Abstract
Background
Residual systemic inflammation, which is associated with non-AIDS clinical outcomes, may persist despite viral suppression. We assessed the effect of antiretroviral (ART) adherence interruptions on systemic inflammation among Ugandans living with HIV who were virally suppressed.
Setting
We evaluated adults initiating first-line ART at a regional referral hospital clinic in Mbarara, Uganda.
Methods
Plasma concentrations of interleukin-6 (IL-6), D-dimer, soluble sCD14, sCD163, the kynurenine/tryptophan (K/T) ratio, and CD8+ T-cell activation (HLA-DR+/CD38+ co-expression) were measured at baseline and 6 months following ART initiation among participants who achieved viral suppression (VL<400) at 6 months. ART adherence was monitored electronically. Time spent in an adherence interruption was computed as the percentage of days when the running average adherence was ≥10%. We fit adjusted linear regressions to evaluate the effect of time spent in an interruption on the log-transformed plasma concentrations of the inflammation biomarkers.
Results
Of 282 participants, 70% were female and median age was 34 years. At baseline, median CD4 and median log viral load were 135 cells/μl and 5.1 copies/ml, respectively. In the adjusted analysis, a running average adherence <10% was associated with higher sCD14 (+3%, p<0.008), sCD163 (+5%, p=0.002), D-dimer (+10%, p=0.007), HLA-DR+/CD8+ (+3%, p<0.025), IL-6 (+14%, p=0.008), and K:T ratio (+5%, p=0.002). These findings were largely robust to adjustment for average adherence, as well as higher thresholds of running average adherence, albeit with decreased statistical significance.
Conclusion
Increased time spent in adherence interruptions is associated with increased levels of inflammation despite viral suppression above and beyond average adherence.
Keywords: adherence, treatment interruption, inflammation, antiretroviral therapy, Uganda
Introduction
Antiretroviral therapy (ART) prevents HIV from progressing to AIDS, decreases mortality, and reduces HIV transmission [1, 2]. Suppression of HIV viral load achieved through sustained ART adherence is a major predictor of ART effectiveness [3, 4]. However, even when viral suppression has been achieved, residual systemic inflammation may persist, as has been shown with biomarkers of inflammation (i.e., IL-6, IFN-gamma), immune activation (i.e., sCD14, sCD163 and KT ratio) [5, 6] coagulation (i.e., d-dimer) [7, 8], and T-cell activation (i.e., percentage of HLA-DR-CD38 among CD4 and CD8+ T cells) [9, 10]. Such inflammation may lead to non-AIDS mortality and morbidity, including cardiovascular disease [11-14].
Recently, we demonstrated an inverse association between average ART adherence and biomarkers of inflammation among Ugandans living with HIV who had achieved virologic suppression early after initiation of ART [15]. These findings suggest that incomplete adherence may lead to intermittent low-level replication despite clinically undetectable plasma HIV RNA levels at the time of sampling. Importantly, we and others have shown that sustained ART adherence interruptions can be more strongly associated with loss of viral suppression than average adherence [16] (i.e., number of missed doses over a given time period), and that this relationship can be independent of the level of average adherence [17]. It is also possible that prolonged interruptions might have an even greater impact on persistent inflammation than poor average adherence (i.e., missing 8 days in a row vs. one missed day per week for 8 weeks). For example, once sustained viral replication occurs, extracellular virions can be deposited and persist on follicular dendritic cells in secondary lymphoid tissue, where they may persist for several months after viral replication is re-suppressed [18].
Based on these considerations, we hypothesized that time spent in adherence interruptions may be strongly associated with heightened inflammatory biomarker levels, even in the setting of viral suppression, and that this association may persist independent of the number of missed doses (average adherence). We explore this hypothesis in the same prospective cohort of Ugandans living with HIV and initiating ART as we used in our initial analysis [15].
Methods
Participants
We evaluated adults living with HIV and initiating first-line ART between 2005 and 2010 at a regional referral hospital clinic in Mbarara, Uganda; all participants were enrolled in the Uganda AIDS Rural Treatment Outcomes (UARTO) prospective cohort study (; [19, 20]). In the UARTO study, participants were seen by study staff every 3–4 months and at each visit a questionnaire was administered and blood drawn for plasma and cell isolation. This analysis involves all participants who had a) inflammatory biomarker levels measured prior to treatment initiation and after 6 (±1) months on ART, b) an HIV viral load <400 copies/ml at the 6-month blood draw and, c) ART adherence data for at least the first 3 months after enrolment [15].
Adherence measurement and time spent in adherence interruption
ART adherence was measured by the medication event monitoring system (MEMS, (Aardex Group, Switzerland), a pill bottle with an electronic microchip in the cap that records the date and time at each bottle opening. As with our initial analysis [15], periods of participant-reported device non-usage were censored from adherence computations and average ART adherence was computed as the total number of openings within the 6-month study period divided by the total prescribed doses within the period. Adherence was capped at 100% when the numerator exceeded the denominator.
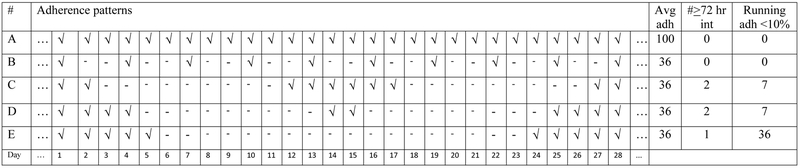
Our primary predictor variable of interest was the time spent in adherence interruptions during the first six months of therapy. To define time spent in an interruption, we first computed a running average adherence per participant per day as the average of the surrounding 9 days, starting at 4 days prior and ending 4 days after the day in question. The time spent in an interruption was defined as the proportion of days when the running average was less than or equal 10%. Unlike the more standard, simple count of interruptions longer than a certain length (e.g., 72 hours), the running average mitigates the effects of variations in the duration of adherence interruptions, which may impact treatment outcomes differently [16]. Specifically, our prior research indicates odds of 1.25 (95% CI: 1.06, 1.47) for viral replication with each day of an adherence interruption beyond 48 hours [17]. Moreover, the duration of the interruption and dose resumption should be considered in light of plausible virological effects. The hypothetical data in Figure 1 provides further explanation of this technique. Adherence pattern “A” shows consistent daily adherence (average 100%), while patterns “B”, “C”, “D” and “E” all show 18/28 missed doses in different patterns (average 36%). For example, pattern “E” shows the longest sustained adherence interruption, which is more likely to lead to viral replication than pattern “B”, which shows isolated missed doses. Additionally, pattern “D” shows a two-day separation of two otherwise sustained interruptions. Treating this interruption as two distinct interruptions may not reflect the same impact on viral replication as the more temporally separated interruptions in pattern “C”. The final two columns of the figure show the distinction between the standard and the running average approaches to computing treatment interruptions. In the former, adherence is lower with increased number of treatment interruptions regardless of temporal separation between the interruptions. With the latter, the longer the interruption is sustained, the lower the adherence. In the Appendix Table 4a, we demonstrate how the running averages are computed based on the hypothetical adherence patterns described in Figure 1. In the analysis dataset, a 1% increase in the primary predictor, time spent in an adherence interruption, corresponds to 1.8 days or 1% of 180 days (6 months). Since the effect of a 1% increase would be small and of unclear clinical significance, we report a 5% increase in ART adherence interruption, which is equivalent to 9 days (i.e., 1.8 days x 5).
Figure 1.
The symbol “√” indicates that a pill was taken on a given day; “-” indicates no pill taken. Adherence pattern “A” represents 100% adherence, while patterns “B”, “C”, “D” and “E“ represent varying distributions of the same number of missed doses (18/28, or 36%). The final three columns show the average adherence, the number of ≤72-hour interruptions, and the percentage of days when the running average adherence was <10% (See Appendix Table 4a and 4b for further details). For simplicity’s sake, we assume perfect adherence prior to and after these 28 days.
Biomarkers of inflammation, coagulopathy and CD8+ T-cell activation
As previously reported [9, 15, 21, 22], D-dimer (Diagnostico Stago), interleukin 6 (IL-6; Human IL-6 Ultra-Sensitive Kit, Meso Scale Diagnostics), soluble (s)CD14 (sCD14; R&D Systems), sCD163 (sCD163; Trillium Diagnostics), and the kynurenine/tryptophan ratio (K/T ratio) were measured in thawed plasma samples and the percentage of HLA-DR+/CD38+ CD8+ T-cells measured in fresh whole-blood specimens processed on the day of collection. About 95% of samples were stored in acid citrate dextrose (ACD) and the rest in ethylenediaminetetraacetic acid. All plasma collected in ACD was diluted by 1.5ml for storage; we therefore used an adjustment factor of 1.276 for these biomarkers to reflect the true concentration. Plasma was centrifuged and stored at −80°C until analysis [21].
Statistical Analysis
Participant demographic and clinical characteristics were summarized using medians (interquartile range [IQR]) for continuous variables and frequencies (percent) for categorical variables. Our dependent variables of interest were inflammatory biomarker concentrations, and our main predictor of interest was time spent in the adherence interruption computed as the percentage of days during the first 6 months of observation when the 9-day running average adherence was ≥10%. We assessed the effect of the time spent in adherence interruptions on the inflammatory biomarker using several different modeling approaches.
Effect of time spent in adherence interruptions
First, we fit multivariable linear regression models with each biomarker as the outcome of interest and the time spent in the adherence interruption as the primary explanatory variable of interest plus the following potential confounders that have been shown to influence adherence in prior analyses: baseline inflammation [23], use of a nevirapine-based regimen versus efavirenz [24], duration of viral suppression [25], age [26], gender [27], CD4+ T-cell count [26], baseline log viral load [28], depression [27, 29] as assessed by the Hopkin’s depression check list [30], and heavy alcohol use [27, 31] as assessed by the AUDIT-C (score≤4 for males or score≤3 for females). These models are hereinafter referred to as the primary models.
Effect of average percentage adherence
In the secondary models, we refit the multivariable linear regression models above for each biomarker, but with average percentage adherence as the explanatory variable of interest.
Next, to assess the effect of time spent in adherence interruptions adjusted for average percentage adherence and vice versa, we fit models including both explanatory variables (i.e time spent in an adherence interruption and average percentage adherence).
In all the models, duration of viral suppression at month 6 was measured as the number of days since viral suppression at month 3; participants with an unsuppressed or missing month 3 viral load were considered unsuppressed (0 suppression days).
Sensitivity analyses
We performed sensitivity analyses on the primary models in which the time spent in adherence interruptions was computed as the percentage of days when the running average was ≥20%, ≥30%, and ≥50%. Additionally, we provide results from the models using the standard ≤72-hour treatment interruptions as the outcome of interest. Finally, we performed an additional sensitivity analysis on the primary models, in which participants without month 3 viral suppression data were excluded from the models.
Model diagnostics
The distribution of all biomarker concentrations was assessed for normality by normal plots; all biomarker concentrations were log-transformed prior to performing the regressions. To assess for linear regression model fit, we performed sensitivity analyses, on the primary models, excluding high influence observations with DFBETA values outside the ± 2/√n range or Cook’s D value >1.0 [15]. Statistical analysis was performed using Stata 13 (StataCorp, College Station, TX).
Ethical Considerations
UARTO study procedures were approved by the Ugandan National Council of Science and Technology, and the institutional review boards of Mbarara University of Science and Technology, and Partners Healthcare/Massachusetts General Hospital. All participants provided written informed consent.
Results
Participant characteristics
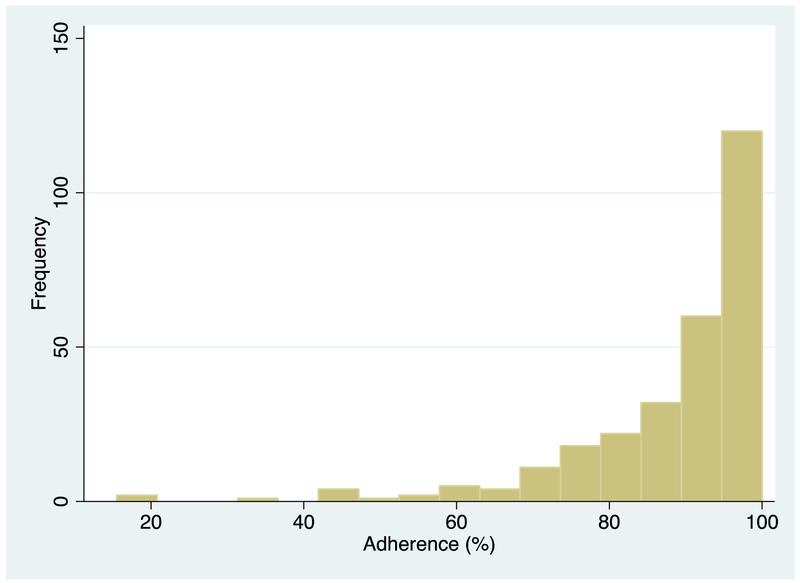
Among the 282 participants who met eligibility criteria, 196 (70%) were female and the median age was 34 years (29, 39). As shown in Figure 2, the median (IQR) adherence at month 6 was 93% (85, 99). At baseline, the median (IQR) CD4 was 135 cells/μl (83, 199) and median log viral load 5.1 copies/ml (4.6, 5.5). The majority (80%) were literate, less than half (43%) were married, and a quarter (25%) unemployed. At enrollment, 262 (93%) were on nevirapine-based regimen, 93 (33%) were depressed and 39 (14%) were heavy drinkers. Pre-treatment and 6-month inflammation samples were present for at least 95% of the participants for all biomarkers except HLA-DR+/CD8+ (Appendix Table 1). At the Month 3 study visit, 231 (82%) were virologically suppressed, 21 (7%) were unsuppressed, and 30 (11%) did not have viral load data.
Figure 2:
Distribution of the 6-month average percentage adherence.
Effect of time spent in adherence interruptions
In the primary models, as shown in Table 1, each unit increase (equivalent to 9 days) in the time spent in an adherence interruption was associated with higher levels of sCD14 (2.6%, p<0.008), sCD163 (4.6%, p=0.002), D-dimer (10.1%, p=0.007), HLA-DR+/CD8+ (+2.7%, p=0.025), IL-6 (14.1%, p=0.008), and K:T ratio (4.9%, p=0.002). When we adjust for average percentage adherence, the effect sizes for sCD14 (4.4%, p=0.014), sCD163 (9.8%, p<0.001), and K/T ratio (8.3%, p=0.006) increased while maintaining statistical significance; other effect sizes decreased and lost statistical significance, but remained positive.
Table 1:
Effect of adherence interruptions on inflammatory biomarkers. Both sets of models adjust for potential confounders (baseline inflammation level, use of a nevirapine versus efavirenz-based regimen, duration of viral suppression, age, gender, baseline log viral load, depression, and heavy alcohol use); the second model also adjusts for average adherence.
| Effect of treatment interruption on biomarkers of inflammation |
||||
|---|---|---|---|---|
| Biomarker | Without adjustment for average adherence |
With adjustment for average adherence |
||
| Effect (95% CI) | P | Effect (95% CI) | P | |
| sCD14 | 2.6 (0.7, 4.6) | 0.008 | 4.4 (0.9, 8.0) | 0.014 |
| sCD163 | 4.6 (1.7, 7.6) | 0.002 | 9.8 (5.0, 14.9) | <0.001 |
| D-dimer | 10.1 (2.6, 18.0) | 0.007 | 2.4 (−9.4, 15.8) | 0.70 |
| HLA−DR+/CD8+ | 2.7 (0.3, 5.1) | 0.025 | 0.7 (−3.2, 4.7) | 0.74 |
| IL-6 | 14.1 (3.5, 25.8) | 0.008 | 5.9 (−8.5, 22.6) | 0.44 |
| K/T ratio | 4.9 (1.9, 8.1) | 0.002 | 8.3 (2.3, 14.6) | 0.006 |
Effect of average percentage adherence
In the secondary models (Appendix Table 2), those biomarkers that were significantly associated with average adherence before accounting for time spent in adherence interruptions (i.e., D-dimer [−1.2%, p=0.008], HLA-DR+/CD8+ [−0.4%, p=0.023], IL-6 [−1.6%, p=0.003]) lost statistical significance after we adjusted for time spent in adherence interruptions (i.e., D-dimer [−1.0%, p=0.22], HLA-DR+/CD8+ [−0.3%, p=0.23], IL-6 [−1.0%, p=0.16]). However, while sCD163 was not significantly associated with adherence prior to accounting for time spent in adherence interruptions (−0.16%, p=0.45), we did see a significant association after accounting for time spent in adherence interruptions (0.7%, p=0.027).
Sensitivity analyses
When we redefined time spent in a adherence interruption as the percentage of days when the running average was ≥20%, ≥30%, and ≥50%, biomarker effect sizes attenuated in size towards the null and lost statistical significance with increasing thresholds (Appendix Table 3b). As shown in Appendix Table 4b, the higher the threshold (especially beyond 30%), the more similar the patterns of interruption (B, C, D, and E) become and therefore the less effective the running average approach becomes in distinguishing among these patterns. Of note, biomarker effect sizes from the standard ≤72-hour adherence interruptions show a pattern similar to that from the running average approach (Appendix Table 3b), with D-dimer and IL-6 having the largest estimates, while the rest showed relatively smaller sizes.
Results from the sensitivity analyses excluding the records without month 3 viral suppression data were not materially different than those from the primary analyses with the exception of D-dimer, which was no longer statistically significant and whose effect size attenuated (Appendix Table 3a).
Model diagnostics
In exploring the influence of outliers, only one record for IL6 was excluded for a Cook’s distance >1 and no significant differences were seen in our results. However, when considering the DFBETA for the running average >2/√n, we excluded 7–14 records for the various biomarkers. Effect estimates were stable for all biomarkers, although statistical significance was lost (results not shown).
Discussion
Among individuals who achieved virologic suppression 6 months after ART initiation, an increase in the time spent in an ART adherence interruption was associated with increased levels of biomarkers of inflammation, coagulopathy, and CD8+ T-cell activation. For all biomarkers assessed, a unit (9-day) increase in adherence interruption was associated with a 2.6–14.1% increase in the level of the biomarker. This association almost doubled and maintained statistical significance for sCD14, sCD163, and K/T ratio, after controlling for average adherence. Our findings suggest that among those who have achieved virologic suppression, increased time spent in ART adherence interruptions may result in higher residual inflammation and immune activation regardless of the number of doses missed (average adherence).
Our present findings expand on our previous work, which demonstrated an inverse relationship between average adherence and biomarkers of inflammation, immune activation and coagulopathy [15], in the broader sense that adherence is associated with biomarker levels. However, these new findings differ in that they underscore the importance of adherence interruptions as potentially the bigger driver of the association between ART adherence and inflammatory biomarkers. When adherence interruptions were accounted for; average adherence was no longer significantly associated with levels of biomarkers in all but one biomarker, even though the effect sizes remained relatively similar. Importantly, the effect sizes were also similar, but reduced, when considering standard adherence interruptions (i.e., ≤72 hours) and higher thresholds for the running average; indeed, a dose-dependent relationship was seen with the latter. These findings highlight the more sensitive nature of the running average for identifying adherence patterns that can reflect the biological effects on the virus. To our knowledge, this is the first demonstration of a direct relationship between adherence interruptions and residual inflammation. Among the potential explanations for our findings is that longer adherence interruptions could lead to increased seeding of virus in lymph node follicles, which retain virus much longer than it persists in plasma after ART re-initiation [12, 18]. Intermittently missed doses likely will not have time for such effects, particularly with the long half-lives of modern ART regimens.
Our findings have important clinical implications for chronic HIV care, because the effects of adherence interruptions on inflammation and immune activation are likely to occur over years, along with other diseases of aging (e.g., non-communicable diseases [NCDs] like cardiovascular disease). As more research explores the relationship of inflammation and NCDs in chronic HIV infection [8, 32, 33], the role of adherence (particularly interruptions) needs to be explored. Additionally, the effect might be different in drugs with different half-lives and penetration into tissues and sanctuary sites.
In addition to these clinical implications, our findings also emphasize the importance of robust adherence monitoring in promoting sustained and durable adherence and avoiding adherence interruptions. Importantly, these results suggest that viral load monitoring might not be sufficient to detect effects of non-adherence that occur without viral replication. Adherence methods usually employed to detect non-adherence in clinical care, which typically include self-report and pharmacy refill, do not assess patterns of adherence such as sustained non-adherence [34]. Electronic adherence monitoring [35] and more recently developed ingestion monitors [36] allow for detection of day-to-day adherence patterns, often in real-time, and are readily linked to interventions to those in need of support. Additionally, future advancements in viral load assay technology with lower detection thresholds might aid in combating low-level replication, which is not detectable with current HIV RNA monitoring.
Some strengths of this analysis include the use of an objective adherence measure and consideration of multiple biomarkers of systemic inflammation, immune activation and coagulopathy. Among the main limitations is the use of a high plasma viral load cutoff (<400 copies/mL) to define viral suppression. However, heightened inflammation has been shown to be associated with lower adherence among virally suppressed individuals at lower thresholds [23, 37]. Another is the limited generalizability of our findings which may only be specific to participants from similar settings and who have been on non-nucleoside reverse transcriptase-based ART regimen for only 6 months and with only two viral load measurements (i.e., at months 3 and 6 following ART initiation). Additionally, our findings may have biases from residual confounding from variables not included in our regression models including those omitted because of a high prevalence of missing data. Future studies may explore lower viral load cutoffs, longer duration of viral suppression and associations with alternative ART regimen formulations.
In conclusion, this analysis indicates that during the first 6 months of ART, increasing time spent in ART adherence interruptions is associated with increased levels of biomarkers of systemic inflammation, immune activation and coagulopathy among treatment-naïve Ugandans who achieved suppression after the first 6 months of treatment. This relationship persisted for some biomarkers after controlling for average ART adherence. These findings underscore the importance of sustained ART adherence support with lifelong ART therapy even among individuals who achieve virologic suppression.
Supplementary Material
Acknowledgements
The authors would like to thank the UARTO participants and staff, Huyen Cao, Russell Tracy and Kenneth Williams who made aspects of this study possible.
Source of Funding: The Uganda AIDS Rural Treatment Outcomes Study was funded by U.S. National Institutes of Health (NIH) R01 MH54907, P30 AI27763, UM1 CA181255 and the Sullivan Family Foundation.
Footnotes
Conflicts of Interest: The authors report no conflict of interest.
Meetings at which parts of the data were presented: International Association of Providers of AIDS Care (IAPAC) conference held in Miami between June 8–10, 2018
References
- 1.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012,367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med 2016,375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Zyl GU, van der Merwe L, Claassen M, Cotton MF, Rabie H, Prozesky HW, et al. Protease inhibitor resistance in South African children with virologic failure. Pediatr Infect Dis J 2009,28:1125–1127. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Hughes MD, Lockman S, Benson CA, Hosseinipour MC, Campbell TB, et al. Antiretroviral therapy and efficacy after virologic failure on first-line boosted protease inhibitor regimens. Clin Infect Dis 2014,59:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Halloran JA, Dunne E, Gurwith M, Lambert JS, Sheehan GJ, Feeney ER, et al. The effect of initiation of antiretroviral therapy on monocyte, endothelial and platelet function in HIV-1 infection. HIV Med 2015,16:608–619. [DOI] [PubMed] [Google Scholar]
- 6.Siedner MJ, Zanni M, Tracy RP, Kwon DS, Tsai AC, Kakuhire B, et al. Increased Systemic Inflammation and Gut Permeability Among Women With Treated HIV Infection in Rural Uganda. J Infect Dis 2018,218:922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freiberg MS, Bebu I, Tracy R, So-Armah K, Okulicz J, Ganesan A, et al. D-Dimer Levels before HIV Seroconversion Remain Elevated Even after Viral Suppression and Are Associated with an Increased Risk of Non-AIDS Events. PLoS One 2016,11:e0152588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuhaus J, Jacobs DR Jr., Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010,201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003,187:1534–1543. [DOI] [PubMed] [Google Scholar]
- 10.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008,197:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis 2011,217:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 2015,112:E1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebnord EW, Strand E, Midttun O, Svingen GFT, Christensen MHE, Ueland PM, et al. The kynurenine:tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia 2017,60:1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 2010,115:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo-Mancilla JR, Morrow M, Boum Y, Byakwaga H, Haberer JE, Martin JN, et al. Brief Report: Higher ART Adherence Is Associated With Lower Systemic Inflammation in Treatment-Naive Ugandans Who Achieve Virologic Suppression. J Acquir Immune Defic Syndr 2018,77:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genberg BL, Wilson IB, Bangsberg DR, Arnsten J, Goggin K, Remien RH, et al. Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS 2012,26:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberer JE, Musinguzi N, Boum Y 2nd, Siedner MJ, Mocello AR, Hunt PW, et al. Duration of Antiretroviral Therapy Adherence Interruption Is Associated With Risk of Virologic Rebound as Determined by Real-Time Adherence Monitoring in Rural Uganda. J Acquir Immune Defic Syndr 2015,70:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol 2008,82:5548–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adakun SA, Siedner MJ, Muzoora C, Haberer JE, Tsai AC, Hunt PW, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr 2013,62:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musinguzi N, Mocello RA, Boum Y 2nd, Hunt PW, Martin JN, Haberer JE, et al. Duration of Viral Suppression and Risk of Rebound Viremia with First-Line Antiretroviral Therapy in Rural Uganda. AIDS Behav 2017,21:1735–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siedner MJ, Kim JH, Nakku RS, Bibangambah P, Hemphill L, Triant VA, et al. Persistent Immune Activation and Carotid Atherosclerosis in HIV-Infected Ugandans Receiving Antiretroviral Therapy. J Infect Dis 2016,213:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byakwaga H, Boum Y 2nd, Huang Y, Muzoora C, Kembabazi A, Weiser SD, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2014,210:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo-Mancilla JR, Brown TT, Erlandson KM, Palella FJ Jr., Gardner EM, Macatangay BJ, et al. Suboptimal Adherence to Combination Antiretroviral Therapy Is Associated With Higher Levels of Inflammation Despite HIV Suppression. Clin Infect Dis 2016,63:1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nachega JB, Hislop M, Dowdy DW, Gallant JE, Chaisson RE, Regensberg L, et al. Efavirenz versus nevirapine-based initial treatment of HIV infection: clinical and virological outcomes in Southern African adults. AIDS 2008,22:2117–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raboud JM, Harris M, Rae S, Montaner JS. Impact of adherence on duration of virological suppression among patients receiving combination antiretroviral therapy. HIV Med 2002,3:118–124. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor JL, Gardner EM, Mannheimer SB, Lifson AR, Esser S, Telzak EE, et al. Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. J Infect Dis 2013,208:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-Saharan Africa: a systematic review. BMJ Glob Health 2016,1:e000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood E, Hogg RS, Yip B, Moore D, Harrigan PR, Montaner JS. Impact of baseline viral load and adherence on survival of HIV-infected adults with baseline CD4 cell counts > or = 200 cells/microl. AIDS 2006,20:1117–1123. [DOI] [PubMed] [Google Scholar]
- 29.Grenard JL, Munjas BA, Adams JL, Suttorp M, Maglione M, McGlynn EA, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med 2011,26:1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep 2014,11:291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Detsis M, Tsioutis C, Karageorgos SA, Sideroglou T, Hatzakis A, Mylonakis E. Factors Associated with HIV Testing and HIV Treatment Adherence: A Systematic Review. Curr Pharm Des 2017,23:2568–2578. [DOI] [PubMed] [Google Scholar]
- 32.Borges AH, Silverberg MJ, Wentworth D, Grulich AE, Fatkenheuer G, Mitsuyasu R, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013,27:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008,5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005,353:487–497. [DOI] [PubMed] [Google Scholar]
- 35.Haberer JE, Kiwanuka J, Nansera D, Muzoora C, Hunt PW, So J, et al. Realtime adherence monitoring of antiretroviral therapy among HIV-infected adults and children in rural Uganda. AIDS 2013,27:2166–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai PR, Rosen RK, Boyer EW. Ingestible Biosensors for Real-Time Medical Adherence Monitoring: MyTMed. Proc Annu Hawaii Int Conf Syst Sci 2016,2016:3416–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castillo-Mancilla JR, Phillips AN, Neaton JD, Neuhaus J, Collins S, Mannheimer S, et al. Association of Suboptimal Antiretroviral Therapy Adherence With Inflammation in Virologically Suppressed Individuals Enrolled in the SMART Study. Open Forum Infect Dis 2018,5:ofx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.