Abstract
Most of the adult population are infected with Epstein–Barr virus (EBV), but as EBV replication is usually under immune system control, the majority of individuals remain asymptomatic. On the other hand, some individuals continuously retain a high EBV antibody titer and a high EBV DNA load in their blood, suggesting a defect of EBV replication control. To date, only a limited number of reports have addressed the relationship between this chronic form of EBV infection and renal involvement. Here, we describe an 80-year-old woman who developed acute kidney injury shortly after an episode of mosquito bites, accompanied by a severe skin rash, which raised a suspicion of chronic EBV infection. She was subsequently diagnosed as having chronic replicative EBV infection. Renal biopsy revealed a diagnosis of IgA nephropathy with crescent formation. Although the relationship between IgA nephropathy and EBV infection has been discussed, no substantial understanding has yet emerged. The patient’s characteristic clinical course suggested that the renal failure may have been partly attributable to chronic EBV infection. This case suggests that physicians may need to consider the possibility that chronic EBV infection may affect the clinical course of IgA nephropathy, or exacerbate the disease.
Keywords: Epstein–Barr virus, IgA nephropathy, Glomerulonephritis, Renal failure
Introduction
Epstein–Barr virus (EBV) is a member of the human herpesvirus family. Infectious mononucleosis, a primary infection caused by EBV often occurring in adolescence, is usually a self-limiting benign disease characterized by symptoms such as fever, pharyngitis, lymphadenopathy or hepatosplenomegaly [1]. However, the replication of EBV is usually controlled after the primary infection has subsided, and most of the general population remain asymptomatic thereafter, although latent EBV persists in lymphocytes throughout the life of the host. Occasionally, however, some patients retain a high EBV antibody titer and high levels of EBV DNA in their blood, and EBV genome-positive cells are sometimes present in affected tissues. Although the underlying mechanism remains to be clarified, this long-term persistent EBV replication is known to be associated with various diseases or conditions, such as chronic active Epstein–Barr virus (CAEBV) disease [2], hypersensitivity to mosquito bite (HMB) [3–5], or certain hematologic malignancies [6].
However, only a limited number of reports have addressed the relationship between chronic EBV infection and renal complications. Although tubulointerstitial nephritis as a renal complication of EBV infection has been well described [7–9], previous reports of glomerular disease in chronic EBV infection have been limited [10–12]. Furthermore, as EBV usually affects children or adolescents, descriptions of adult cases have been sparse.
Here, we report an elderly female patient who rapidly developed renal failure shortly after recurrent episodes of mosquito bites. The skin rash that subsequently developed raised the possibility of chronic EBV infection, and subsequent investigation confirmed the diagnosis of chronic replicative EBV infection. Renal biopsy demonstrated IgA nephropathy with crescent formation. The characteristic clinical course of this patient suggested that the chronic EBV infection may have had a close relationship with the observed deterioration of her renal function.
Case report
An 80-year-old woman had been followed up for hypertension at the outpatient department of another hospital. Her blood pressure had generally been well controlled. She had no history of immunodeficiency and had not received immunosuppressants. Urinalysis had revealed slight abnormalities (mild occult blood and proteinuria) for at least several years, but her renal function had been almost stable and not investigated further. Since the age of 42 she had begun to develop intense skin reactions at the sites of mosquito bites, along with general symptoms such as fever and malaise. However, between the bite episodes, she had been completely asymptomatic.
One month prior to admission, the patient had suffered mosquito bites on the face while working outside. Shortly thereafter, she had developed severe skin reactions (Fig. 1), followed gradually by bilateral leg edema. Furthermore, she had suffered further bites on the thigh 2 weeks prior to admission. During this period, her body weight had increased by 4 kg, and her renal function had declined from a usual serum creatinine value of 1.2 mg/dl to one of 4.0 mg/dl.
Fig. 1.
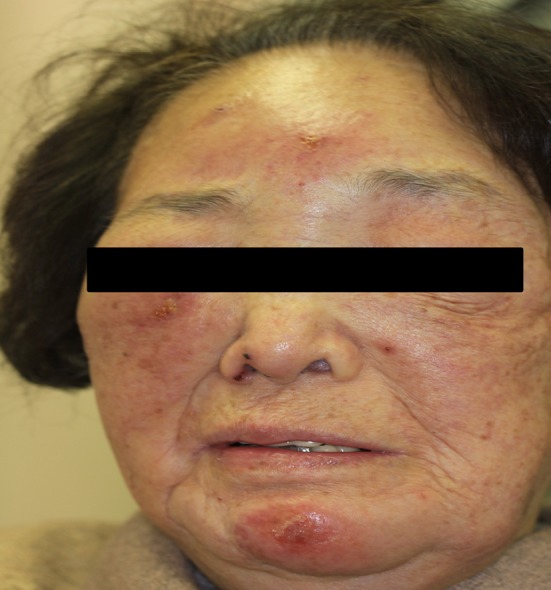
The mosquito bite sites at 2 weeks after the bite episode; intense skin reactions are evident on the face
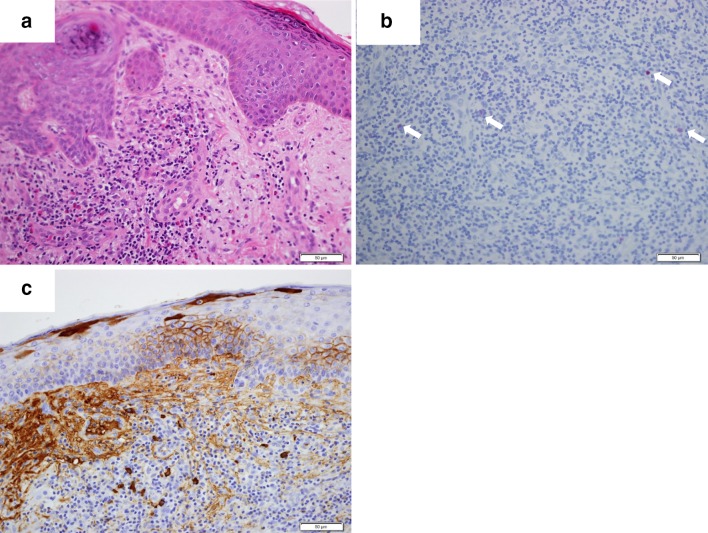
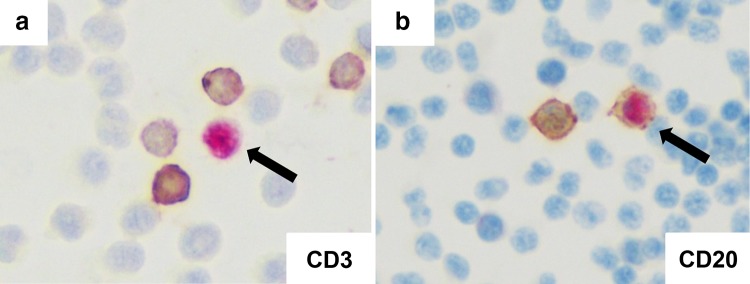
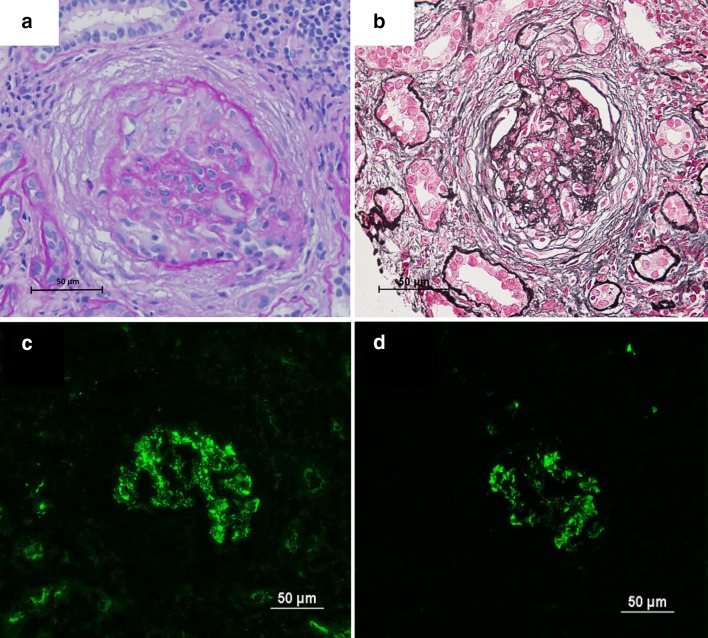
She was referred to our hospital because of rapidly progressive severe edema and declining renal function. On admission, her blood pressure was 145/66 mmHg, pulse rate 93/min, and body temperature 37.7 °C. Skin rashes were present on the face and thigh. She had no evident signs of lymphadenopathy, hepatosplenomegaly or pharyngitis. Her laboratory data on admission were: serum creatinine 7.65 mg/dl, blood urea nitrogen 87.5 mg/dl, white blood cell count 14,060/µl, hemoglobin concentration 8.8 g/dl, platelet count 161,000/µl, C-reactive protein 1.68 mg/dl, ferritin 242 ng/ml, immunoglobulin (Ig) G 2898 mg/dl, IgA 919 mg/dl and IgM 192 mg/dl. IgE was markedly elevated at 72,369 IU/ml. The liver aminotransferase levels were normal. Hepatitis virus B and C were both negative. Results of immunological tests were all unremarkable, including myeloperoxidase antineutrophil cytoplasmic antibody (ANCA), proteinase 3 ANCA, antinuclear antibody and anti-glomerular basement membrane antibody. The serum complement CH50 titer was normal at 53 U/ml (normal range 32–58). Immunoelectrophoresis using a serum sample revealed no monoclonal protein. No Bence-Jones protein was present in her urine. Microscopic examination of her urine demonstrated > 100 red blood cells and 1–4 white blood cells per high-power field. Urinary protein excretion was 1.6 g/day. Computed tomography revealed bilateral pleural effusion and bilaterally normal-sized kidneys. She was started and continued on hemodialysis therapy from the second hospital day. We suspected that she had HMB because of intense skin reactions at the affected sites with general symptoms including fever or malaise. A skin biopsy sample from a bite site on her chin demonstrated massive lymphocyte and eosinophil infiltration (Fig. 2a). As HMB is known to be associated with chronic EBV infection, we carried out further investigation to ascertain whether the latter was present. Additional blood tests were ordered, and these revealed negativity for anti-EBV viral capsid antigen (VCA) IgM antibody (< 1:20), marked elevation of anti-EBV-VCA IgG antibody (1:640), elevation of EBV-VCA IgA (1:40), and only slight positivity for EBV nuclear antigen (NA) (1:40). In addition, real-time polymerase chain reaction using peripheral blood mononuclear cells demonstrated mild elevation of EBV DNA at 217 copies/106 cells. These findings were suggestive of chronic replicative EBV infection. EBV-encoded RNA 1 (EBER-1) in situ hybridization using the skin biopsy specimen demonstrated only a few positive lymphocytes (Fig. 2b). Immunohistochemistry using a blood smear showed that the EBER-1 positive cells were stained for cluster of differentiation (CD) 20 (Fig. 3b), but not for CD3 (Fig. 3a). Finally, we diagnosed her as having a replicate form of EBV infection in B cells; however, as EBV infection of T/NK cells was not demonstrated, a diagnosis of HMB could not be proven. A comprehensive work-up revealed no sign of any hematologic malignancy. We carried out renal biopsy on the 14th hospital day. The biopsy sample contained 10 glomeruli, one of which showed global sclerosis and remainder mild mesangial proliferation. Two glomeruli had cellular crescents and the other showed adhesion and segmental sclerosis (Fig. 4a, b). EBER-1 in situ hybridization gave a negative result for the kidney tissue. Immunofluorescence revealed mesangial granular deposits of IgA and C3c (Fig. 4c, d). Therefore, we diagnosed the patient as having IgA nephropathy. The amount of renal tissue we obtained was insufficient for electron microscopy analysis. We also examined the skin biopsy specimen using immunohistochemistry and this demonstrated extensive IgA deposition (Fig. 2c). We started the patient on prednisolone at 30 mg daily, which dramatically improved her skin lesions. However, her renal function did not recover and regular hemodialysis became necessary. As the patient was elderly and there were no evident signs of hematologic neoplasms or immunodeficiency, we decided to closely observe her without any specific treatments for her chronic EBV infection.
Fig. 2.
Skin biopsy from the mosquito bite site on the chin. The skin tissue shows marked infiltration by lymphocytes and eosinophils (a hematoxylin–eosin, magnification ×200). EBER-1 in situ hybridization shows a small number of EBV-positive cells in the skin (b). Immunohistochemistry reveals extensive IgA deposition (c magnification ×200)
Fig. 3.
EBER-1 in situ hybridization using a peripheral blood smear; EBER-1-positive cells are not stained for CD3 (a), but are stained for CD20 (b) by immunochemistry
Fig. 4.
Renal biopsy findings. Light microscopy reveals a cellular crescent (a periodic acid-Schiff, magnification × 200), adhesion and mild mesangial matrix accumulation (b periodic acid silver methenamine–Masson trichrome, magnification × 200). Immunofluorescence reveals mesangial granular deposition of IgA and C3c (c IgA, d C3c)
After 3 months, her serum profile for anti-EBV antibody and EBV DNA in blood showed no change from that at admission, thus confirming the diagnosis of chronic infection with the replicative form of EBV. Furthermore, the patient’s serum IgA level at 6 months after admission was still elevated at 707 mg/dl, suggesting persistent IgA production.
Discussion
Here, we have described an elderly female patient with IgA nephropathy and the chronic replicative form of EBV infection who developed acute kidney injury. Considering the rapid worsening of her renal function shortly after suffering mosquito bites, we speculated that these two factors may have been related, against an underlying pathophysiology of chronic EBV infection.
The present case suggests that chronic EBV infection may affect the clinical course of IgA nephropathy. Patients with chronic EBV infection and glomerular disease have been reported, albeit occasionally (Table 1). Among them, however, none have had IgA nephropathy. Joh et al. reported a patient with recurrent EBV infection who developed immune complex-type glomerulonephritis [10]. They suggested that the continuously high titer of antibodies against EBV may have been associated with the glomerulonephritis. Although this explanation may have partially applied to our patient, who had a persistently high titer of anti-EBV antibodies, immunofluorescence in their study had revealed mesangial and glomerular basement membrane deposition of IgG, IgM and C3, unlike the situation in our patient, suggesting a different mechanism of disease progression.
Table 1.
Clinical summary of glomerular disease in patients with chronic EBV infection
| Author | Age and sex | Type of EBV infection | Type of EBV-infected lymphocytes | Renal involvement | EBV detection in kidney | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Joh et al. 1998 [10] | 7-year-old girl | CAEBV | Not described | Immune complex-mediated GN, TIN | Detected in the tubular epithelial cells | Methylprednisolone pulse | Not described |
| Okada et al. 2002 [11] | 70-year-old male | CAEBV | Not described | MCNS, acute TIN | Detected in kidney-infiltrating lymphocytes | Observation | Renal; spontaneous recovery |
| Kano et al. 2005 [12] | 11-year-old girl | CAEBV | T cells | Mesangium proliferative GN (with cellular crescents) | Detected in intracapillary spaces of glomeruli and in intravascular spaces surrounding renal tubules | Prednisolone, etoposide, cyclosporine A | Death due to interstitial pneumonia and NK cell leukemia |
| Present case | 80-year-old female | Chronic infection in B cells | B cells | IgA nephropathy with crescent formation | Not detected | Oral prednisolone | Maintenance hemodialysis |
CAEBV chronic active Epstein–Barr virus infection, GN glomerulonephritis, TIN tubulointerstitial nephritis, MCNS minimal change nephrotic syndrome
As the present patient had chronic EBV infection in B cells, she may have developed hypergammaglobulinemia including marked elevation of IgA because of B cell transformation by EBV [13], and this may have contributed to disease progression by producing much more pathogenic IgA. Indeed, it has been reported that EBV-transformation of B cells in patients with IgA nephropathy produces more IgA relative to IgG, in comparison to normal controls [14]. There is also a possibility that, in patients with IgA nephropathy, the population of B cells susceptible to EBV activation is increased [14]. Furthermore, although both IgA1 and IgA2 are increased by EBV-transformation of B cells, production of only the IgA1 isotype involved in the pathogenesis of IgA nephropathy is significantly increased in B cells from patients with IgA nephropathy in comparison with controls [15]. These data suggest that chronic EBV infection may affect the progression of IgA nephropathy. However, the patient’s serum IgA level had not been examined at the former facility before this episode of renal exacerbation. Therefore, we were unable to conclude definitively that the exacerbation of IgA nephropathy had occurred through the mechanism mentioned above. However, we did confirm that the IgA value was elevated at 6 months after hospitalization. We speculate that this persistently higher IgA value lends support to the above mechanism. In addition, as there was extensive IgA deposition in skin at the bite site, we speculate that the skin reaction may have been related to the pathogenic mechanism underlying the exacerbation of IgA nephropathy. Recently, it has been shown that galactose-deficient IgA1 plays a role in the pathogenesis of IgA nephropathy. Although galactose-deficient IgA1 is produced in components of the mucosal immune system such as the tonsils or gastrointestinal tract under conditions of infection, it has been reported that IgA1-producing B cells in patients with IgA nephropathy have a low capacity for galactosylation. Two possible mechanisms for reduced galactosylation have been reported: elevated expression of ST6 N-acetylgalactosaminide α-2,6-sialyltransferase 2 (ST6GALNAC2), which prematurely sialylates N-acetylgalactosamine (GalNAc) and prevents addition of galactose to GalNAc, and decreased expression or lower activity of core 1 β1,3-galactosyltransferase (C1GALT1) [16]. Therefore, we speculate that EBV-transformation of the affected patient’s B cells may have produced galactose-deficient IgA1 in the absence of mucosal infection. Although the glomerular IgA deposition in our patient may have merely represented non-specific trapping due to the high serum level of IgA, C3c deposition was concurrently distributed, and therefore we speculate that the IgA deposition may have been involved in the pathogenesis.
EBV DNA has also been detected in tissue lysates of renal biopsy specimens from patients with various glomerular diseases such as IgA nephropathy, membranous nephropathy and focal/segmental lesions [17]. The EBV DNA detection rate was reportedly higher in subgroups with mesangial injury or immunoglobulin deposition than in subgroups without these features, suggesting that EBV might damage the glomerular mesangium through an immunoglobulin-mediated mechanism [17]. Although this does not necessarily mean that EBV is causative, it is conceivable that EBV might play a role in the progression of IgA nephropathy. Although we found no EBV genome-positive cells in the renal tissue, as glomerular complications may develop through an antibody-mediated humoral mechanism, we believe that paucity of the EBV genome in kidney tissue might not in itself be contradictory. Taken together, the evidence suggests that IgA nephropathy, and also other glomerular diseases, might develop from—or be affected by—EBV infection.
In the present patient, renal function deteriorated rapidly despite steroid therapy, possibly reflecting the advanced renal damage demonstrated in the biopsy sample. The urinalysis abnormalities seen in the present case had preceded hospitalization for at least several years, suggesting that the glomerular injury related to EBV infection may have been present several years before presentation, thus explaining the substantial histological damage. Although we considered a more potent treatment such as steroid pulse therapy, in view of the progression of the patient’s renal biopsy findings, we were concerned that potentially harmful side effects might outweigh any gains, and therefore we decided to administer only oral steroid. Treatment may have been more successful if the diagnosis had been made earlier when renal damage was less advanced.
In this case, the role of mosquito bites in disease development remained unknown. The patient developed an intense skin lesion after suffering the mosquito bites, and a markedly high IgE level, which made us suspect HMB, which is now recognized to develop with chronic EBV infection [3, 4]. However, as reported cases of HMB are usually of the T/NK-cell type [18], the present case was atypical, and we were unable to diagnose it as HMB. Considering the temporal relationship between the mosquito bites and deterioration of renal function, we speculate that immune responses induced by the mosquito bites, such as a cytokine storm or increased production of gamma-globulin, may have triggered exacerbation of her disease in a background of chronic EBV infection. However, as no previous reports have discussed the relationship between such renal complications and mosquito bites, any direct role of the latter in the IgA nephropathy needs to be carefully considered.
CAEBV is a potentially lethal hematologic disorder associated with the chronic replicative form of EBV infection in which T/NK cells are usually infected [19]. Patients with this condition are usually children or adolescents, and they manifest recurrent infectious mononucleosis-like symptoms. In the present case, as only B cells were positive for the EBV genome and no infectious mononucleosis-like symptoms were evident, the proposed diagnostic criteria were not met [20].
Many points remain to be clarified regarding renal involvement in chronic EBV infection. We speculate that only a proportion of patients with IgA nephropathy may be affected by EBV infection. However, we are unable to draw any conclusions regarding differences between patients with IgA nephropathy who are affected by EBV and those who are not. It is also uncertain why many normal individuals harboring latent EBV infection lack urinary abnormalities. We speculate that the replicative form of EBV infection, as seen in this case characterized by increases in both viral genome copy number and EBV antibody titer, may contribute to or affect disease progression through immune responses. Therefore, we speculate that the status of EBV infection—latent or replicative—may need to be considered in the pathogenesis of glomerular complication. However, no sufficiently convincing explanation for the relationship between IgA nephropathy and EBV infection has yet been established, and therefore we are unable to make any definitive statement about the contribution of EBV infection.
In conclusion, we have described a case of acute kidney injury in an adult patient with IgA nephropathy and chronic EBV infection. Physicians may need to consider the possibility that chronic EBV infection may affect the clinical course of IgA nephropathy.
Abbreviations
- EBV
Epstein–Barr virus
- CAEBV
Chronic active Epstein–Barr virus
- HMB
Hypersensitivity to mosquito bite
- Ig
Immunoglobulin
- ANCA
Antineutrophil cytoplasmic antibody
- VCA
Viral capsid antigen
- NA
Nuclear antigen
- EBER-1
EBV encoded RNA 1
- CD
Cluster of differentiation
Compliance with ethical standards
Conflict of interest
The authors declare that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balfour HH, Jr, Holman CJ, Hokanson KM, et al. A prospective clinical study of Epstein-Barr virus and host interactions during acute infectious mononucleosis. J Infect Dis. 2005;192:1505–1512. doi: 10.1086/491740. [DOI] [PubMed] [Google Scholar]
- 2.Kimura H, Hoshino Y, Kanegane H, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98:280–286. doi: 10.1182/blood.V98.2.280. [DOI] [PubMed] [Google Scholar]
- 3.Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145–152. doi: 10.1016/j.jdermsci.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara S, Okada S, Wakiguchi H, et al. Clonal lymphoproliferation following chronic active Epstein-Barr virus infection and hypersensitivity to mosquito bites. Am J Hematol. 1997;54:276–281. doi: 10.1002/(SICI)1096-8652(199704)54:4<276::AID-AJH3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Chiu TM, Lin YM, Wang SC, Tsai YG. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613–616. doi: 10.1016/j.jmii.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H, Ito Y, Kawabe S, Nakamura S. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673–686. doi: 10.1182/blood-2011-10-381921. [DOI] [PubMed] [Google Scholar]
- 7.Becker JL, Miller F, Nuovo GJ, et al. Epstein-Barr virus infection of renal proximal tubule cells: possible role in chronic interstitial nephritis. J Clin Invest. 1999;104:1673–1681. doi: 10.1172/JCI7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cataudella JA, Young ID, Iliescu EA. Epstein-Barr virus-associated acute interstitial nephritis: infection or immunologic phenomenon? Nephron. 2002;92:437–439. doi: 10.1159/000063320. [DOI] [PubMed] [Google Scholar]
- 9.Mansur A, Little MA, Oh WC, et al. Immune profile and Epstein-Barr virus infection in acute interstitial nephritis: an immunohistochemical study in 78 patients. Nephron Clin Pract. 2011;119:c293–c300. doi: 10.1159/000329671. [DOI] [PubMed] [Google Scholar]
- 10.Joh K, Kanetsuna Y, Ishikawa Y, et al. Epstein-Barr virus genome-positive tubulointerstitial nephritis associated with immune complex-mediated glomerulonephritis in chronic active EB virus infection. Virchows Arch. 1998;432:567–573. doi: 10.1007/s004280050207. [DOI] [PubMed] [Google Scholar]
- 11.Okada H, Ikeda N, Kobayashi T, et al. An atypical pattern of Epstein-Barr virus infection in a case with idiopathic tubulointerstitial nephritis. Nephron. 2002;92:440–444. doi: 10.1159/000063322. [DOI] [PubMed] [Google Scholar]
- 12.Kano K, Yamada Y, Sato Y, et al. Glomerulonephritis in a patient with chronic active Epstein-Barr virus infection. Pediatr Nephrol. 2005;20:89–92. doi: 10.1007/s00467-004-1645-3. [DOI] [PubMed] [Google Scholar]
- 13.Straus SE, Cohen JI, Tosato G, Meier J. NIH conference. Epstein-Barr virus infections: biology, pathogenesis, and management. Ann Intern Med. 1993;118:45–58. doi: 10.7326/0003-4819-118-1-199301010-00009. [DOI] [PubMed] [Google Scholar]
- 14.Jackson S, Galla JH, Kirk KA, et al. Epstein-Barr virus transformation of B lymphocytes from IgA nephropathy patients and first-degree relatives results in increased immunoglobulin synthesis not restricted to IgA. Am J Kidney Dis. 1991;17:55–61. doi: 10.1016/S0272-6386(12)80251-4. [DOI] [PubMed] [Google Scholar]
- 15.Layward L, Allen AC, Harper SJ, Feehally J. Increased IgA and decreased IgG production by Epstein-Barr virus transformed B cells in culture in IgA nephropathy. Exp Nephrol. 1994;2:24–29. [PubMed] [Google Scholar]
- 16.Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwama H, Horikoshi S, Shirato I, Tomino Y. Epstein-Barr virus detection in kidney biopsy specimens correlates with glomerular mesangial injury. Am J Kidney Dis. 1998;32:785–793. doi: 10.1016/S0272-6386(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 18.Asada H. Hypersensitivity to mosquito bites: a unique pathogenic mechanism linking Epstein-Barr virus infection, allergy and oncogenesis. J Dermatol Sci. 2007;45:153–160. doi: 10.1016/j.jdermsci.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Cohen JI. Chronic active Epstein-Barr virus disease. Front Immunol. 2017;8:1867. doi: 10.3389/fimmu.2017.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80:64–69. doi: 10.1002/ajh.20398. [DOI] [PubMed] [Google Scholar]