Background:
Four of the largest HIV prevention trials have been conducted in sub-Saharan Africa, enrolling hundreds of thousands of participants in catchment areas of millions of people. The trials have focused on community-level interventions to increase diagnosis and initiation of antiretroviral therapy (ART) to improve health and reduce HIV transmission. Universal test-and-treat strategies are deployed to achieve viral suppression thereby reducing risk to uninfected persons, known as treatment as prevention (TasP).
Purpose:
We review the work that found HIV plasma load to correlate with transmission risk, demonstrated that ART could reduce genital tract viral expression, and showed early treatment to be beneficial for persons living with HIV, and that HIV-uninfected sexual partners were protected from infection. We review the seemingly inconsistent findings of the major TasP trials: the TasP [National Agency for AIDS Research (ANRS) 12249] study in South Africa, the SEARCH trial in Kenya and Uganda, the Botswana Combination Prevention Project Ya Tsie study, and the HIV Prevention Trials Network 071 (PopART) trial in Zambia and South Africa.
Findings:
All the trials reinforce the critical need to identify approaches to optimize programs and incentivize uptake and engagement in HIV testing and ART-based care in ways that consistently reduce HIV transmission. That other chronic conditions can be screened for and treated in the same infrastructures suggests added value of HIV investments.
Conclusions:
Implementation challenges are a principal frontier in the global struggle to reduce HIV transmission and mortality using TasP, complementing efforts to find a cure for HIV and an effective, deployable vaccine.
Key Words: HIV, prevention, treatment as prevention, universal test and treat, 90-90-90 targets, HIV care continuum, randomized trials, Africa
To accelerate HIV prevention progress, the Joint United Nations Programme on HIV and AIDS (UNAIDS) and the World Health Organization (WHO) announced new goals to jumpstart efforts to end AIDS as a public health threat in 2014–2015.1 The goal was coined “90-90-90,” with specific goals to have 90% of all people living with HIV know their status, 90% of all HIV-infected persons who knew their status taking antiretroviral therapy (ART), and 90% of those on ART being virally suppressed, all by the year 2020. The 2030 goal is “95-95-95.” To facilitate these goals, the WHO encouraged treatment1,2 for all persons living with HIV (PLHIV), regardless of their CD4+ cell counts. These goals represented a more aggressive and streamlined approach to increasing coverage of antiretroviral treatment (ART). They were based on recognition that early treatment yielded better clinical outcomes, and that Treatment as Prevention (TasP) was a viable HIV prevention and control strategy, by preventing onward HIV transmission (we use TasP and universal testing and treatment interchangeably).3 Here, we seek to present the conceptual foundation for TasP, summarize the key findings from 4 large-scale community-based randomized trials designed to assess the impact of large-scale deployment of TasP on HIV transmission, and discuss the challenges remaining in our understanding and implementation of TasP.
THE SCIENTIFIC RATIONALE FOR TasP
The virological and clinical basis for TasP was established by 2 key studies from Quinn et al in 20004 and Fideli et al in 2001.5 These 2 observational studies followed serodiscordant heterosexual couples in Uganda (N = 415 couples)4 and Zambia (N = 1022 couples)5 and found that lower viral load in the partner living with HIV was significantly associated with lower risk of onward transmission to HIV-uninfected partners. In the Ugandan study, incidence was 11.8/100 person-years,4 and in the Zambian study, incidence was 8.6/100 person-years.5 The Zambian study used phylogenetic analyses to confirm transmission pairs, excluding nongenetically linked pairs whose transmission should not be associated with the viral load of the HIV-infected partner. In both studies, there was no transmission observed between sexual partners when the infected partner's viral load was low; the threshold for protection from infection in the Zambian study was under 3000 viral copies/mL.
Subsequent studies of genital tract samples demonstrated that treatment-induced suppression of plasma viral load also suppressed the genital viral load, which in turn, reduced viral shedding.6–8 These studies suggested that reduced viral loads in plasma and the genital tract would be likely to reduce the risk of onward sexual HIV transmission. That lower viral load was associated with lower transmission risk, and that ART-reduced genital tract viral load led to the launch of the seminal HIV Prevention Trials Network (HPTN) 052 trial to examine the effect of earlier ART initiation on the risk of transmission of HIV from HIV+ individuals to their sexual partners.9,10 Serodiscordant couples from 13 sites in 9 countries were enrolled in HPTN 052 and randomly assigned to receive either early ART (initiation upon study enrollment) or delayed ART (initiation after either 2 consecutive CD4+ counts <250/μL, which was later changed to <350/μL as national guidelines changed, or an AIDS-defining illness). An interim analysis of the study data in 2011 after a median follow-up of 1.7 years found that early ART, at a median CD4 count of 442 cells/μL, was associated with a 96% lower risk of infection transmission compared with delayed ART, at a median CD4 count of 221 cells/μL.10 Based on the overwhelming benefit observed with early ART, all study participants in the delayed ART arm were offered ART, regardless of their CD4+ count. The trial continued to follow participants through the end of the study in 2015 and found that the overwhelming protective effect of early ART on transmission was maintained throughout the study period.9
As the clinical effectiveness of TasP was established by individual-level controlled trials and modeling studies,3 researchers also examined the effectiveness of expanded treatment in larger, uncontrolled population level studies in such diverse settings as Western Canada11 and KwaZulu-Natal, South Africa.12 These studies suggested that expansion of ART in “real-world” uncontrolled conditions could still produce a significant decline in HIV incidence.11,12 TasP seemed to hold the same promise as prevention of mother-to-child transmission (PMTCT) in which treating an infected mother can prevent vertical HIV transmission to the infant.13 Indeed, many lessons learned from the PMTCT experience following the WHO recommendation that pregnant women living with HIV be treated with ART during and after pregnancy (Option B+) could be applied to the logistical challenges in deploying TasP to achieve 90-90-90 goals.14
WHEN DEPLOYED AT SCALE, WHAT IS THE EFFECTIVENESS OF TasP?
There have been 4 large-scale trials of TasP and 90-90-90 with HIV incidence endpoints 15: the TasP/ANRS 12249 trial in South Africa,16–19 the Sustainable East Africa Research in Community Health (SEARCH) study in Kenya and Uganda,20–27 the Botswana Combination Prevention Project (BCPP) Ya Tsie study,28–30 and the HPTN 071 (PopART) study in South Africa and Zambia.31–35 Each of these studies highlights different opportunities and challenges in reaching the 90-90-90 goals and using TasP to make meaningful reductions in HIV incidence in sub-Saharan Africa. Details on the context and methods used in these trials have been compiled and compared elsewhere.15
The TasP Study
The TasP (ANRS 12249) study conducted by the Africa Centre (now within the African Health Research Institute) employed a cluster-randomized design to assess the effectiveness of TasP on HIV incidence in KwaZulu-Natal, South Africa, where HIV seroprevalence has been estimated at ∼30%.17 Repeated home-based HIV testing of adults was conducted in all clusters. Clusters were randomized to either immediate ART initiation (intervention) or initiation according to national guidelines (control) after HIV diagnosis. The home-based testing was well accepted and reached the first 90 target,19 despite challenges reaching men. However, compared with the control arm, linkage to care, ART initiation, and viral suppression saw only modest increases that fell far short of the second and third 90 goals. Specifically, linkage to care and initiation of ART among those diagnosed was low in both arms, with 53.4% ART coverage in the intervention arm and 52.8% in the control arm, P = 0.67.
The differences in HIV incidence between the control and intervention groups were not substantial and were not statistically significant.16 During the study, 565 individuals acquired HIV (244 in the intervention arm and 321 in the control arm). Of these, 1 year after seroconversion, 22% migrated out from the study area, 57% were aware of their HIV status, 27% were actively in HIV care, 12% were on ART, and 10% were virally suppressed. The cascade was similar for both trial arms, except for ART coverage, which was marginally higher in the intervention arm (15%) than the control arm (10%).36 A key lesson learned from the TasP trial was that the intervention did not address the critical barrier in this setting, namely a long delay between HIV diagnosis and ART initiation, which may have led to reduction in HIV incidence. Individuals who had never been in HIV care before referral were significantly less likely to link to care than those who had previously been in care.16 Linkage to care was also lower among students than among employed adults, among adults who completed some or all secondary school compared to those with a primary school education or less, among those who lived closer to TasP clinics, and those who were referred to the clinic after 2 or more contacts compared to those who were referred at first contact. Linkage to care was higher in adults who reported knowledge of a family member living with HIV versus not, and among those who said that they would take ART as soon as possible after receiving an HIV diagnosis versus not.16 These findings suggested that future TasP efforts would need to develop and/or adapt approaches to reach, engage, and retain multiple heterogenous groups.18
The SEARCH Study
The SEARCH study was conducted by a Kenya–Uganda–US team and embedded TasP within a more horizontal/integrated multidisease, patient-centered care model. The goals were to reduce HIV incidence, improve linkage to care, and improve overall community health compared with the usual care model of more vertical/siloed health programming and restrictive ART usage at the time of the study, based on CD4+ count thresholds.23 Intervention communities received the following: community health fairs at baseline and annually where HIV and noncommunicable disease (NCD) screening was conducted, home-based testing for those who did not want to be tested at fairs, immediate ART start upon HIV diagnosis, and a patient-centered chronic care model for HIV and NCD care, clinics with trained and sensitized providers and flexible operating hours, and mobile phone triage and appointment reminders. Control communities received the following: health fair for testing and diagnosis at baseline only, home-based testing upon request, ART initiation based on national guidelines (which expanded over time), and access to the national standard-of-care for HIV and NCDs.26
The SEARCH intervention communities experienced a significant decline in mortality among both PLHIV and HIV-uninfected individuals, as well as substantial improvements in hypertension control (a key NCD target) relative to the control communities. Viral suppression among HIV+ individuals in the intervention communities also significantly improved from baseline (42%) to year 3 measurements (79.7%; RR: 1.17, 95% CI: 1.11 to 1.22, P < 0.001) relative to the control communities (42% at baseline and 68.4% at year 3).37 After 2 years of follow-up, there were some disparities in the intervention communities in viral suppression based on age and sex; 76.2% of males compared with 82.2% of females (difference, 6.0%; 95% CI: 4.3 to 7.7) and 64.5% of youth aged 15–24 years compared with 81.5% of adults older than 24 years (difference, 17.0%; 95% CI: 13.6 to 20.4) had achieved viral suppression.20 Despite the positive outcomes measured in SEARCH intervention communities, there was no significant difference in the 3-year cumulative HIV incidence rate between the intervention (0.77%) and control (0.81%) communities (RR = 0.95, 95% CI: 0.77 to 1.17, P = 0.60).
This study is harder to interpret than the Africa Centre TasP study; we believe, since with improved viral suppression, theory suggests that an incidence reduction should follow. There are several hypotheses to consider as to why no reduction in HIV incidence was achieved in SEARCH. One hypothesis might suggest that the control communities also received some elements of the intervention package through the baseline health fairs, which could have increased health-seeking behaviors, including testing and ART initiation, after the baseline. However, the different levels of viral suppression between the arms at the end of the study argue against this interpretation. A second hypothesis is that ART eligibility guidelines shifted over the course of the study, with ART eligibility becoming “near universal” within a year. The mathematical model developed by the SEARCH team predicted a 10% reduction in HIV incidence, which may not have been detectable in context of possible increased health-seeking and wider ART eligibility. This classic dilemma of the control group benefiting from their participation in the study, resembling the intervention group, and leading to bias toward the null and reduction in planned statistical power is a hazard of multiyear prevention clinical trials.38,39 Another potential explanation is that the new infections in the intervention group may have arisen from individuals never exposed to the full intervention, including mobile populations (who also tend to be younger),40 youth, individuals during the early stages of new infection and with a high level of infectiousness, and/or a subset of individuals with unsuppressed viral loads, believed to be particularly salient for communities in Kenya.41 Newly infected individuals may not yet have had an opportunity to be diagnosed, may have tested negative on an antibody test (despite having been recently infected), and/or may be those involved with higher numbers of concurrently partnerships. If a high proportion of all new infections arise in these situations, it may be that an annual health fair approach may not reach enough persons to slow transmission. One must also consider random imbalances in the control compared with intervention clusters that may result in bias toward the null,42 although SEARCH had 32 clusters randomized into just 2 groups.
The BCPP: The Ya Tsie Study
The Ya Tsie study in Botswana used a pair-matched community-randomized trial in 30 communities, with 15 communities in the intervention arm and 15 in standard of care. Intervention communities received the following package: community mobilization to encourage HIV testing and counseling and male circumcision, home-based and other mobile HIV testing campaigns, linkage to care support, scaled-up linkage to male circumcision services, and expanded ART at government clinics to cover HIV+ individuals with either CD4 counts of >350–500 cells/μL, or CD4+>500 cells/μL and HIV-1 RNA ≥10,000 copies/mL. The standard of care communities received ART according to national guidelines at the time of the study, that is, CD4+ ≤350, WHO III/IV disease, or pregnancy. In June 2016, midway through the Ya Tsie study period, the Ministry of Health of Botswana began offering universal ART, which was implemented in all intervention and control communities.28 Given the shifting guidelines and the period over which the study was conducted, the rates of ART initiation were very similar between the intervention and control sites throughout the study, reminiscent of arm B of the HPTN 071 (PopART) study described below.
The Ya Tsie study found a significant 30% reduction in HIV incidence in the intervention communities compared with the control communities. An important difference between the other TasP trials is Ya Tsie's focus on combination prevention (eg, male circumcision in addition to TasP). It will be important for the investigators to clarify how they handled loss to follow-up and death in their analysis. Bias away from the null could have occurred if those infected disproportionately were sicker, died, or were lost to follow-up in the intervention group, perhaps because of stigma and heightened surveillance. There were also difficulties in fully enumerating all inhabited households, such that 20% of age- and residency-eligible members of enumerated households were not enrolled. The most common reasons for nonparticipation were absenteeism and refusal, and it is possible that people not found or not consenting in a household might differ from enrolled participants.28 Similarly, just as random imbalances of characteristics at baseline could have led to a null result in SEARCH, random imbalances at baseline could have resulted in a positive result in Ya Tsie, for example, if the intervention group included participants with higher baseline viral suppression, circumcision, and/or ART coverage. Post hoc multivariable adjustment can be used to try to confirm or refute this speculation. The Ya Tsie study finding was remarkable since Botswana programs had come close to achieving 90-90-90 goals even at study baseline, reducing power to detect an intervention effect.29 Nevertheless, the findings from Botswana suggest that it is possible to increase ART coverage and reduce HIV incidence in a high (29%) prevalence context, even with high baseline ART coverage, providing important lessons for other countries in sub-Saharan Africa.
We speculate that increased Ya Tsie study coverage might have managed to reach persons who were marginalized within the previous health strategies such as men or youth but were brought into care with the Ya Tsie intervention, although it is not yet known whether this was, in fact, the case. Key factors contributing to Botswana's overall extraordinary programmatic successes have been summarized elsewhere43 and include rapid point-of-care testing, free and decentralized access to ART, and routine access to viral load monitoring. It will be most helpful to understand more fully the cost and cost effectiveness of the BCPP/Ya Tsie intervention package, the relative effectiveness of specific intervention components (possibly varying within subgroups), and the feasibility for successful and cost-effective intervention components to be delivered at scale within and outside Botswana.
The HPTN 071 (PopART) Study
The HPTN 071 (PopART) study was a 3-arm cluster-randomized trial with 21 communities in South Africa and Zambia (Fig. 1), with a primary outcome of HIV incidence between 12 and 36 months.44 Arm A provided the full PopART intervention package, which included ART initiation irrespective of CD4+ cell count at a local health center, annual rounds of home-based HIV testing by community HIV-care providers (CHiPs), active referral and/or retention in care support by CHiPs, and referrals/enhanced linkage to male circumcision, PMTCT, adolescent and male engagement, use of community engagement boards and community dialogue processes, and screening/treatment of tuberculosis and sexually transmitted infections (STIs). Although CHiPs home-based visits sought to offer testing to persons who did not know their current HIV status, the linking and treatment elements targeted all PLHIV whether newly diagnosed, in care but not receiving ART, or seropositive, but out of care. Arm B provided this same intervention package, except that ART initiation was based on national guidelines (initially, ART if CD4 count <350 cells/μL), which changed to universal ART midway through the study, at which time arm B was identical to arm A. Arm C served as the control, providing standard of care including ART initiation consistent with national guidelines and treatment support from the respective Ministries of Health aided by resources from the US President's Emergency Response for AIDS Relief (PEPFAR) and others. Interim findings from this study contributed insights on using enhanced quality assurance measures to overcome challenges related to poor sensitivity of community-based point-of-care testing,32 successfully scaling up testing to reach the first 90,33,34 and efforts to achieve the second 90 (linkage to care).31,33,45 The final findings revealed that, surprisingly, there was no difference between the A and C arms, but there was a significant 30% decrease in new HIV infections between the B and C arms.35 The HPTN 071 (PopART) trial included 48,301 participants in the population cohort used to estimate incidence rates. Baseline HIV prevalence was 21%–22% across study arms, and from months 12–36 of the study, 553 new HIV infections within 39,702 person-years (py) were observed (incidence 1.4/100 py), about twice as high in women than men.35 The adjusted rate ratio for arm A vs. C was 0.93 (95% CI: 0.74 to 1.18, P = 0.51) and arm B vs. C was 0.70 (95% CI: 0.55 to 0.88, P = 0.006). Viral suppression at 24 months was 72.1% in arm A, compared to 67.9% in arm B, and 62.5% in arm C. ART coverage at 36 months did not differ in arm A (81%) vs. arm B (80%).35
FIGURE 1.
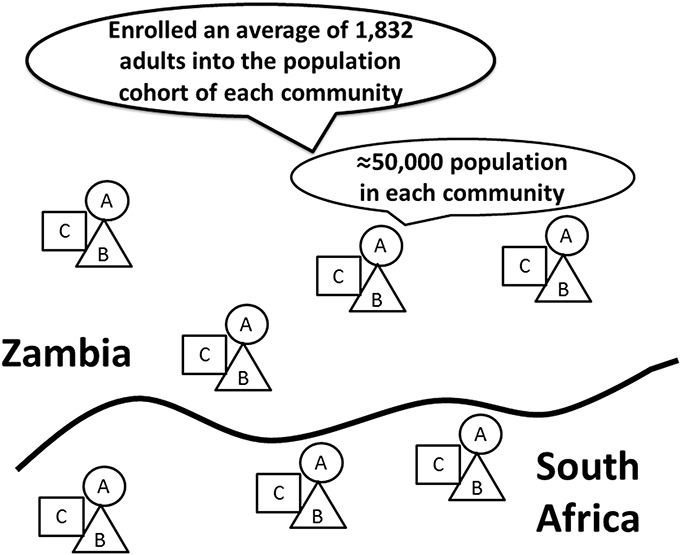
Schematic outline of the cluster-randomization approach used in HPTN 071 (PopART). Three communities with similar characteristics (eg, HIV prevalence; urban, periurban, or rural) were then randomized into study arms A, B, or C. Four clusters of 3 communities in Zambia and 3 clusters of 3 communities in South Africa were randomized. Within each community, an estimated 15,000–30,000 people resided from whom an average of 1832 persons were enrolled into the population cohort from which incidence was measured. Figure adapted from Refs. 44, 81.
The HPTN 071 (PopART) results were surprising and will require significant ongoing work to better understand. Given the similarity between the interventions delivered in arms A and B, it is possible that the differences between these 2 arms were a chance finding, and that their combined difference with arm C (approximately a 20% reduction in incidence) reflects the key, albeit post hoc, finding of the trial. This effect was significant, but smaller than hypothesized.35 In addition, it is possible that there was imbalance after randomization of the communities assigned to each arm sufficiently different to confound the results. For example, 38% of arm B participants living with HIV were already on ART at baseline, while only 31%–32% of those in the other 2 arms were. Multivariate adjusted analysis can address this explanation. It is also possible that the immediate implementation of the full intervention in arm A overburdened the already stretched health care infrastructure, compromising the quality and continuity of care. The more gradual roll-out of the intervention in arm B may have allowed clinics and staff more time to adapt and may have results in improved quality and retention; while other aspects of the delivery of the home-based intervention may have more successful in arm B early in the trial than in arm A. Although there were challenges in reaching some key groups, particularly young adults,33 the distribution of young people was similar across the 3 arms. Early indications are that while migration might reduce 90-90-90 coverage, differential migration across study arms was not apparent. As we speculate might have occurred in the SEARCH trial, the “last unreached 25%” may be those most responsible for a disproportionately high number of transmissions, preventing adequate coverage to enable the levels of reduction in population viral load that were hoped for. Perhaps the 3-year study duration was not long enough to fully observe the effect of the intervention, given the low linkage-to-care rates in the first half of the study. It will take careful secondary data analyses to determine the most likely explanations for the findings across all 3 arms of the study.
MODELS OF THE DYNAMICS OF HIV TRANSMISSION AND TasP
STIs in general, including HIV, are not spread homogenously in communities.1,46–52 Persons with many and/or concurrent sexual partners contribute disproportionately to transmission.40,53–55 Currently, mathematical models adopt a variety of ways to represent such individuals within models, but this poses several challenges, especially in sub-Saharan Africa where, for example, periods of risk vary across the life course, and definitions of sex work are complex. The interactions of high-risk subgroups and coverage of HIV testing, linking to ART-based care, and viral suppression must be examined further, since access to or utilization of HIV testing and treatment services may differ for these subgroups. Even if 90-90-90 metrics were achieved, fully 27% of individuals would not be virally suppressed (if 90% were tested, 90% were on ART, and 90% were virally suppressed, multiplicative). It is plausible, therefore, that this hardest-to-reach minority is more influential in epidemic dynamics and in achieving the population impact of TasP that might have been predicted.56 Thus, models that suggested that TasP would reduce community viral transmission may have been overly optimistic.56 New models that can better take into account heterogeneity of risk and ART coverage may be needed to guide predictions of outcomes for various approaches to HIV prevention.
A second potential challenge to HIV transmission modeling is that we do not know the time that interventions take to make an imprint on community spread of HIV. Models assuming impact in just 2–4 years may have underestimated the time that it takes to actually deploy new field logistics and to achieve desired coverage since low- and middle-income countries have few examples of having actually achieved 90-90-90 coverage. Although we have no data to back up our speculation, it is possible that studies may have ended just as their impact might be the greatest and most measurable. It will be helpful if completed studies estimate and report effect modification by time since randomization.
A third concern about the existing models is their inattention to and/or inability to estimate in- and out-migration. When a substantial proportion of individuals leaves or enters a study zone disproportionately in one arm versus another, there can be a dramatic dilution of the intervention/treatment effect.
90-90-90 IMPLEMENTATION STUDIES
In addition to the large-scale clinical trials of TasP described above, there have been an increasing number of studies examining the process of implementation of the 90-90-90 goals.57–61 Such studies are an anchor of HIV implementation science efforts, and we highlight 3 of the larger such studies—MaxART62 in Swaziland, Project Shikamana63 in Tanzania, and the SAPPH-IRe intervention in Zimbabwe.64
MaxART was a stepped-wedge randomized trial across 14 public-sector clinical sites in Swaziland's HhoHho Region, which aimed to assess the impact of TasP compared with standard of care on patient retention and viral suppression. This study examined impact of TasP on those who initiated ART early themselves, rather than on HIV incidence among others. Sites transitioned 2 at a time from the standard of care to the intervention, which was early access to ART. This study employed mixed methods and was designed to determine the feasibility, acceptability, clinical outcomes, affordability, and scalability of offering early ART to all HIV+ individuals in a public-sector health system. Early qualitative findings provided further context for challenges associated with treatment retention for mobile populations,65 as well as the challenges associated with increasing numbers of patients and task-shifting in public clinics.66 Results of MaxART found that TasP modestly improved retention, improved client perceptions of care quality, and increased retention and viral suppression rates combined fivefold, thus establishing that rather than being harmful to patients initiated on ART early, this strategy is strongly beneficial to them.67 Final results from MaxART are expected to be published later in 2019.
Project Shikamana was a phase 2 community-randomized trial of a community-based model of combination HIV prevention to improve TasP among high-risk female sex workers (FSWs) in the Iringa region of Tanzania.63 Participants were recruited from entertainment venues, and time-location sampling was used to enroll a cohort of 203 HIV+ and 293 HIV− women. Time-location sampling entailed identifying times when the target population gathered at venues, constructing sampling frames of venues and daytime units, randomly selecting and visiting venues and daytime units, and systematically collecting information from eligible women. The intervention package included community-led peer education, condom distribution, and HIV testing in accessible entertainment venues; peer navigation to facilitate ART uptake and retention in care; sensitivity training for HIV clinical providers; SMS to promote awareness, solidarity, and adherence; and a community-led center with activities to promote social cohesion and community mobilization to address challenges specific to FSWs. Pre-exposure prophylaxis (PrEP) was not part of the intervention. The study assessed feasibility and acceptance of the package as well as preliminary effectiveness. Baseline data are available.63,68 Results presented at conferences suggest that intervention participants were significantly less likely to become infected with HIV at follow-up, had lower HIV incidence, reported more consistent condom use, and experienced less gender-based violence compared with participants in the control group.69,70 Final results are forthcoming.71
The SAPPH-IRe (Sisters Antiretroviral Prevention Programme—an Integrated Response) trial was a pair-matched, parallel, cluster-randomized study nested within the Sisters with a Voice national sex work program in Zimbabwe.64 The primary outcome was the proportion of FSWs with HIV viral loads of ≥1000 copies/mL. An additional 9 secondary outcomes related to aspects of treatment and prevention intended to be affected by the intervention were assessed. In clusters receiving the standard-of-care, the Sisters program provided FSWs with free condoms and contraception, free HIV testing and counseling, STI treatment, health education, community mobilization, and legal advice. These services were provided at drop-in centers in primary care clinics and supported by peer educators. Women testing HIV-positive were referred to government clinical services. In the intervention clusters, an enhanced version of the Sisters program was implemented. This enhanced version provided additional community mobilization activities aimed at raising awareness of the benefits of ART and PrEP, strengthened support networks to encourage health-promoting behaviors and developing leadership skills. In addition, ART and PrEP users were encouraged to join a community-based Adherence Sisters program, which allowed them to nominate a trusted “sister” to serve as their adherence supporter and to attend Adherence Sisters training together. The program also included activities to encourage HIV testing every 6 months among HIV− women, including mobile phone messaging reminders. Clinical services were also improved to enable initiation of ART or PrEP on site, in compliance with local and international guidelines. Clinical and social support services were delivered by clinical staff, with text messages and follow-up phone calls used to support clinic attendance. A representative sample for outcome surveys was sought through respondent-driven sampling.
Between study baseline and the end of the assessment period, the proportions of women with viral loads of ≥1000 copies/mL dropped in both the control (35.1% reduction) and intervention (45.6% reduction) clusters. However, the weighted percentage risk difference suggested little difference between the groups. Among the secondary outcomes, the proportions of HIV+ women who reported being aware of their status, taking ART, and being virally suppressed all increased, but to similar degrees between control and intervention groups. The intervention did strengthen engagement of FSWs with services but did not lower overall viral loads in the intervention group relative to the control. Study authors believed their results supported a conclusion that FSWs, if supported, will access services in the public sector. They also identified improvements that could be made in testing and ART uptake. Since the Sisters approach did not specifically seek to identify highly vulnerable sex workers, they may not have reached these individuals, reducing the impact of the intervention.
CONCLUSIONS
Large scale prevention research to assess the impact of TasP at a community scale is complex. For example, each of the major TasP trials contended with treatment guideline expansion outside the intervention arm, which reduced the power of all the studies. Given a lack of highly consistent findings, variation in the fidelity of the interventions could be at play, with challenges in rigorous adherence to protocols over time, process outcomes, data quality, and other critical “on the ground” details. Indeed, in large scale observational studies outside the trial setting, HIV treatment guideline expansion has been shown to increase the timely uptake of ART, especially those expansions from CD4 <350 cells/μL to 500 cells/μL,72 and from 500 cells/μL to universal treatment.73 In one large study of patients enrolling in HIV care from 6 sub-Saharan African countries, 82% of patients initiated ART within 30 days.73 That the TasP/ANRS trial in KwaZulu-Natal had such low linkage-to-care rates was unfortunate given the high background incidence rates and ability to assess the impact of TasP in a large community setting. The suboptimally low median CD4+ cell counts at ART initiation and a slowly improving trend74,75 suggest a critical need for identifying and scaling implementation strategies aimed at earlier diagnosis and more effective linkage to care of those found to be HIV-positive. Given that national HIV treatment guidelines have expanded to universal treatment in nearly every country in the world,76 and that early data from a multicountry study in sub-Saharan Africa suggest >80% of those linked to care in SSA initiate ART rapidly,73 it is important that the TasP trials be carefully evaluated in more detail, with continued follow-up to garner lessons learned around how best to implement and scale testing and linkage strategies that are successful at promoting timely achievement of HIV care continuum outcomes. Indeed, others have called for a focus on identifying strategies for earlier diagnosis and linkage for key and underserved populations in the sub-Saharan African region,76–78 as well as a need for new metrics to help focus our implementation efforts on these critical prerequisites to maintain continued impact on HIV incidence and mortality and, ultimately, end the epidemic (ie, for those with newly diagnosed HIV, 90% diagnosed with CD4 >500 cells/μL, 90% enrolling in care with CD4 >500 cells/μL, and 90% initiating ART with CD4 >500 cells/μL).75
It is puzzling that the findings from the large TasP trials been inconsistent. TasP holds much promise for reducing HIV incidence, as the Ya Tsie study and the arm B vs. arm C (or post hoc combined arms A + B to arm C) comparisons in HPTN 071 (PopART) suggest. Yet, many questions remain as to what is needed to achieve goals for global reduction in HIV incidence, and end the HIV epidemic. Modeling has suggested the need to achieve the 90-90-90 seconds to sufficiently reduce HIV incidence, but these models have made assumptions about population homogeneity that may not hold up in real-world contexts. The HPTN 052 study may, therefore, overestimate the potential population impact of TasP given real-world field logistics and inability to reach the highest behavioral risk subset of PLHIV.
The large-scale clinical trials of TasP suggest the need for additional data on implementation factors and process outcomes at the individual, community, and facility levels that could have reduced testing uptake, linkage and retention in care, and adherence to ART. Anthropologists, sociologists, psychologists, and health systems quality improvement experts must be engaged to learn more about why individuals with undiagnosed HIV infection do not get diagnosed sooner, linked to care earlier, and/or become virally suppressed. Analogous questions arise in the use of antiretroviral oral or topical PrEP. Social-behavioral data, qualitative assessments, and health systems data are needed to fully understand gaps and more effectively tailor interventions to direct individuals into appropriate treatment or prevention modalities.79,80 Although the evidence for TasP indicates some promise, there is considerable need for refinement and targeting of strategies that ensure implementation and achievement of TasP and the 90-90-90 goals.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Sarah Fidler's helpful review and comments on a draft of this manuscript.
Footnotes
Supported by National Institutes of Health grants K12HS023000 (MAB), R01AI112339 (D.S. and S.H.V.), P30MH062294 (M.A.B., D.S., and S.H.V.), UM1AI068619 (J.H. and S.H.V.), U01AI096299 (D.N.), P30MH043520 (D.N.), and P30AI124414 (D.N.). This publication resulted in part from research supported by the Penn Center for AIDS Research (CFAR) (P30 AI 045008-Ronald Collman, PI), the Penn Mental Health AIDS Research Center (PMHARC) (P30 MH 097488-Dwight Evans, PI) and the CFAR Social & Behavioral Science Research Network National Scientific Meeting (SBSRN) (R13 HD 074468-Michael Blank, PI).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Granich R, Williams B, Montaner J, et al. 90-90-90 and ending AIDS: necessary and feasible. Lancet. 2017;390:341–343. [DOI] [PubMed] [Google Scholar]
- 2.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009;301:2380–2382. [DOI] [PubMed] [Google Scholar]
- 3.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. [DOI] [PubMed] [Google Scholar]
- 5.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernazza PL, Kashuba AD, Cohen MS. Biological correlates of sexual transmission of HIV practical consequences and potential targets for public health: practical consequences and potential targets for public health. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2002;45:277–285. [DOI] [PubMed] [Google Scholar]
- 7.Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000;14:117–121. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Miller WC. Sexually transmitted diseases and human immunodeficiency virus infection: cause, effect, or both? Int J Infect Dis. 1998;3:1–4. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaner JS, Lima VD, Harrigan PR, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” experience in a Canadian setting. PLoS One. 2014;9:e87872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanser F, Barnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachega JB, Uthman OA, Mills EJ, et al. Adherence to antiretroviral therapy for the success of emerging interventions to prevent HIV transmission: a wake up call. J AIDS Clin Res. 2013;2012(suppl 4):pii: 007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forhan SE, Modi S, Houston JC, et al. Moving toward test and start: learning from the experience of universal antiretroviral therapy programs for HIV-infected pregnant/breastfeeding women. AIDS. 2017;31:1489–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perriat D, Balzer L, Hayes R, et al. Comparative assessment of five trials of universal HIV testing and treatment in sub-Saharan Africa. J Int AIDS Soc. 2018;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plazy M, Farouki KE, Iwuji C, et al. Access to HIV care in the context of universal test and treat: challenges within the ANRS 12249 TasP cluster-randomized trial in rural South Africa. J Int AIDS Soc. 2016;19:20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwuji CC, Orne-Gliemann J, Tanser F, et al. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013;14:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larmarange J, Diallo MH, McGrath N, et al. The impact of population dynamics on the population HIV care cascade: results from the ANRS 12249 Treatment as Prevention trial in rural KwaZulu-Natal (South Africa). J Int AIDS Soc. 2018;21(suppl 4):e25128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Uptake of home-based HIV testing, linkage to care, and community attitudes about ART in rural KwaZulu-Natal, South Africa: descriptive results from the first phase of the ANRS 12249 TasP cluster-randomised trial. PLoS Med. 2016;13:e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen M, Balzer L, Kwarsiima D, et al. Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in East Africa. JAMA. 2017;317:2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowski A, Snyman K, Kwarisiima D, et al. High CD4 counts associated with better economic outcomes for HIV-positive adults and their HIV-negative household members in the SEARCH Trial. PLoS One. 2018;13:e0198912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown LB, Havlir DV, Ayieko J, et al. High levels of retention in care with streamlined care and universal test and treat in East Africa. AIDS. 2016;30:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamie G, Clark TD, Kabami J, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV. 2016;3:e111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang W, Chamie G, Mwai D, et al. Implementation and operational research: cost and efficiency of a hybrid mobile multidisease testing approach with high HIV testing coverage in East Africa. J Acquir Immune Defic Syndr. 2016;73:e39–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown LB, Getahun M, Ayieko J, et al. Factors predictive of successful retention in care among HIV-infected men in a universal test-and-treat setting in Uganda and Kenya: a mixed methods analysis. PLoS One. 2019;14:e0210126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwarisiima D, Kamya MR, Owaraganise A, et al. High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya. J Int AIDS Soc. 2017;20(suppl 4):21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Havlir DV, Balzer LB, Charlebois ED, et al. HIV testing and treatment with the use of a community health approach in rural Africa. N Engl J Med. 2019;381:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaolathe T, Wirth KE, Holme MP, et al. Botswana's progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV. 2016;3:e221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyo S, Gaseitsiwe S, Mohammed T, et al. Cross-sectional estimates revealed high HIV incidence in Botswana rural communities in the era of successful ART scale-up in 2013–2015. PLoS One. 2018;13:e0204840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med. 2019;381:230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabapathy K, Mubekapi-Musadaidzwa C, Mulubwa C, et al. Predictors of timely linkage-to-ART within universal test and treat in the HPTN 071 (PopART) trial in Zambia and South Africa: findings from a nested case-control study. J Int AIDS Soc. 2017;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bock P, Phiri C, Piwowar-Manning E, et al. Understanding low sensitivity of community-based HIV rapid testing: experiences from the HPTN 071 (PopART) trial in Zambia and South Africa. J Int AIDS Soc. 2017;20(suppl 6):21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes R, Floyd S, Schaap A, et al. A universal testing and treatment intervention to improve HIV control: one-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med. 2017;14:e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanaube K, Schaap A, Floyd S, et al. What works—reaching universal HIV testing: lessons from HPTN 071 (PopART) trial in Zambia. AIDS. 2017;31:1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes R, Donnell D, Floyd S, et al. Impact of a universal testing and treatment intervention on HIV incidence in Zambia and South Africa: results of the HPTN 071 (PopART) community randomized trial. N Engl J Med. 2019;381:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larmarange J, Diallo MH, Iwuji CC, et al. Cascade of care of HIV seroconverters in the context of universal “test and treat”. Conference on Retroviruses and Opportunistic Infections (CROI), February 14, 2017, Seattle, WA.
- 37.Havlir D, Charlebois E, Balzer L, et al. SEARCH community cluster randomized study of HIV “test and treat” using multi-disease approach and streamlined care in rural Uganda and Kenya. Paper presented at: Journal of the International AIDS Society July 23–27, 2018.
- 38.Atienza AA, King AC. Community-based health intervention trials: an overview of methodological issues. Epidemiol Rev. 2002;24:72–79. [DOI] [PubMed] [Google Scholar]
- 39.Colditz GA, Taylor PR. Prevention trials: their place in how we understand the value of prevention strategies. Annu Rev Public Health. 2010;31:105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camlin CS, Akullian A, Neilands TB, et al. Population mobility associated with higher risk sexual behaviour in eastern African communities participating in a Universal Testing and Treatment trial. J Int AIDS Soc. 2018;21(suppl 4):e25115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain V, Petersen ML, Liegler T, et al. Population levels and geographical distribution of HIV RNA in rural Ugandan and Kenyan communities, including serodiscordant couples: a cross-sectional analysis. Lancet HIV. 2017;4:e122–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes RJ, Moulton LH. Cluster Randomised Trials. Boca Raton, FL: Chapman and Hall/CRC; 2017. [Google Scholar]
- 43.Marukutira T, Stoove M, Lockman S, et al. A tale of two countries: progress towards UNAIDS 90-90-90 targets in Botswana and Australia. J Int AIDS Soc. 2018;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes R, Ayles H, Beyers N, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials. 2014;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwuji C, Newell ML. Towards control of the global HIV epidemic: addressing the middle-90 challenge in the UNAIDS 90-90-90 target. PLoS Med. 2017;14:e1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galvani AP, Pandey A, Fitzpatrick MC, et al. Defining control of HIV epidemics. Lancet HIV. 2018;5:e667–e670. [DOI] [PubMed] [Google Scholar]
- 47.Okano JT, Robbins D, Palk L, et al. Testing the hypothesis that treatment can eliminate HIV: a nationwide, population-based study of the Danish HIV epidemic in men who have sex with men. Lancet Infect Dis. 2016;16:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okano JT, Gerstoft J, Obel N, et al. HIV elimination and population viral load. Lancet HIV. 2016;3:e507–e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delva W, Eaton JW, Meng F, et al. HIV treatment as prevention: optimising the impact of expanded HIV treatment programmes. PLoS Med. 2012;9:e1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eaton JW, Bacaër N, Bershteyn A, et al. Assessment of epidemic projections using recent HIV survey data in South Africa: a validation analysis of ten mathematical models of HIV epidemiology in the antiretroviral therapy era. Lancet Glob Health. 2015;3:e598–e608. [DOI] [PubMed] [Google Scholar]
- 51.Lou J, Hu P, Qian HZ, et al. Expanded antiretroviral treatment, sexual networks, and condom use: treatment as prevention unlikely to succeed without partner reduction among men who have sex with men in China. PLoS One. 2017;12:e0171295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lou J, Blevins M, Ruan Y, et al. Modeling the impact on HIV incidence of combination prevention strategies among men who have sex with men in Beijing, China. PLoS One. 2014;9:e90985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaefer R, Gregson S, Eaton JW, et al. Age-disparate relationships and HIV incidence in adolescent girls and young women: evidence from Zimbabwe. AIDS. 2017;31:1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hakim A, Patnaik P, Telly N, et al. High prevalence of concurrent male-male partnerships in the context of low human immunodeficiency virus testing among men who have sex with men in Bamako, Mali. Sex Transm Dis. 2017;44:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamanis TJ, Fisher JC, Moody JW, et al. Young men's social network characteristics and associations with sexual partnership concurrency in Tanzania. AIDS Behav. 2016;20:1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cori A, Ayles H, Beyers N, et al. HPTN 071 (PopART): a cluster-randomized trial of the population impact of an HIV combination prevention intervention including universal testing and treatment: mathematical model. PLoS One. 2014;9:e84511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowman AS, Mehta M, Lerebours Nadal L, et al. Strengthening the HIV care continuum in the Dominican republic: application of a triadic implementation framework to meet the UNAIDS 90-90-90 treatment goal. AIDS Patient Care STDs. 2017;31:407–412. [DOI] [PubMed] [Google Scholar]
- 58.Wechsberg WM, Ndirangu JW, Speizer IS, et al. An implementation science protocol of the Women's Health CoOp in healthcare settings in Cape Town, South Africa: a stepped-wedge design. BMC Womens Health. 2017;17:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dorward J, Garrett N, Quame-Amaglo J, et al. Protocol for a randomised controlled implementation trial of point-of-care viral load testing and task shifting: the Simplifying HIV TREAtment and Monitoring (STREAM) study. BMJ Open. 2017;7:e017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gamble T, Branson B, Donnell D, et al. Design of the HPTN 065 (TLC-Plus) study: a study to evaluate the feasibility of an enhanced test, link-to-care, plus treat approach for HIV prevention in the United States. Clin Trials. 2017;14:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia Z, Mao Y, Zhang F, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003-11): a national observational cohort study. Lancet. 2013;382:1195–1203. [DOI] [PubMed] [Google Scholar]
- 62.Walsh FJ, Barnighausen T, Delva W, et al. Impact of early initiation versus national standard of care of antiretroviral therapy in Swaziland's public sector health system: study protocol for a stepped-wedge randomized trial. Trials. 2017;18:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerrigan D, Mbwambo J, Likindikoki S, et al. Project Shikamana: baseline findings from a community empowerment–based combination HIV prevention trial among female sex workers in Iringa, Tanzania. J Acquir Immune Defic Syndr. 2017;74:S60–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cowan FM, Davey C, Fearon E, et al. Targeted combination prevention to support female sex workers in Zimbabwe accessing and adhering to antiretrovirals for treatment and prevention of HIV (SAPPH-IRe): a cluster-randomised trial. Lancet HIV. 2018;5:e417–e426. [DOI] [PubMed] [Google Scholar]
- 65.Shabalala FS, Vernooij E, Pell C, et al. Understanding reasons for discontinued antiretroviral treatment among clients in test and treat: a qualitative study in Swaziland. J Int AIDS Soc. 2018;21:e25120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dlamini-Simelane T, Moyer E. Task shifting or shifting care practices? The impact of task shifting on patients' experiences and health care arrangements in Swaziland. BMC Health Serv Res. 2017;17:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan S, Spiegelman D, Walsh F, et al. Universal test and treat (UTT) versus standard of care for access to antiretroviral therapy in HIV clients: the MaxART stepped-wedge randomized controlled health systems trial in Swaziland. Paper presented at: The International AIDS Society 2018; Amsterdam, Netherlands.
- 68.Hendrickson ZM, Leddy AM, Galai N, et al. Work-related mobility and experiences of gender-based violence among female sex workers in Iringa, Tanzania: a cross-sectional analysis of baseline data from Project Shikamana. BMJ Open. 2018;8:e022621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerrigan DL, Mbwambo JK, Likindikoki S, et al. Let's Stick Together: community empowerment approach significantly impacts multiple HIV and sexual and reproductive health outcomes among female sex workers in Tanzania. Oral Presentation presented at 22nd International AIDS Conference; July 23–27, 2018; Amsterdam, Netherlands.
- 70.Kerrigan DL, Mbwambo JK, Likindikoki S, et al. Shikamana Intervention Significantly Reduces HIV Incidence Among FSW in Tanzania. Poster Presentation presented at CROI; March 5, 2019; Seattle, WA.
- 71.Kerrigan DL, Mbwambo JK, Likindikoki S, et al. Project Shikamana: a community empowerment model of combination prevention significantly impacts HIV incidence and care continuum outcomes among female sex workers in Iringa, Tanzania. J Acquir Immune Defic Syndr [in press]. [DOI] [PMC free article] [PubMed]
- 72.Tymejczyk O, Brazier E, Yiannoutsos C, et al. HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: a metaregression analysis of programmatic data from 22 countries. PLoS Med. 2018;15:e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tymejczyk O, Brazier B, Yiannoutsos CT, et al. Rapid HIV treatment initiation improves with Treat All adoption in six sub-Saharan African countries: regression discontinuity analysis. Conference on Retroviruses and Opportunistic Infections (CROI); March 5, 2019; Seattle, WA. Abstract No: 1016.
- 74.IeDea, Collaborations CC. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis. 2018;66:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nash D, Robertson M. How to evolve the response to the global HIV epidemic with metrics and targets based on pre-treatment CD4 counts. Curr HIV/AIDS Rep. 2019. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nash D, Yotebieng M, Sohn AH. Treating all people with HIV infection in sub-Saharan Africa: a new era calling for new approaches. J Viral Erad. 2018;4(suppl 2):1–4. [PMC free article] [PubMed] [Google Scholar]
- 77.Yotebieng M, Brazier E, Addison D, et al. Research priorities to inform “Treat All” policy implementation for people living with HIV in sub-Saharan Africa: a Consensus Statement from the International Epidemiology Databases to Evaluate AIDS (IeDEA). J Int AIDS Soc. 2019;22:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hensen B, Taoka S, Lewis JJ, et al. Systematic review of strategies to increase men's HIV-testing in sub-Saharan Africa. AIDS. 2014;28:2133–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Camlin CS, Seeley J. Qualitative research on community experiences in large HIV research trials: what have we learned? J Int AIDS Soc. 2018;21(suppl 7):e25173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camlin CS, Seeley J, Viljoen L, et al. Strengthening universal HIV “test-and-treat” approaches with social science research. AIDS. 2016;30:969–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vermund SH, Fidler SJ, Ayles H, et al. Can combination prevention strategies reduce HIV transmission in generalized epidemic settings in Africa? The HPTN 071 (PopART) study plan in South Africa and Zambia. J Acquir Immune Defic Syndr. 2013;63(suppl 2):S221–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]


