Abstract
This phase Ib clinical trial evaluated whether pretargeting of CD20+ clonogenic myeloma precursor cells (CMPCs) with anti-CD3 × anti-CD20 bispecific antibody-armed T cells (BATs) before autologous stem cell transplantation (SCT) in patients with standard-risk and high-risk multiple myeloma would induce antimyeloma immunity that could be detected and boosted after SCT. All 12 patients enrolled in this study received 2 BATs infusions before SCT, and 4 patients received a booster infusion of BATs after SCT. Pretargeting CD138−/CD20+ CMPCs with BATs before SCT was safe and reduced levels of CMPCs by up to 58% in the postinfusion bone marrow in patients who remained in remission. Four of 5 patients who remained in remission had a >5-fold increase in IFN-γ enzyme-linked immunospot responses. SOX2 antibody increased after BATs infusions and persisted after SCT. The median anti-SOX2 level at 3 months after SCT was 28.1 ng/mL (range, 4.6 to 256 ng/mL) in patients who relapsed and 46 ng/mL (range, 28.3 to 73.3 ng/mL) in patients who remained in remission. The immune correlates suggest that infusions of targeted T cells given before SCT were able to reduce CMPC levels and induced cellular and humoral antimyeloma immunity that could be transferred and boosted after SCT.
Keywords: Activated T cells, Bispecific antibodies, Multiple myeloma
INTRODUCTION
Although high-dose chemotherapy (HDC) followed by autologous stem cell transplantation (SCT) can induce complete responses in patients with multiple myeloma (MM), there are no curative therapies [1-10]. The induction of anti-MM immune responses may improve disease-free survival and prevent relapse after SCT. Early reports identified CD138−/CD34− cells with the expression of CD19+, CD20+, and CD27+ as clonogenic myeloma precursor cells (CMPCs) [11-13] with self-renewal, drug resistance, and proliferative properties that can lead to progression. Although lenalidomide kills myeloma drug-resistant populations [14], it is ineffective against other CMPC subpopulations [12], and rituximab is clinically ineffective [11] in inhibiting CMPCs.
Therefore, nontoxic strategies to target drug-resistant tumor-initiating CMPCs could prevent disease progression in high-risk MM patients.
Because CD20 is one of the molecules expressed on these cells, we used anti-CD3 × anti-CD20 bispecific antibody-armed T cells (BATs) to target CMPCs. Our approach combines the potent non–MHC-restricted cellular cytotoxicity mediated by activated T cells (ATCs) with the retargeting function of the anti-CD3 × anti-CD20 bispecific antibody (CD20Bi) [15]. Arming with CD20Bi converts ATCs into CD20-specific cytotoxic T lymphocytes (CTLs). Our preclinical studies have shown that BATs exhibit high levels of specific cytotoxicity against CD20+ B9C (immortalized normal B cell line), Raji (Burkitt’s lymphoma cell line), and ARH-77 (a rituximab-resistant cell line) [15]. Furthermore, such targeting is effective even when directed at tumor cell lines that express low or no surface antigen [16-18].
In a small pilot study using a vaccinate and boost strategy, 8 patients with metastatic breast cancer received anti-CD3 × anti-HER2 BATs (HER2 BATs). After the BATs infusions,the patients underwent leukapheresis to activate and expand “immune Tcells” that were reinfused after SCT [19]. The data show that ATCs expanded after “vaccination” would kill breast cancer cells, and that patients who received immune ATCs developed specific cytotoxicity and anti–breast cancer antibody responses.
Based on the data in the proof-of-principle study in breast cancer patients, the hypothesis emerged that if BATs could target CD20+ CMPCs, they could induce MM-specific immune responses before SCT, and that such responses could be transferred in the stem cell product without the need for booster immunizations. We further hypothesized that if the transfer were successful, then the transferred immunity could be boosted by a subsequent infusion of BATs.
In this study, patients received only 2 infusions of 1010 BATs spaced 1 week apart before SCT. The rationale for choosing 2 doses of 1010 BATs was based on the results of earlier phase I clinical trials [20-22] that consistently showed induction of immune responses after 2 weeks even after 1 infusion per week.
METHODS
Trial Design
The study protocol and consent forms were approved by the Wayne State University Institutional Review Board and the US Food and Drug Administration (FDA). The study protocol (WSU 2008-106) was conducted in accordance with guidelines set forth in the Declaration of Helsinki. BATs were produced under an FDA Investigational New Drug application sponsored by L.G.L., and the study was monitored by the Karmanos Cancer Institute’s Data Safety Monitoring Committee. Figure 1A shows the protocol schema, which involved 2 infusions of 1010 BATs given 1 week apart. Twelve patients were enrolled in the proof-of-concept protocol, and 4 of these 12 patients were reconsented for an amended protocol after approval by the FDA and the Wayne State University Institutional Review Board to receive a booster infusion of 1010 BATs.
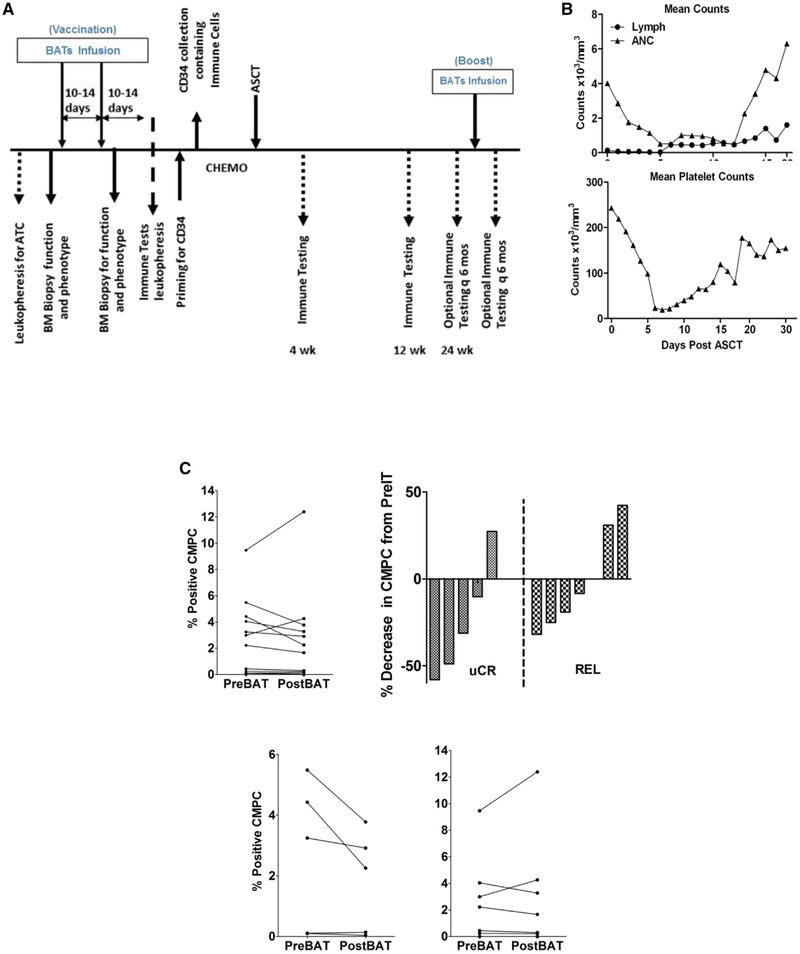
Figure 1.
(A) Treatment schema showing leukapheresis to obtain T cells for expansion, followed by baseline bone marrow biopsy and phenotyping. After expansion ex vivo and arming of ATCs from the patients, 10 × 109 BATs were infused on 2 occasions 1 week apart, followed by a second biopsy to evaluate changes in the BM. Immune testing was performed as indicated at the various time points after SCT. Four patients received BATs booster infusions. (B) (Upper) Daily lymphocyte (Lymph) and ANC counts in 12 patients. (Lower) Daily mean platelet counts. (C) (Upper, Left) CMPC population at 2 weeks post-BATs compared with pre-BATs in all 12 patients. (Upper, Right) Waterfall chart of percent change in CMPC population at 2 weeks post-BATs compared with pre-BATs in patients who remained in remission (uCR) and those who relapsed (REL). (Lower) Absolute percent positive CMPC population in patients who remained in remission (uCR) and those who relapsed (REL).
Clinical follow-up as of November 30, 2015, is reported herein. Tumor evaluation was based on serologic testing results; bone marrow samples were not required, in accordance with our institutional tumor evaluation criteria. This trial was registered with ClinicalTrials.gov (Identifier: ).
Patient Eligibility
Standard SCT inclusion criteria were applied for enrollment into the protocol. Patients with MM age ≥18 years who were candidates for SCT with no morphological evidence of myelodysplasia in pretreatment bone marrow specimens were eligible. (Details of patient eligibility are provided in the Supplementary Data.)
Preparative Regimen
All 12 patients received a preparative regimen consisting of melphalan 200 mg/m2 and supportive care [7,23].
Stem Cell Mobilization
The patients received 16 mg/kg of granulocyte colony-stimulating factor (G-CSF) for 4 days to mobilize stem cells, followed by 1 or 2 days of leukapheresis to obtain a minimum dose of 2 × 106 CD34+ cells for SCT.
Production of Heteroconjugated CD20Bi, Leukapheresis, and T Cell Expansion
Rituximabwas heteroconjugated to anti-CD3 (OKT3) to produce CD20Bi as described previously [16]. A separate leukapheresis was performed to obtain peripheral blood mononuclear cells (PBMCs) for ATC expansion before G-CSF mobilization of peripheral blood stem cells as described previously [16]. (Details are provided in Supplementary Data.)
BATs Infusion and Monitoring
Patients received 2 infusions of 1010 BATs. Cryopreserved BATs were thawed and infused in 2 infusions given 1 week apart. Infusions were given over 5 to 15 minutes, with monitoring of vital signs as described previously [24].
BATs Infusion–Related Toxicities
Side effects were recorded on the patient charts based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0 toxicity table. No patient was removed from the study. (See Supplementary Data for criteria for study removal.)
Immune Monitoring Studies
Methods for phenotyping of bone marrow mononuclear cells and PBMCs, IFN-γ enzyme-linked immunospot (ELISPOT) responses, serum cytokines, and quantitation of anti-tetanus toxoid (anti-TT) and anti-SOX2 IgG are described in the Supplementary Data. An anti-SOX2 antibody level >20 ng/mL(>1.5-fold increase) was defined as positive, and a level <20 ng/mL (<1.5-fold increase) was defined as negative.
Statistical Analyses
Repeated-measures ANOVA and Dunnett’s multiple-comparison test were used to compare differences in IFN-γ responses directed at RPMI-8226, Daudi, and K562 cells before BATs infusion (pre-BATs), the highest observed value within 3 weeks after BATs infusion (post-BATs), and the highest observed value within 0.5 to 3 months and 6 to 12 months post-SCT using Prism version 7.00 for Windows (GraphPad Software, San Diego, CA). The Wilcoxon signed-rank test was used to compare the proportions of CMPCs in pre-BATs and post-BATs bone marrow samples and anti-SOX2 antibody levels in pre-BATs and 3 weeks post-BATs or pre-BATs and post-SCT serum samples.
RESULTS
Clinical Characteristics, and Stem Cell and BATs Doses
Table 1 presents data on patient age, sex, M protein Ig class, previous courses of chemotherapy, disease status at SCT, BATs dose, peripheral blood stem cell dose, disease status at SCT, day of engraftment, time to progression (TTP), and survival after SCT.
Table 1.
Clinical Data, Transplantation Data, and BATs and Stem Cell Doses
| Patient | Sex | Age, yr | M Protein |
Previous Chemotherapy | Disease Status at SCT |
Risk Status |
BATs Dose, 109 |
Stem Cell Dose, 106/kg |
Current Disease Status |
Days to Engraftment |
TTP Post- SCT, mo |
Survival Post-SCT, mo |
Maintenance Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 66 | IgG | (B + D) × 2 | VGPR | Int | 24 | 6 | REL | 11 | 6.4 | 26.7* | Revlimid |
| 2 | M | 41 | IgG | (L + D) × 4 | CR | ND | 30 | 7.58 | uCR | 13 | 69.30 | Revlimid | |
| 3 | F | 54 | IgA | (B, dox, D) × 6 | PR | High | 20 | 10.26 | REL | 17 | 26.2 | 66.93 | Revlimid |
| 4 | F | 49 | IgG | (B, ex, L) × 3 | PR | High | 20 | 14 | REL->SD | 23 | 3.3 | 64.90 | Revlimid |
| 5 | M | 59 | IgA | (L + D) × 4 | CR | Low | 20 | 7.89 | **REL-alloBMT | 17 | 15.8 | 63.67 | Revlimid |
| 6 | F | 40 | IgA | (B + dox, D) × 3 | VGPR | High | 30 | 7.17 | REL | 13 | 42.4 | 63.97 | Revlimid |
| 7 | M | 66 | IgG | (B + D) × 6 | PR | ND | 20 | 20.87 | uCR | 21 | 62.73 | Revlimid | |
| 8 | F | 66 | IgG | (B+ TI + D × 3 | PR | ND | 20 | 8.18 | uCR | 12 | 62.57 | Revlimid | |
| 9 | F | 66 | IgG | (B + D) × 4 | PR | ND | 20 | 7.96 | uCR | 18 | 62.50 | Revlimid | |
| 10 | M | 62 | IgA | (B + D) × 8 | VGPR | Low | 20 | 11.7 | REL | 16 | 14.8 | 57.67 | No maintenance |
| 11 | M | 57 | IgG | (B + D) × 3 | VGPR | Low | 30 | 7.56 | REL | 18 | 22.3 | 56.90 | No maintenance |
| 12 | M | 53 | Non-S | (T, B + D + T, B + D) | CR | Low | 30 | 5.05 | uCR | 14 | 56.50 | No maintenance | |
| Median | 58 | 15.8 | 62.6 |
B indicates bortezomib; VGPR, very good partial remission; Int, intermediate risk; L, lenalidomide; D, dexamethasone; CR, complete remission based on bone marrow biopsy; ND, not determined owing to the lack of cytogenetics at the time of diagnosis; uCR, complete remission based on lack of serum MM protein detected because bone marrow biospies were not part of the standard of care at the institution; dox, doxorubicin; PR, partial remission; BMT, bone marrow transplantation; T, thalidomide; Non-S, nonsecretory.
Bold text indicate the patients who received booster infusions.
Patient expired from progressive disease.
Underwent allogeneic SCT while in PR.
BATs Infusions
All 12 patients received 2 BATs infusions. Characteristics of ATCs are presented in Table S1 in the Supplementary Data. Regimen-related and cell-based toxicities are listed in Table 2. Patients were premedicated, prehydrated, and treated for side effects on an outpatient basis. Cell infusions were well tolerated, with no dose-limiting toxicities (see Supplementary Data for details).
Table 2.
Incidence of Toxic Reactions by CTCAE Version 3.0 Grade
| Reaction | Number (%) of Patients Affected |
Total Number of Episodes by CTCAE Version 3.0 Grade |
|||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Hypotension | 9(75) | 1 | 9 | ||
| Chills | 10 (83) | 1 | 16 | 3 | |
| Fatigue | 8 (67) | 11 | |||
| Fever | 7 (58) | 6 | 2 | ||
| Rash | 1(8) | 1 | |||
| Pain | 4(33) | 7 | 1 | ||
| Dyspnea | 1(8) | 1 | |||
| Edema, limb | 1(8) | 1 | |||
| Deep vein thrombosis | 1(8) | 1 | |||
CTCAE indicates Common Terminology Criteria for Adverse Events.
Engraftment of Lymphocytes, Neutrophils, and Platelets
All 12 patients engrafted, with a median time to engraftment (defined as an absolute neutrophil count [ANC] ≥500/mm3) of 17 days (95% CI, 15.0 to 19.2 days) and a median time to a platelet count of ≥20 × 103/mm3 of 18 days (95% CI, 16.5 to 23.8 days). Eleven patients had a median time to lymphocyte engraftment of ≥500/mm3 of 14 days (95% CI, 11 to 19 days) (Figure 1B). All patients engrafted without any complications or dose-limiting toxicities.
Bone Marrow CMPC Population and Progression after SCT
We examined the effect of targeting the CD20+/CD138−/CD34− CMPC population by OKT3 × CD20 BATs at 2 weeks post-BATs. Figure 1C shows the percentage of positive CMPCs and percent change in the proportion of CMPCs in bone marrow. A reduced proportion of CMPCs was observed in 8 of 12 patients post-BATs compared with pre-BATs; however, there was no statistically significant difference in the percentage of positive CMPCs in pre-BATs and post-BATs bone marrow (Figure 1C, Upper). Bone marrow samples from patients who remained in remission showed a reduction in percent of the CMPC populations post-BATs (median, 2.26; range, 0.04 to 3.7) compared with pre-BATs (median, 3.2; range, 0.1 to 5.4). In contrast, there was either an increase or no change in percent of CMPCs in post-BATs bone marrow samples (median, 1.6; range, 0 to 12.4) compared with pre-BATs bone marrow samples (median, 2.2; range, 0 to 9.4) in patients who relapsed (Figure 1C, Upper and Lower).
Immune Responses after BATs Infusions
ELISPOT analyses showed the highest numbers of IFN-γ–producing T cells at 1 to 3 weeks post-BATs. Increased ELISPOT IFN-γ values were seen after stimulation with MM-specific RPMI 8226 cells at post-BATs time points compared with pre-BATs samples (Figure 2A, Lower). Figure S2 shows the IFN-γ ELISPOT results in PBMCs of the 12 patients against RPMI 8226, Daudi, and K562 at pre-BATs, post-BATs, and post-SCT time points.
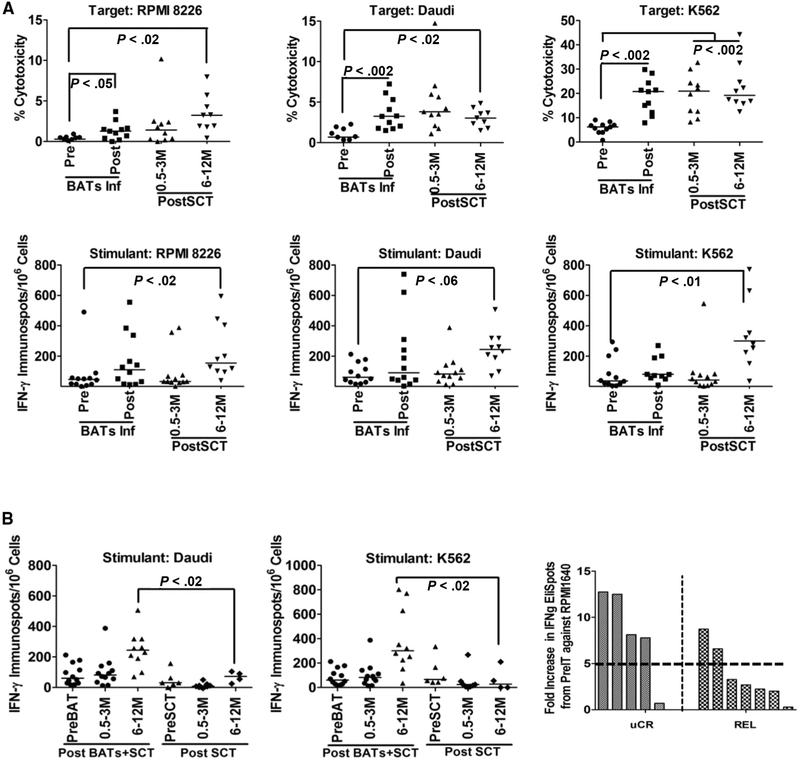
Figure 2.
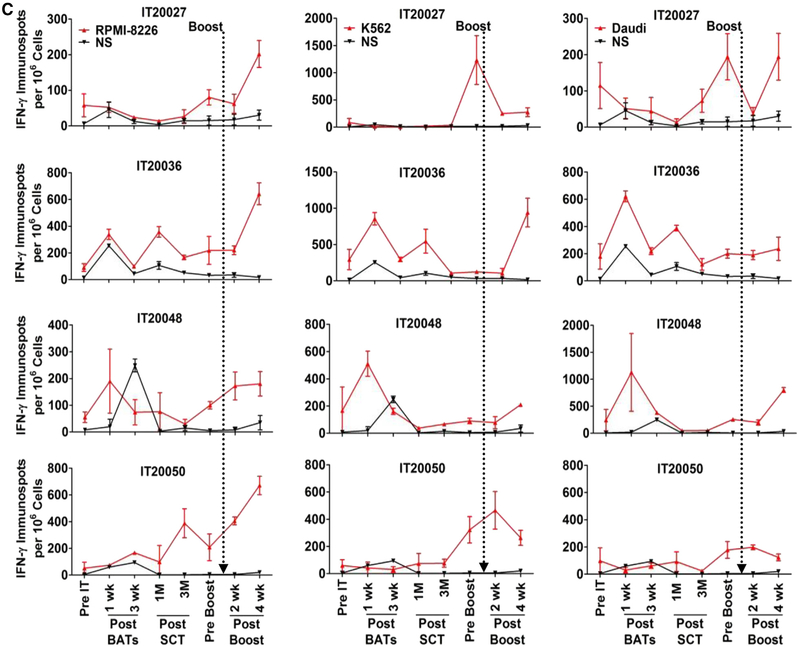
(A) Cytotoxicity and IFN-γ ELISPOTs directed at RPMI 8226, Daudi, and K562 targets after BATs infusions (Upper) and after SCT (Lower) in 12 patients. (B) (Left and Middle) IFN-γ ELISPOTs in patients who received BATs + SCT compared with patients who received SCT only (control group). (Right) The fold increase in IFN-γ ELISPOTs against RPMI 8226 at 6 to 12 months post-SCT in patients who remained in remission (uCR) compared with patients who relapsed (REL). (C) Data for 4 patients who received a single “booster” infusion of BATs at 6 to 15 months post-SCT. Booster infusion of BATs induced increases in IFN-γ ELISPOT responses when stimulated with MM-specific targets (RPMI 8226) and NK targets (K562) in all 4 patients at 2 and 4 weeks after booster BATs infusions.
Immune Responses after SCT
IFN-γ ELISPOT responses to RPMI 8226 cells were consistently higher at post-SCT time points compared with baseline (P < .02). Likewise, T cells in PBMCs stimulated with K562 (P < .01) showed significantly higher IFN-γ ELISPOT responses compared with pre-BATs values (Figure 2A, Lower). To determine whether these responses were due to “noise” from immune reconstitution, we compared these patients with a control group of patients who received autologous SCT alone (ie, did not receive BATs). Figure 2B shows the IFN-γ ELISPOT responses in patients who received BATs + SCT compared with those who received SCT only (control group). IFN-γ ELISPOT responses to Daudi (P < .02) and K562 (P < .02) targets were significantly higher than baseline at 6 to 12 months post-SCT in patients who received BATs + SCT compared with those who received SCT only. The percentage of patients with a ≥2-fold increase for IFN-γ ELISPOT responses to RPMI 8226, Daudi, and K562 was 8%, 17%, and 17%, respectively, at 3 months post-after SCT and 64%, 36%, and 55%, respectively, at 6 months post-SCT. Table S2 in Supplementary Data shows the pattern of immune responses to RPMI 8226, Daudi, and K562 as a function of time after SCT. ELISPOT was scored as positive if the ELISPOT/106 PBMCs plated were ≥30 spots; a positive increase was defined as a ≥2-fold (+), 3- to 5-fold (++), or >5-fold (+++) increase in spots over the pre-BATs baseline value.
IFN-γ ELISPOT Responses and Progression after SCT
Four of 5 patients who remained in remission as of November 30, 2015, had a median 8.1-fold increase in IFN-γ ELISPOT values against RPMI 8226 from pre-SCT to post-SCT (6 to 12 months), compared with 2.6-fold in patients who relapsed (Figure 2B, Left and Table S2).
IFN-γ ELISPOT Responses to RPMI 8226 Boosted by BATs Infusion
In 4 patients, booster infusions at 15.2, 10.5, 6.5, and 5.6 months post-SCT increased IFN-γ ELISPOT responses to RPMI 8226 (fold increases of 3.3, 3.5, 6.0, and 12.9, respectively) above the pre-boost baseline (Figure 2C). Compared with RPMI 8226, fold increases in response to Daudi were lower (0.3, 1.3, 1.3, and 1.7, respectively), whereas fold increases in response to K562 were 3.3, 4.4, 14.4, and 37.9, respectively. These data show stronger innate (K562) and anti-MM (RPMI 8226) immune responses than the response against Daudi (Figure 2C).
Anti-SOX2 IgG Antibody Levels Post-SCT
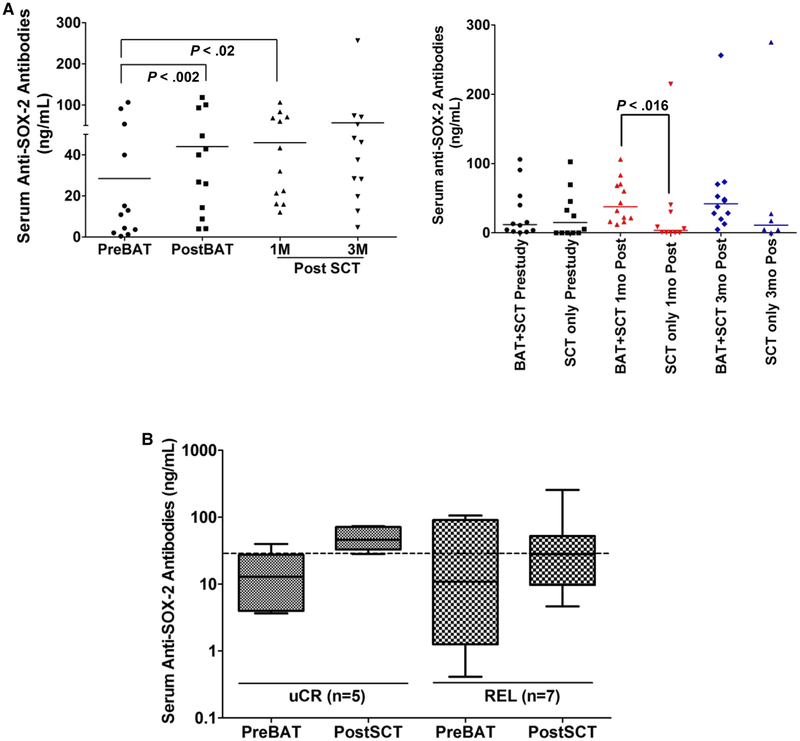
Anti-SOX2 levels were screened in all 12 patients at pre-BATs, post-BATs, and 1 month and 3 months post-SCT. The control group included 12 SCT recipients who did not receive BATs infusions. Serum was evaluated pre-SCT (n = 12) and at ~1 month (n = 12) and 3 months (n = 6) post-SCT. Anti-SOX2 levels were significantly higher in 9 of 12 patients post-BATs and remained high at 1 month post-SCT (P < .02) (Figure 3A, Left). Serum samples from control SCT recipients who did not receive BATs infusions showed no statistically significant difference in anti-SOX2 levels between pre-SCT and 1 month post-SCT or between pre-SCT and 3 months post-SCT (Figure 3A, Right). Interestingly, anti-SOX2 levels were significantly higher (P < .016) in patients who received BATs before SCT at 1 month post-SCT compared with control patients who did not receive BATs (Figure 3A, Right).
Figure 3.
Transfer and reconstitution of anti-SOX2 IgG. The results for individual patients are presented. (A) (Right) Anti-SOX2 IgG antibodies in all 12 patients at pre-BATs, post-BATs, and 1 month and 3 months post-SCT. (Left) Control autologous SCT patients who did not receive BATs showed no difference in serum anti-SOX2 antibody levels between pre-SCT and 1 month post-SCT or pre-SCT and 3 months post-SCT. (B) Shows the boxplots of the serum anti-SOX2 titers of those who are in uCR and those who progressed (REL), they are nearly nonoverlapping.
Anti-SOX2 and Progression after SCT
To determine whether there was an association between the level of anti-SOX2 and clinical outcome, anti-SOX2 levels were plotted in patients who did and did not progress. Despite small numbers in each group, the boxplots appear to separate (Figure 3B). The median anti-SOX2 level was 10.8 ng/mL (range, 0.4 to 106 ng/mL) pre-BATs, compared with 28.1 ng/mL (range, 4.6 to 256 ng/mL) at 3 months post-SCT after a median follow-up of 62.6 months for the patients who progressed. In comparison, the patients who remained in remission had a median anti-SOX2 level of 12.9 ng/mL (range, 3.6 to 39 ng/mL) at pre-BATs and 46.06 ng/mL (range, 28.3 to 73.3 ng/mL) at 3 months post-SCT. These data suggest a trend toward higher SOX2 levels possibly providing a longer TTP (P < .06, Wilcoxon matched-pairs signed-rank test). When the total number of SOX2-positive (>20 ng/mL and a > 1.5-fold increase) and negative (<20 ng/mL and a <1.5-fold increase) levels in those who remained in remission versus those who relapsed showed that a significantly higher number of patients who remained in remission (all 5) had SOX2-positive levels (P < .02, Fisher’s exact test) compared with those who relapsed (2 of 7).
Quantitative IgG and Anti-TT IgG Serum Levels and Cytokine Levels
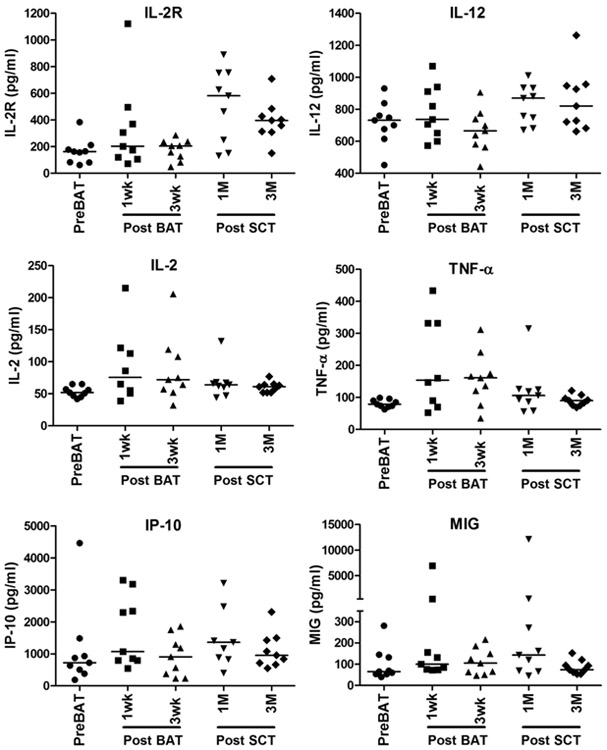
Serum IgG levels and anti-TT levels are shown in Figure S3A and B. In 9 of the 12 patients, levels of serum IL-2–, IL-2R–, IL-12–, TNF-α–, and IFN-γ–induced chemokines CXCL10 and CXCL9 were elevated above baseline, but not significantly so (Figure 4). Levels of chemokines MIP-1α, MIP-1 β, and MCP-1 showed no change from baseline to post-BATs or post-SCT (data not shown).
Figure 4.
Serum cytokine and chemokine profiles. Analysis of sequential serum samples after BATs infusions and after SCT show increased IL-2R and IL-12 levels at 1 to 3 months post-SCT. IL-2 and TNF-α levels increased post-BATs, but not post-SCT, whereas chemokines CXCL9 (MIG) and CXCL10 (IP-10) increased at 1 month post-SCT.
Clinical Responses
The number of patients in this study did not allow a statistical comparison with historical controls for progression-free survival or overall survival. Five patients remained in complete remission (CR) at 69.3, 62.7, 62.5, 62.5, and 56.9 months post-SCT as of November 30, 2015. Two patients, at 62.5 and 56.9 months post-SCT, did not receive Revlimid maintenance. Seven patients progressed, at 3.3, 6.4, 14.8, 15.8, 22.3, 26.7, and 42.4 months post-SCT. The median overall survival for the entire group was not reached with a median follow-up of 62.5 months. The median TTP was between 26.4 and 42.5 months. Three of 9 patients who were in partial remission at the time of SCT are now in CR, and the 2 patients who were CR at the time of SCT are still in CR.
Clinical Correlates between CMPC and SOX2 Levels and Clinical Status
Table 3 summarizes clinical responses and changes in percent of CMPC and anti-SOX2 antibody levels post-BATs and post-SCT. Interestingly, 4 of 5 patients who remained in remission showed a reduction in the proportion of CMPCs, and all 5 had increased anti-SOX2 antibodies. In contrast, 3 of 7 patients who relapsed showed either no change or an increase in percent of CMPCs, and 3 of 7 showed a decrease in anti-SOX2 antibodies.
Table 3.
Clinical and Immune Responses in Individual Patients
| Patient | BATs Dose, 109 |
Current Disease Status |
Bone Marrow CMPC Status Post-BATs |
Anti-SOX2 Level Post-SCT |
TTP Post- SCT, mo |
Survival Post-SCT, mo |
|---|---|---|---|---|---|---|
| 1 | 24 | REL |  |
 |
6.4 | 26.7 |
| 2 | 30 * | uCR |  |
 |
69.30 | |
| 3 | 20 | REL |  |
 |
26.2 | 66.93 |
| 4 | 20 | REL->SD | NC |  |
3.3 | 64.90 |
| 5 | 20 | REL-alloBMT† |  |
 |
15.8 | 63.67 |
| 6 | 30* | REL |  |
 |
42.4 | 63.97 |
| 7 | 20 | uCR |  |
 |
62.73 | |
| 8 | 20 | uCR |  |
 |
62.57 | |
| 9 | 20 | uCR |  |
 |
62.50 | |
| 10 | 20 | REL |  |
 |
22.3 | 57.67 |
| 11 | 30* | REL |  |
 |
14.8 | 56.90 |
| 12 | 30* | uCR |  |
 |
56.50 |
NC indicates no change; alloBMT, allogeneic bone marrow transplantation.
Received a 109 BATs booster dose.
Received allogeneic SCT while in partial remission.
DISCUSSION
Although SCT can profoundly reduce the tumor burden in most patients with MM, relapse is inevitable. We hypothesized that MM patients could benefit from targeted T cell immunotherapy that would shift the immune response against MM after SCT. HDC provides “immunologic space,” allowing anti-MM memory T and B cells to expand in autografts and provide the anti-MM response. Our results show that BATs infusions before SCT can pretarget CD20+ CMPCs, leading to a decreased proportion of CMPCs within 2 weeks of the last BATs infusion.
Targeted killing of CMPCs releases MM antigens, inducing endogenous immune responses that could be detected after SCT. Anti-MM IFN-γ ELISPOT responses developed not only at 3 weeks after the BATs infusions, but also at 6 to 12 months post-SCT, and these responses could be boosted by a single BATs infusion. Similarly, humoral responses to SOX2 were detected after immunotherapy and during the process of immune reconstitution after SCT. Given that the significant increase in CTL activity was seen only after 6 months, it is likely that the extent of “vaccination” to MM antigens might not have been sufficiently strong to induce significant CTL activity before 6 months post-SCT using only 2 BATs infusions. Furthermore, the transferred helper and CTL memory cells for MM might not have been present in sufficient numbers to respond, particularly during the first 3 months of immune reconstitution, without immediate booster immunization after SCT due to immune deficiency and T cell suppression present during the early post-SCT period. Therefore, the specific transferred immune responses without the immediate transfer of immune cells would appear 6 months or later during immune reconstitution after SCT.
Our findings from the present study shows that pretargeting CD19+/CD20+/CD138−/CD34− CMPCs with only 2 infusions of BATs was sufficient to change the proportion of CMPCs detected in the follow-up bone marrow specimens. Testing of the entire group revealed no statistical differences in CMPCs before and after immunotherapy; however, patients who remained in remission showed a trend toward a decreasing proportion of CMPCs after BATs infusion. The significant increase in anti-SOX2 level after BATs infusions and the development and persistence of anti-SOX2 levels post-SCT is quite remarkable. Anti-SOX2 and IFN-γ responses are reportedly associated with a reduced risk of progression of monoclonal gammaopathy of undetermined significance (MGUS) to MM [25]. SOX2 (an embryonal stem cell marker) is expressed on a small population of CD138− progenitors in patients with MGUS; these patients frequently mount humoral and cellular immune responses to SOX2. A small subpopulation of CD138+ cells also acquire SOX2 expression in patients with advanced MM [25]. In a large anti-SOX2 screening study, 92.6% (63 of 68) of serum samples from patients with MM who underwent allogeneic SCT were seropositive for anti-SOX2, whereas only small proportions of untreated chemotherapy-naïve MM patients (4.4%; 3 of 68), conventional chemotherapy-treated MM patients (0%; 0 of 68), and patients who underwent SCT (2.9%; 2 of 68) were seropositive for anti-SOX2 [26].
The biological function and clinical significance of anti-SOX2 antibodies in MM remain unclear. The high seropositivity after allogeneic SCT suggests that allogeneic SCT breaks tolerance toward SOX2 [26]. Higher baseline anti-SOX2 levels before SCT might prove to be a biomarker. On the other hand, SOX2 expression in cancer stem cell–like or tumor progenitor cells from solid tumors have been reported to play roles in tumor recurrence, metastasis, and resistance to chemotherapeutic agents [27-29]. The increase in anti-TT levels at 3 months post-SCT suggests that pretargeting of CD20+ cells with infusions of anti-CD3 × anti-CD20 BATs did not impair the reconstitution of CD20+ or CD19+ B cells in phenotyping of blood or the development of serum IgG levels and anti-TT levels by 100 days post-SCT. The levels of anti-TT are comparable to those seen in transfer of specific immunity after allogeneic SCT under nonboosted conditions [30-32].
As mentioned earlier, both CD19+ and CD20+ cells are rare self-replenishing drug-resistant populations, and thus CD19 and CD20 cells are not considered conventional immunotherapeutic targets. Our study shows that targeting this rare CD20+ population decreased the proportion of CD138−CD20+ cells in the bone marrow in patients who remained in remission, and induced cellular and humoral anti-MM immunity that could be detected after SCT. Similar to our findings, a recent case report described a durable complete response in a patient with advanced, refractory MM who had failed 9 previous lines of therapy. Infusion of autologous T cells expressing a CD3-zeta/CD137–based anti-CD19 chimeric antigen receptor after treatment with high-dose melphalan and autologous SCT showed no evidence of monoclonal immunoglobulin in serum and urine immunofixation and no clinical signs or symptoms of MM.
Consistent with our results reported here, there were no signs of the cytokine release syndrome after the CD19 chimeric antibody receptor T cell (CART) infusion [33]. However, there are major differences between the approach using gene-transduced CARTs into anti-CD3/anti-CD28–activated T cells [34] and our approach of using anti-CD3/IL-2–activated T cells armed with bispecific antibodies. CARTs offer rapid expansion of target antigen engagement and tumor lysis, leading to the development of a sustained antileukemia effect [35]. In contrast, BATs are designed to mediate acute cytotoxicity with short-term proliferation and Th1 cytokine/chemokine and tumor antigen release by directed cytotoxicity at the tumor site, resulting in the recruitment of endogenous T and B cells to become long-term tumor-specific memory cells via the in situ vaccination process [36].
In summary, our proof-of-principal study suggests that in patients with MM, pretargeting CD138−/CD20+ cells with BATs infusions before SCT is safe, decreases the proportion of CD138−CD20+ cells in the bone marrow in patients who remain in remission, and induces cellular and humoral anti-MM immunity and anti-SOX2 antibodies that can be detected after SCT. The presence of anti-SOX2 antibodies may predict a protective effect of BATs infusions. The increases in anti-SOX2 IgG and anti-MM IFN-γ ELISPOT responses directed at CD20− RPMI 8226 occurred after 2 BATs infusions, persisted for longer than 3 months after SCT, and could be boosted in 3 of 4 patients. The robust immune responses to CD20− RPMI 8226 cells indicate antigen and possibly epitope spreading in the targeting and vaccination process, because the BATs targeted CD20. Furthermore, anti-SOX2 antibody levels and IFN-γ ELISPOT responses appear to be lower in the patients who relapsed than in those who did not relapse. Higher baseline anti-SOX2 levels may become a useful prognostic biomarker. Although the numbers are small, 3 of 9 patients who had residual disease (partial response or very good partial response) at the time of SCT had no evidence of progression at 62.6 to 65.7 months post-SCT, and 2 of 3 patients who were in CR at the time of SCT remained in remission at 56.9 to 69.3 months post-SCT. These results provide a strong rationale for a “vaccinate and boost” approach with chemotherapy alone or with HDC and SCT to optimize anti-MM immune responses to improve clinical results.
Supplementary Material
ACKNOWLEDGMENTS
We thank the nurse practitioners, physician assistants, and BMT inpatient and outpatient nursing staff for their dedication to the BMT and immunotherapy program’s patients.
Financial disclosure: L.G.L was supported in part by grants from the Leukemia and Lymphoma Society (TRP 6066-06 and TRP 6092-09) and the National Cancer Institute (R01 CA 092344 and R01 CA 140314). We acknowledge support from the Ray and Lynn Wood Neag Foundation and the Young Family Foundation. J.A.Z received startup funding from the Karmanos Cancer Institute.
Footnotes
Conflict of interest statement: L.G.L. is a cofounder of Transtarget Inc. A.T. is a cofounder of NovaImmune Platform. M.H.A. has received honoraria from Seattle Genetics and Millennium. S.V.K., A.D., Z.A., J.A., L.A., E.N.T., P.A.S., D.L.S., A.M., H.Y.,J.A.Z.,J.P.U., and V.R. have no conflicts of interest to report.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at doi: 10.1016/j.bbmt.2015.12.030.
Financial disclosure: See Acknowledgments on page 877.
REFERENCES
- 1.McElwain TJ, Powles RL High-dose intravenous melphalan for plasmacell leukaemia and myeloma. Lancet. 1983;2:822–824. [DOI] [PubMed] [Google Scholar]
- 2.Raab MS, Podar K, Breitkreutz I, et al. Multiple myeloma. Lancet. 2009; 374:324–339. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Fraçais du Myélome. N Engl J Med. 1996;335:91–97. [DOI] [PubMed] [Google Scholar]
- 4.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–9233. [DOI] [PubMed] [Google Scholar]
- 5.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. [DOI] [PubMed] [Google Scholar]
- 6.Bladé J, Rosiñol L, Cibeira MT, et al. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115: 3655–3663. [DOI] [PubMed] [Google Scholar]
- 7.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002;99:731–735. [DOI] [PubMed] [Google Scholar]
- 8.Samuels BL, Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–1799. [DOI] [PubMed] [Google Scholar]
- 9.Barlogie B, Jagannath S, Desikan KR, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93: 55–65. [PubMed] [Google Scholar]
- 10.Moreau P, Hullin C, Garban F, et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99-04 protocol. Blood. 2006;107: 397–403. [DOI] [PubMed] [Google Scholar]
- 11.Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher K, Parquet N, Widen R, et al. Stemness of B-cell progenitors in multiple myeloma bone marrow. Clin Cancer Res. 2012;18:6155–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosen N, Matsuoka Y, Kishida S, et al. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia. 2012;26:2135–2141. [DOI] [PubMed] [Google Scholar]
- 14.Jakubikova J, Adamia S, Kost-Alimova M, et al. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011;117:4409–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gall JM Davol PA, Grabert RC, et al. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B-cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol. 2005;33:452–459. [DOI] [PubMed] [Google Scholar]
- 16.Sen M, Wankowski DM, Garlie NK, et al. Use of anti-CD3 × anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu+ tumors. J Hematother Stem Cell Res. 2001;10: 247–260. [DOI] [PubMed] [Google Scholar]
- 17.Lum HE, Miller M, Davol PA, et al. Preclinical studies comparing different bispecific antibodies for redirecting T cell cytotoxicity to extracellular antigens on prostate carcinomas. Anticancer Res. 2005;25: 43–52. [PubMed] [Google Scholar]
- 18.Chan JK, Hamilton CA, Cheung MK, et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clin Cancer Res. 2006;12: 1859–1867. [DOI] [PubMed] [Google Scholar]
- 19.Thakur A, Lum LG, Rathore R, et al. Transfer of anti-breast cancer immunity induced by infusions of bispecific antibody armed T cells and boosted with ex vivo primed T cells after stem cell transplant in metastatic breast cancer patients [abstract]. J Clin Oncol. 2015; 33(Suppl):3076. [Google Scholar]
- 20.Vaishampayan U, Thakur A, Rathore R, et al. Phase I study of anti-CD3 × anti-HER2 bispecific antibody in metastatic castrate resistance prostate cancer patients. Prostate Cancer. 2015;2015:285193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lum LG, Choi M, Thakur A, et al. Five advanced pancreatic cancer patients in a phase I study of anti-CD3 × anti-EGFR bispecific antibody armed activated T-cells (BATs). J Immunother Cancer. 2015;3(Suppl 2): P55. [Google Scholar]
- 22.Lum LG, Thakur A, Liu Q, et al. CD20-targeted T cells after stem cell transplantation for high risk and refractory non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2013;19:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau P, Kergueris MF, Milpied N, et al. A pilot study of 220 mg/m2 melphalan followed by autologous stem cell transplantation in patients with advanced haematological malignancies: pharmacokinetics and toxicity. Br J Haematol. 1996;95:527–530. [DOI] [PubMed] [Google Scholar]
- 24.Lum LG, Thakur A, Al-Kadhimi Z, et al. Targeted T-cell therapy in stage IV breast cancer: a phase I clinical trial. Clin Cancer Res. 2015;21: 2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spisek R, Kukreja A, Chen LC, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobold S, Tams S, Luetkens T, et al. Patients with multiple myeloma develop SOX2-specific autoantibodies after allogeneic stem cell transplantation. Clin Dev Immunol. 2011;2011:302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikegame A, Ozaki S, Tsuji D, et al. Small molecule antibody targeting HLA class I inhibits myeloma cancer stem cells by repressing pluripotency-associated transcription factors. Leukemia. 2012;26: 2124–2134. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Xu Y, Chen Y, et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/β-catenin signal network. Cancer Lett. 2013;336:379–389. [DOI] [PubMed] [Google Scholar]
- 29.Tian T, Zhang Y, Wang S, et al. Sox2 enhances the tumorigenicity and chemoresistance of cancer stem-like cells derived from gastric cancer. J Biomed Res. 2012;26:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lum LG, Munn NA, Schanfield MS, et al. The detection of specific antibody formation to recall antigens after human bone marrow transplantation. Blood. 1986;67:582–587. [PubMed] [Google Scholar]
- 31.Lum LG, Seigneuret MC, Shiobara S, et al. Adoptively transferred immunity persists in human marrow graft recipients In: Truitt RL, Gale RP, Bortin MM, editors. Cellular Immunotherapy of Cancer. New York: Alan R. Liss; 1987. p. 449–460. [PubMed] [Google Scholar]
- 32.Lum LG, Noges JE, Beatty P, et al. Transfer of specific immunity in marrow recipients given HLA-mismatched, T cell–depleted, or HLA-identical marrow grafts. Bone Marrow Transplant. 1988;3:399–406. [PubMed] [Google Scholar]
- 33.Garfall AL, Maus MV, Hwang WT, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373: 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter DL, Kalos M, Zheng Z, et al. Chimeric antigen receptor therapy for B-cell malignancies. J Cancer. 2011;2:331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thakur A, Lum LG. Bispecific targeted T cell therapy in breast cancer. OncoImmunology. 2015. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.