, chloroquine and TNF inhibitors interfere with metabolic processes could provide useful clues as to how metabolism is connected to RA and its comorbidities133. Evolving therapeutic strategies that exploit metabolic regulation as a therapeutic target in rheumatic diseases are reviewed by Rhoads et al.134 in this Focus issue.
Analysis of the metabolic pathways in RA widens the pathogenic concept of this disease beyond a narrow view of autoimmunity triggered by the recognition of an autoantigen. An increased knowledge of metabolism promises to yield insights into the relationship between genes and environment in this decade-long disease process, to enable an integrated view of host–microbiota interactions and to invigorate the discussion of nutraceuticals as novel therapeutic agents135.
Key points.
A fundamental abnormality in rheumatoid arthritis is the inappropriate growth of immune cells and stromal cells, imposing high metabolic demands to generate energy and biosynthetic precursors.
In rheumatoid arthritis, immune cells and stromal cells undergo metabolic adaptations to generate biomass.
The disease process in rheumatoid arthritis involves several stages and multiple tissue sites (e.g. lymphoid organs, joints, etc), each with a distinct metabolic environment.
A metabolic signature associated with rheumatoid arthritis is the dampening of glycolytic flux and the shunting of glucose into the pentose phosphate pathway in CD4 T cells.
In the rheumatoid joint, metabolic intermediates function as signalling molecules and facilitate cell-cell communication, amplifying inflammatory tissue damage.
The dependence of the rheumatoid disease process on metabolic activity identifies metabolic interference as a potential therapeutic strategy.
Acknowledgements
This work was supported by grants from the NIH (R01 AR042527, R01 HL 117913, R01 AI108906 and P01 HL129941 to C.M.W. and R01 AI108891, R01 AG045779, U19 AI057266, and I01 BX001669 to J.J.G.).
Glossary terms
- Glycolysis
Glycolysis describes the step-wise breakdown of a glucose molecule (6-carbon sugar) into two pyruvate molecules (3-carbon sugar) to generate two molecules of the energy carrier ATP and two molecules of the electron donor NADH. Glycolysis is an evolutionary ancient pathway that is shared by essentially all organisms and can proceed in the absence and presence of oxygen.
- Tricarboxylic acid (TCA) cycle
The TCA cycle, also known as the Krebs cycle or the citric acid cycle, is a highly efficient cyclic pathway that occurs in the matrix of mitochondria and oxidizes acetyl-CoA to generate ATP, NADH, FADH2 and metabolic intermediates used in the biosynthesis of amino acids, fatty acids, cholesterol, hemoproteins etc. NADH and FADH2 serve as an electron donor in the electron transport chain.
- Oxidative phosphorylation (OXPHOS)
OXPHOS is the process through which cells synthesize ATP in the inner membrane of mitochondria. In the electron transport chain, NADH and FADH2, produced in the TCA cycle, donate electrons to oxygen to reduce it to water. The generation of a proton motive force allows for the conversion of ADP plus phosphate into ATP.
- Hexosamine biosynthesis pathway
The hexosamine biosynthesis pathway is a side branch of glycolysis used to synthesize nucleotide sugars from fructose-6-phosphate and glutamine, such as uridine diphosphate N-acetylglucosamine (UDP-GlcNAc). UDP-GlcNAc functions as a glycosyl donor in post-translational modification of biomolecules, required for the synthesis of glycolipids, glycosaminoglycans, and proteoglycans.
- Pentose phosphate pathway (PPP)
Cells utilize the PPP to convert glucose into ribulose-5-phopshate and NADPH. Ribulose-5-phophate is required as a precursor for nucleotides (DNA, RNA) and coenzymes (ATP, NAD, FAD, CoA). NADPH is the cell’s most important donor of reducing equivalents, sustains anabolic reactions and protects the cytoplasmic redox balance.
- Warburg effect
Named after the 1931 Nobel laureate Otto Warburg, the Warburg effect describes the high utilization of glycolysis in rapidly proliferating cancer cells and the release of lactate into the extracellular milieu by such glucose-addicted cells. Warburg also hypothesized that the mitochondrial processing of pyruvate was inadequate and proposed that cancer cell growth could be manipulate by interfering with glucose metabolism.
Biography
Cornelia M. Weyand is Chief of the Division of Immunology and Rheumatology at the Stanford University School of Medicine, Stanford, California, USA. Her clinical expertise is in the management of patients with autoimmune disease, specifically those with refractory rheumatoid arthritis and those with large-vessel vasculitis. Her research has focused on molecular mechanisms of tolerance loss, the immuno-stromal interactions that cause tissue damage and the role of maladaptive immune aging in tissue inflammation. Her research team has described metabolic signatures in T cells that acquire autoimmune effector functions and has identified molecular defects in DNA damage repair and in telomere maintenance that lead to premature T-cell aging and T-cell-dependent tissue inflammation.
Jörg J. Goronzy is Professor of Medicine at the Stanford University School of Medicine, Stanford, California, USA. His main research interest is to understand the effect of age on the deterioration of protective immunity and the contribution of age to developing autoimmune disease. His research has focused on understanding the age-related remodeling of the T cell system, driven by failing regenerative capacity and by chronic antigenic stimulation and to identifying molecular pathways that can be targeted to improve T memory cell generation after vaccination. His clinical interest lies in the treatment of rheumatoid arthritis and the improvement of vaccine responses.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Rantapaa-Dahlqvist S et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48, 2741–2749 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Majka DS & Holers VM Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 48, 2701–2705 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Arbuckle MR et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 349, 1526–1533 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Kimpimaki T & Knip M Disease-associated autoantibodies as predictive markers of type 1 diabetes mellitus in siblings of affected children. J Pediatr Endocrinol Metab. 14 Suppl 1, 575–587 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Knip M et al. Prediction of type 1 diabetes in the general population. Diabetes Care. 33, 1206–1212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlag DM, Norris JM & Tak PP Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxford). 55, 607–614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law SC, Benham H, Reid HH, Rossjohn J & Thomas R Identification of self-antigen-specific T cells reflecting loss of tolerance in autoimmune disease underpins preventative immunotherapeutic strategies in rheumatoid arthritis. Rheum Dis Clin North Am. 40, 735–752 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Conigliaro P et al. Autoantibodies in inflammatory arthritis. Autoimmun Rev. 15, 673–683 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Koppejan H et al. Role of Anti-Carbamylated Protein Antibodies Compared to Anti-Citrullinated Protein Antibodies in Indigenous North Americans With Rheumatoid Arthritis, Their First-Degree Relatives, and Healthy Controls. Arthritis Rheumatol. 68, 2090–2098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekkers J, Toes RE, Huizinga TW & van der Woude D The role of anticitrullinated protein antibodies in the early stages of rheumatoid arthritis. Curr Opin Rheumatol. 28, 275–281 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Imboden JB The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol. 4, 417–434 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Grimbacher B, Warnatz K, Yong PF, Korganow AS & Peter HH The crossroads of autoimmunity and immunodeficiency: Lessons from polygenic traits and monogenic defects. J Allergy Clin Immunol. 137, 3–17; quiz 18 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Szekanecz Z & Koch AE Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 3, 635–643 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Koch AE Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 62 Suppl 2, ii60–67 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprent J & Surh CD Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 12, 478–484 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surh CD & Sprent J Homeostasis of naive and memory T cells. Immunity. 29, 848–862 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Dziurla R et al. Effects of hypoxia and/or lack of glucose on cellular energy metabolism and cytokine production in stimulated human CD4+ T lymphocytes. Immunol Lett. 131, 97–105 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Tripmacher R et al. Human CD4(+) T cells maintain specific functions even under conditions of extremely restricted ATP production. Eur J Immunol. 38, 1631–1642 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Maciolek JA, Pasternak JA & Wilson HL Metabolism of activated T lymphocytes. Curr Opin Immunol. 27, 60–74 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Wang R & Green DR Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev. 249, 14–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster S, Boley D, Moller P, Stark H & Kaleta C Mathematical models for explaining the Warburg effect: a review focussed on ATP and biomass production. Biochem Soc Trans. 43, 1187–1194 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Stark H, Fichtner M, Konig R, Lorkowski S & Schuster S Causes of upregulation of glycolysis in lymphocytes upon stimulation. A comparison with other cell types. Biochimie. 118, 185–194 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Icard P & Lincet H A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta. 1826, 423–433 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Madeira VM Overview of mitochondrial bioenergetics. Methods Mol Biol. 810, 1–6 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Maldonado EN & Lemasters JJ ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 19 Pt A, 78–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jose C, Bellance N & Rossignol R Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 1807, 552–561 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Fujii H, Mohan SV, Goronzy JJ & Weyand CM Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 210, 2119–2134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 8, 331ra338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clem BF et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 12, 1461–1470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akimoto M et al. Assessment of peripheral blood CD4+ adenosine triphosphate activity in patients with rheumatoid arthritis. Mod Rheumatol. 23, 19–27 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Goronzy JJ & Weyand CM The glycolytic enzyme PFKFB3/phosphofructokinase regulates autophagy. Autophagy. 10, 382–383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Goronzy JJ & Weyand CM Autophagy in autoimmune disease. J Mol Med (Berl). 93, 707–717 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagel AK & Ball LE Intracellular protein O-GlcNAc modification integrates nutrient status with transcriptional and metabolic regulation. Adv Cancer Res. 126, 137–166 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Hanover JA Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J. 15, 1865–1876 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Golks A & Guerini D The O-linked N-acetylglucosamine modification in cellular signalling and the immune system. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 9, 748–753 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigorian A et al. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J Biol Chem. 286, 40133–40141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swamy M et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol. 17, 712–720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forman HJ, Fukuto JM & Torres M Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 287, C246–256 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Sauer H, Wartenberg M & Hescheler J Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 11, 173–186 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Mittler R ROS Are Good. Trends Plant Sci. 22, 11–19 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Paull TT Mechanisms of ATM Activation. Annu Rev Biochem. 84, 711–738 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Holmdahl R, Sareila O, Olsson LM, Backdahl L & Wing K Ncf1 polymorphism reveals oxidative regulation of autoimmune chronic inflammation. Immunol Rev. 269, 228–247 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Yau AC & Holmdahl R Rheumatoid arthritis: identifying and characterising polymorphisms using rat models. Dis Model Mech. 9, 1111–1123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olofsson P & Holmdahl R Positional cloning of Ncf1--a piece in the puzzle of arthritis genetics. Scand J Immunol. 58, 155–164 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Gelderman KA et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J Clin Invest. 117, 3020–3028 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gelderman KA, Hultqvist M, Holmberg J, Olofsson P & Holmdahl R T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci U S A. 103, 12831–12836 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelkka T et al. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxid Redox Signal. 21, 2231–2245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraaij MD et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci U S A. 107, 17686–17691 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelderman KA et al. Rheumatoid arthritis: the role of reactive oxygen species in disease development and therapeutic strategies. Antioxid Redox Signal. 9, 1541–1567 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Kienhofer D, Boeltz S & Hoffmann MH Reactive oxygen homeostasis - the balance for preventing autoimmunity. Lupus. 25, 943–954 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Shirwany NA & Zou MH AMPK: a cellular metabolic and redox sensor. A minireview. Front Biosci (Landmark Ed). 19, 447–474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodson M, Darley-Usmar V & Zhang J Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med. 63, 207–221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan H, Zhou HF, Hu Y & Pham CT Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation. J Rheum Dis Treat. 1, 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang KY et al. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int Immunopharmacol. 16, 85–92 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Thornton CC et al. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: a novel mechanism for vascular protection in chronic systemic inflammation. Ann Rheum Dis. 75, 439–448 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wellen KE & Thompson CB Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 40, 323–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollizzi KN & Powell JD Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 36, 13–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson MO, Siska PJ, Contreras DC & Rathmell JC Nutrients and the microenvironment to feed a T cell army. Semin Immunol. 28, 505–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perl A Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 12, 169–182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delgoffe GM & Powell JD Feeding an army: The metabolism of T cells in activation, anergy, and exhaustion. Mol Immunol. 68, 492–496 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer CS, Ostrowski M, Balderson B, Christian N & Crowe SM Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol 6, 1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fearon U, Canavan M, Biniecka M & Veale DJ Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat Rev Rheumatol. 12, 385–397 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Takemura S et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 167, 1072–1080 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Seyler TM et al. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 115, 3083–3092 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choy E Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 51 Suppl 5, v3–11 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Karmakar S, Kay J & Gravallese EM Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am. 36, 385–404 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang SK, Gu Z & Brenner MB Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 233, 256–266 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Yang Z, Matteson EL, Goronzy JJ & Weyand CM T-cell metabolism in autoimmune disease. Arthritis Res Ther. 17, 29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li G et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 18, 1518–1524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goronzy JJ & Weyand CM Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 14, 428–436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wherry EJ & Kurachi M Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 15, 486–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feldmann M & Maini SR Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 223, 7–19 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Feldmann M & Maini RN Perspectives From Masters in Rheumatology and Autoimmunity: Can We Get Closer to a Cure for Rheumatoid Arthritis? Arthritis Rheumatol. 67, 2283–2291 (2015). [DOI] [PubMed] [Google Scholar]
- 74.LaGory EL & Giaccia AJ The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 18, 356–365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palazon A, Goldrath AW, Nizet V & Johnson RS HIF transcription factors, inflammation, and immunity. Immunity. 41, 518–528 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis DM et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc Natl Acad Sci U S A. 113, 9292–9297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hockel M & Vaupel P Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 93, 266–276 (2001). [DOI] [PubMed] [Google Scholar]
- 78.Hollander AP, Corke KP, Freemont AJ & Lewis CE Expression of hypoxia-inducible factor 1alpha by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 44, 1540–1544 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Muz B, Khan MN, Kiriakidis S & Paleolog EM Hypoxia. The role of hypoxia and HIF-dependent signalling events in rheumatoid arthritis. Arthritis Res Ther. 11, 201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biniecka M et al. Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis. 75, 2192–2200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tas SW, Maracle CX, Balogh E & Szekanecz Z Targeting of proangiogenic signalling pathways in chronic inflammation. Nat Rev Rheumatol. 12, 111–122 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Treuhaft PS & DJ MC Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 14, 475–484 (1971). [DOI] [PubMed] [Google Scholar]
- 83.Thomas DP & Dingle JT In vitro studies of rheumatoid synovium; preliminary metabolic comparison between synovial membrane and villi. Br J Exp Pathol. 36, 195–198 (1955). [PMC free article] [PubMed] [Google Scholar]
- 84.Goetzl EJ et al. A physiological approach to the assessment of disease activity in rheumatoid arthritis. J Clin Invest. 50, 1167–1180 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang XY et al. Energy Metabolism Disorder as a Contributing Factor of Rheumatoid Arthritis: A Comparative Proteomic and Metabolomic Study. PLoS One. 10, e0132695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Carbonell R et al. Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis Rheumatol. 68, 1614–1626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujii W et al. Monocarboxylate transporter 4, associated with the acidification of synovial fluid, is a novel therapeutic target for inflammatory arthritis. Arthritis Rheumatol. 67, 2888–2896 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Zhou R, Wu X, Wang Z, Ge J & Chen F Interleukin-6 enhances acid-induced apoptosis via upregulating acid-sensing ion channel 1a expression and function in rat articular chondrocytes. Int Immunopharmacol. 29, 748–760 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Haas R et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 13, e1002202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veras FP et al. Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci Rep. 5, 15171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colville-Nash PR & Scott DL Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann Rheum Dis. 51, 919–925 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konisti S, Kiriakidis S & Paleolog EM Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 8, 153–162 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Kelly B & O’Neill LA Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weinberg F & Chandel NS Mitochondrial metabolism and cancer. Ann N Y Acad Sci. 1177, 66–73 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Kim S et al. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PLoS One. 9, e97501 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haas R et al. Intermediates of Metabolism: From Bystanders to Signalling Molecules. Trends Biochem Sci. 41, 460–471 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Peti-Peterdi J, Kishore BK & Pluznick JL Regulation of Vascular and Renal Function by Metabolite Receptors. Annu Rev Physiol. 78, 391–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salminen A, Kaarniranta K, Hiltunen M & Kauppinen A Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell Signal. 26, 1598–1603 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Mills EL & O’Neill LA Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol. 46, 13–21 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Tretter L, Patocs A & Chinopoulos C Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta. 1857, 1086–1101 (2016). [DOI] [PubMed] [Google Scholar]
- 101.El Kasmi KC & Stenmark KR Contribution of metabolic reprogramming to macrophage plasticity and function. Semin Immunol. 27, 267–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mills E & O’Neill LA Succinate: a metabolic signal in inflammation. Trends Cell Biol. 24, 313–320 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Bonnet CS et al. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann Rheum Dis. 74, 242–251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Littlewood-Evans A et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 213, 1655–1662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubic T et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 9, 1261–1269 (2008). [DOI] [PubMed] [Google Scholar]
- 106.Tannahill GM et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 496, 238–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shirai T et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 213, 337–354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nefla M, Holzinger D, Berenbaum F & Jacques C The danger from within: alarmins in arthritis. Nat Rev Rheumatol. 12, 669–683 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Lavric M, Miranda-Garcia MA, Holzinger D, Foell D & Wittkowski H Alarmins firing arthritis: Helpful diagnostic tools and promising therapeutic targets. Joint Bone Spine. (2016). [DOI] [PubMed] [Google Scholar]
- 110.Dos Santos Jaques JA et al. Activities of enzymes that hydrolyze adenine nucleotides in lymphocytes from patients with rheumatoid arthritis. Cell Biochem Funct. 31, 395–399 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Arandjelovic S, McKenney KR, Leming SS & Mowen KA ATP induces protein arginine deiminase 2-dependent citrullination in mast cells through the P2X7 purinergic receptor. J Immunol. 189, 4112–4122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caporali F et al. Human rheumatoid synoviocytes express functional P2X7 receptors. J Mol Med (Berl). 86, 937–949 (2008). [DOI] [PubMed] [Google Scholar]
- 113.Fang F et al. Expression of CD39 on Activated T Cells Impairs their Survival in Older Individuals. Cell Rep. 14, 1218–1231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pelka K, Shibata T, Miyake K & Latz E Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol Rev. 269, 60–75 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Berthelot JM, Le Goff B, Neel A, Maugars Y & Hamidou M NETosis: At the crossroads of rheumatoid arthritis, lupus, and vasculitis. Joint Bone Spine. (2016). [DOI] [PubMed] [Google Scholar]
- 116.Dai Y & Hu S Recent insights into the role of autophagy in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 55, 403–410 (2016). [DOI] [PubMed] [Google Scholar]
- 117.van der Burgh R & Boes M Mitochondria in autoinflammation: cause, mediator or bystander? Trends Endocrinol Metab. 26, 263–271 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Weinberg SE, Sena LA & Chandel NS Mitochondria in the regulation of innate and adaptive immunity. Immunity. 42, 406–417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yousri NA et al. Diagnostic and Prognostic Metabolites Identified for Joint Symptoms in the KORA Population. J Proteome Res. 15, 554–562 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Kell DB & Oliver SG The metabolome 18 years on: a concept comes of age. Metabolomics. 12, 148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang M et al. Serum metabolic signatures of four types of human arthritis. J Proteome Res. 12, 3769–3779 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Madsen RK et al. Diagnostic properties of metabolic perturbations in rheumatoid arthritis. Arthritis Res Ther. 13, R19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lauridsen MB et al. 1H NMR spectroscopy-based interventional metabolic phenotyping: a cohort study of rheumatoid arthritis patients. J Proteome Res. 9, 4545–4553 (2010). [DOI] [PubMed] [Google Scholar]
- 124.Young SP et al. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 65, 2015–2023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou J et al. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography-mass spectrometry. J Pharm Biomed Anal. 127, 60–67 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Zabek A et al. Application of (1)H NMR-based serum metabolomic studies for monitoring female patients with rheumatoid arthritis. J Pharm Biomed Anal. 117, 544–550 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Surowiec I et al. Metabolomics study of fatigue in patients with rheumatoid arthritis naive to biological treatment. Rheumatol Int. 36, 703–711 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Weyand CM, Yang Z & Goronzy JJ T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 26, 93–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y et al. Deficient Activity of the Nuclease MRE11A Induces T Cell Aging and Promotes Arthritogenic Effector Functions in Patients with Rheumatoid Arthritis. Immunity. 45, 903–916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Castro Fonseca M, Aguiar CJ, da Rocha Franco JA, Gingold RN & Leite MF GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun Signal. 14, 3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dutta A, Abmayr SM & Workman JL Diverse Activities of Histone Acylations Connect Metabolism to Chromatin Function. Mol Cell. 63, 547–552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tedeschi SK & Costenbader KH Is There a Role for Diet in the Therapy of Rheumatoid Arthritis? Curr Rheumatol Rep. 18, 23 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Nicolau J, Lequerre T, Bacquet H & Vittecoq O Rheumatoid arthritis, insulin resistance, and diabetes. Joint Bone Spine. (2016). [DOI] [PubMed] [Google Scholar]
- 134.Rhoads JP, Major AS & Rathmell JC Fine tuning of immunometabolism for the treatment of rheumatic diseases. Nat Rev Rheumatol. 13, 313–320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsokos GC Metabolic control of arthritis: Switch pathways to treat. Sci Transl Med. 8, 331fs338 (2016). [DOI] [PubMed] [Google Scholar]
- 136.Alexander A et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 107, 4153–4158 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tripathi DN et al. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc Natl Acad Sci U S A. 110, E2950–2957 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]