Abstract
Background
Population modeling and simulations can be used to facilitate the conduct of Phase I studies to develop safe and effective dosing regimens in neonates.
Setting
P1110 is an international, multicenter trial to determine safe and effective raltegravir doses in neonates at risk for HIV infection.
Methods
P1110 used a two-cohort adaptive design incorporating population pharmacokinetic modeling and simulations. An initial cohort of neonates received two single oral doses of raltegravir with standard of care therapy for prevention of perinatal transmission – one within 48 hours of birth and a second at 7–10 days of life. Raltegravir concentration data following administration of these doses were combined with data from a previous study of infants age 4 weeks to 2 years. The combined data base was used for population pharmacokinetic modeling and simulations to select a daily dosing regimen for investigation in a second cohort of neonates.
Results
Raltegravir concentration data from 6 neonates were combined with data from infants age 4 weeks to 2 years receiving raltegravir twice daily. The combined data set allowed successful development of a population pharmacokinetic model with reasonable precision of parameter estimates. Monte Carlo simulations were run to evaluate potential daily dosing regimens from birth to age 6 weeks, allowing selection of a regimen to be evaluated in a subsequent cohort of neonates receiving chronic raltegravir dosing.
Conclusions
An adaptive design incorporating population pharmacokinetic modeling and simulations were used to select a developmentally appropriate neonatal raltegravir dosing regimen in the first 6 weeks of life.
Keywords: raltegravir, neonate, pharmacokinetic modeling, HIV, dose determination
Introduction
Limited data exist to provide dosing recommendations for combination antiretroviral regimens to prevent or treat HIV infection in neonates. The Department of Health and Human Services Perinatal Guidelines recommend the administration of a three-drug antiretroviral regimen for empiric treatment of newborns at highest risk of HIV acquisition and for treatment of neonates with documented HIV infection.1 However, sufficient neonatal pharmacokinetic and safety data are available to allow use in neonates of only a few antiretroviral agents - zidovudine, lamivudine and nevirapine after birth plus lopinavir/ritonavir after 2 weeks of age.1,2
Raltegravir is a potent and selective HIV-1 integrase inhibitor with potential for use for early intensive treatment of infants with HIV infection.2 The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1097 protocol demonstrated that raltegravir readily crosses the placenta and that elimination of trans-placentally acquired raltegravir in infants whose mothers received raltegravir during pregnancy is highly variable and prolonged.3 Raltegravir is metabolized by uridine diphosphate glucuronosyltransferase (UGT) 1A1, whose activity is known to be extremely low immediately after birth followed by a dramatic increase over the first weeks of life.4,5 Both raltegravir and bilirubin are metabolized by UGT1A1 and compete for albumin binding sites.6 Neonatal plasma raltegravir concentrations that exceed typical peak raltegravir concentrations of 5 mg/mL by 50–100 fold could displace sufficient unconjugated bilirubin from albumin to lead to bilirubin induced neurologic dysfunction, including kernicterus, as was seen with sulfisoxazole.6,7
While traditional phase I pharmacokinetic and safety studies are difficult to conduct in neonates, population modeling and simulations can be used to inform selection of initial dosing regimens for study in this vulnerable population. We describe how modeling and simulations were used in IMPAACT P1110 to select a developmentally appropriate neonatal raltegravir dose for use in the first 6 weeks of life.
Methods
IMPAACT P1110 is a phase 1, multicenter non-comparative dose-finding study of raltegravir oral granules for suspension in term infants exposed to HIV-1 at risk of perinatal infection from birth through 6 weeks of life. Eligible infants were at least 37 weeks gestation at delivery with a birth weight of at least 2000 grams. Only infants born to mothers not receiving raltegravir prior to delivery were included in the initial modeling and simulations. Local institutional review boards or in-country ethics committees responsible for oversight of the study granted ethics and regulatory approvals. A two-cohort adaptive design was utilized where pharmacokinetic data from 2 single doses administered 7–10 days apart to infants in cohort 1 were included in population modeling and simulations to guide daily dosing for infants in cohort 2. Cohort 1 infants received raltegravir administered as a single oral dose within 48 hours of birth in addition to standard of care antiretroviral agents (ARVs) for prevention of perinatal transmission, and a second dose was administered at 7– 10 days of life. The initial raltegravir dose studied was 3 mg/kg and doses were adjusted on a rolling basis. PK sampling was obtained around the initial dose (pre-dose, and 1–2 hours, 4–8 hours, 12 hours, and 24 hours post-dose, with random sample on day 3–4 of life) and the second dose (pre-dose and 1–2 hours, 24 hours post-dose). Raltegravir plasma concentrations were measured using a previously published validated, isocratic, reverse-phase high-performance liquid chromatography-tandem mass spectrometry method.8 The linear calibration range was 0.01–10 mg/L from a 200 μL plasma sample.
Protocol exposure targets for each cohort 1 subject were Cmax ≤ 8.7 mg/L (19.6 μM) and AUC0–12 ≤ 20 mg*h/L (≤ 45μM*hr) from a non-compartmental analysis.9 Raltegravir assays and non-compartment pharmacokinetic analysis were performed in real time and results were monitored in an ongoing manner to ensure that raltegravir exposures did not exceed the PK targets (Table 1).
Table 1.
Raltegravir pharmacokinetic parameters from non-compartmental analysis [geometric mean (range)] for Cohort 1 initial doses
| Dose | Age at initial dose (hrs) | Cmax (mg/L) | AUC12 (mg*hr/L) | T ½ (hrs) | Tmax (hrs) | C24h (mg/L) | V2/F (L/kg) | Cl/F (L/kg/hr) |
|---|---|---|---|---|---|---|---|---|
| 3 mg/kg n=6 | 17.2 (9.9–25.4) | 3.36 (2.00–5.32 mg/L) | 29.5 (17.4–44.0 mg*h | 11.8 (7.9–15.7) | 6.5 (4.1–24.0) | 1.44 (0.662–3.65 mg/L) | 0.56 (0.35–0.80) | 0.033 (0.021–0.053) |
Population Modeling
A population pharmacokinetic model was developed incorporating cohort 1 pharmacokinetic data of the first six infants enrolled in the study and enriched with raltegravir concentration data from 24 infants and children ages 4 weeks to < 2 years enrolled in IMPAACT P1066, a phase I/II, multicenter, open-label non-comparative intensive pharmacokinetic treatment study of raltegravir in infants and children.10 Table 2 summarizes demographics of subjects included for pharmacokinetic modeling.
Table 2.
Overview of data used for model development
| Study | P1110 | P1066 | P1066 |
|---|---|---|---|
| Total number of subjects | 6 | 13 | 11 |
| Number of data points | 48 | 121 | 128 |
| Age range (enrollment) | birth | 6 months to < 2 years | 4 weeks to < 6 months |
| Weight range (kg) | 2.9–3.8 | 5.5–14 | 3.7–10.4 |
| Sex (M/F) | 3/3 | 8/5 | 7/4 |
M: Male; F: Female
Population pharmacokinetic modeling was performed using PsN/3.7.6 and NONMEM/7.3.0, applying the first-order conditional estimation method with interaction (FOCEI). Clearances and volumes of distribution were allometrically scaled (Table 3).
Table 3.
Allometric scaling equations applied
| Parameter | Abbreviation | Unit | Allometric Scaling |
|---|---|---|---|
| Volume of distribution (central compartment) | V2 | L | V2 = θV2· (BW/25)1 |
| Clearance | CL | L/hour |
CL = CL(t) ·
(BW/25)0.75 |
| Oral absorption rate constant | KA | 1/hour |
KA =
KA(t) |
| Volume of distribution (peripheral compartment) | V3 | L | V3 = θV3· (BW/25)1 |
| Inter-compartment clearance | Q | L/hour | CL = θQ·(BW/25)0.75 |
BW: Body weight in kg
A preliminary 2-compartment population pharmacokinetic model based on existing model describing raltegravir pharmacokinetic in pediatric and adult patients was used to generate individual predictions (empirical Bayesian estimates or EBEs) of the clearance for each individual for several time intervals.9 Individual clearances were allometrically scaled and subsequently fitted to an exponential function using a non-linear generalized least-squares method:11
| equation (1) |
where CLbase is the clearance at birth, CLmax the maximum change from birth CL and CLtau describes the rate of increase
A similar approach was carried out to describe the time-dependency of the absorption rate constant, a parameter that was assumed independent of body weight (eq. 2)
| equation (2) |
where KAbase is the absorption rate constant at birth, KAmax the change from birth KA and KAtau describes the rate of increase.
Parameters describing both the time-dependent clearance and absorption rate constant functions were implemented in the population pharmacokinetic model and re-estimated simultaneously with all other model parameters during model fit. Inter-individual variability (IIV) was modelled assuming a log-normal distribution for subject-level random effects and was tested on all pharmacokinetic parameters. The residual error of the fit to the observations was described by a combination of additive and proportional components.
Simulations
The resulting population pharmacokinetic model was used to develop simulations of potential dosing regimens predicting the pharmacokinetic profile for the typical individual only. The time of first neonatal dose administration was assumed to be 12 hours post-partum. The growth of the typical neonate was obtained by fitting an exponential function to the body weight (BW) and age (Age) data available from the dataset (eq 3):
| equation (3) |
with BW expressed in kg and Age in years.
Simulations were run for 10 dosing regimens designed for daily treatment during the first 6 weeks of life. Dosing scenarios were designed to be practical with fewest dose regimen changes possible. We evaluated how well each of ten different dosing scenarios met the exposure targets.
Summary pharmacokinetic exposure values for Ctrough, AUC0–24 (once daily dosing), AUC0–12 (twice daily dosing), Cmax and Ctrough pharmacokinetic parameters were calculated for each simulated dosing regimen for each day in a post-processing step using R/3.1.0. The regimen that best met revised pharmacokinetic exposure targets for safety (Cmax ≤ 8.7 mg/L (< 19.6 μM), AUC0–12 ≤ 6–20 mg*hr/L (45 μM.hr), AUC0–24 ≤ 12–40 mg*h/L (90 μM*hr)) and efficacy (Ctrough ≥ 0.033 mg/L or ≥75 nM), obtained from studies in older infants, children, and adults, was selected for evaluation for cohort 2 neonates receiving daily raltegravir dosing.9,10,12,13
Results
Despite the small sample size of 6 neonates, a population pharmacokinetic model was successfully developed with reasonable precision of the parameter estimates as shown in Table 4. 14 The clearance was almost nil at birth and increased to 90% of its maximum capacity at about 13 weeks of life. The absorption rate constant changed from 16% at birth to 90% of its maximum in about two weeks of life. Interindividual variability (IIV) was largest on CLmax, intercompartment clearance (Q), and KAmax, which was accounted for in the final model.
Table 4.
Final model parameter estimates with 95% confidence intervals (CI-95%).
| Parameter | Unit | Value | CI-95% | Parameter | Value | CI-95% | ||
|---|---|---|---|---|---|---|---|---|
| V2 | L | 11.51 | 5.34 | 24.78 | Interindividual variability (IIV) | |||
| V3 | L | 26.47 | 15.75 | 44.47 | IIV-CLmax | 0.18 | 0.08 | 0.27 |
| CLmax | L/hour | 12.73 | 10.60 | 14.86 | IIV-Q | 0.61 | 0.13 | 1.10 |
| Q | L/hour | 1.22 | 0.76 | 1.97 | IIV-KAmax | 0.46 | 0.19 | 0.73 |
| KAmax | 1/hour | 0.76 | 0.32 | 1.20 | Residual unexplained variability (RUV) | |||
| F | - | 1 | RUV-prop | 0.56 | 0.49 | 0.62 | ||
| CLbase | L/hour | 0 | RUV-add | 4.44 | 0 | 82.21 | ||
| CLtau | 1/week | 0.20 | 0.07 | 0.34 | ||||
| KAbase | 1/hour | 0.08 | 0 | 2.66 | ||||
| KAtau | 1/week | 0.95 | 0 | 4.21 | ||||
RUV-prop: proportional RUV; RUV-add: additive RUV
Results of the simulations for all 10 dosing regimens are presented in Table 5. All proposed dosing regimens met the Cmax endpoint criterium (≤8.7 mg/L). Regimen 5, which was initially planned for in the protocol, was predicted to result in overexposure on Day 2 and 3. Regimens 3, 4, 6, 7, and 10 were the only ones meeting all 4 pharmacokinetic endpoint targets. In the once-daily dosing period, regimen 10 achieved the lowest predicted AUC0–24 while maintaining Ctrough above the 0.033 mg/L threshold. When switching to the twice-daily dosing period, the AUC0–12 endpoint with regimens 4 and 6 was considered too close to the target (up to 94% of maximum allowed AUC0–12 of 20mg*hr/L). Regimens 7 and 10 resulted in higher Ctrough concentrations than regimen 3 and the end of week 4.
Table 5.
Simulated dose regimen scenarios with exposure assessments. Exposure targets are Ctrough > 0.033 mg/L, Cmax < 8.72mg/L, AUC0–24 < 40 mg*hr/L and AUC0–12 < 20mg*hr/L. Green cells show that exposure target has been met, red cells denote violation of the exposure target at indicated day(s) and yellow cells denote that exposure is close to the target at the indicated day(s). Raltegravir doses indicated expressed in mg/kg.
| Regimen | Day 1–7 (wk-1) | Day 8–14 (wk-2) | Day 15–21 (wk-3) | Day 22–28 (wk-4) | Day 29–35 (wk-5) | Day 36–42 (wk-6) | Ctrough | Cmax | AUC0–24 (QD) | AUC0–12 (BID) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 QD | 3 BID | Day42 | Days 2+3 | ||||||
| 2 | 3 QD | 3 BID | 4 BID | Day42 | Days 2+3 | |||||
| 3 | 2 QD | 2 BID | 6 BID | Day28 | Days 2+3 | |||||
| 4 | 2 QD | 2 BID | 6 BID | Days 2+3 | Days 14–16 | |||||
| 5 | 3 QD | 3 BID | 6 BID | Day 14 | Days 2+3 | |||||
| 6 | 2 QD | 4 QD | 6 BID | Days 2+3 | Days 14–16 | |||||
| 7 | 2 QD | 3 BID | 6 BID | Days 2+3 | ||||||
| 8 | 2 QD | 6 QD | 6 BID | Day 28 | Days 2+3 | |||||
| 9 | 3 QD | 3 BID | 6 BID | Days 2+3 | ||||||
| 10 | 1.5 QD | 3 BID | 6 BID | |||||||
wk: week; QD: once daily; BID: twice daily; AUC0–24 assessed for days with QD dosing only; AUC0–12 assessed for days with BID dosing only
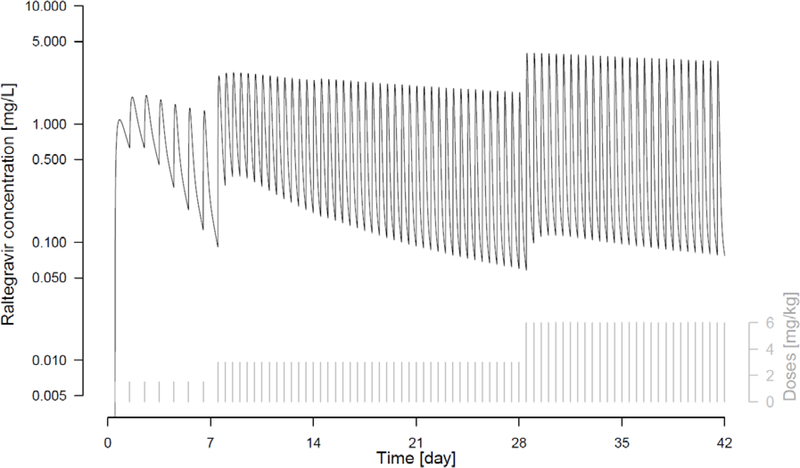
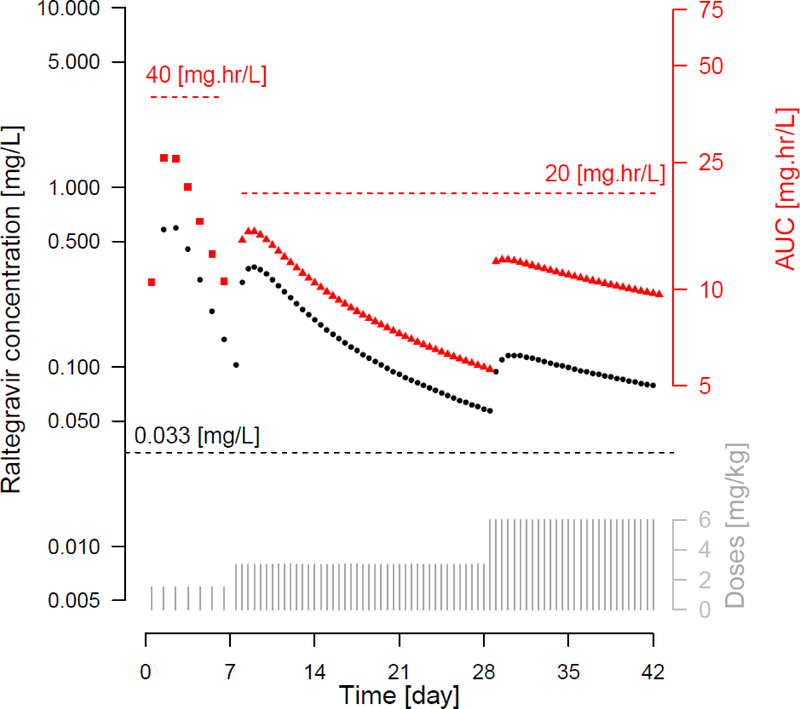
The regimen that best met all of the pharmacokinetic targets was regimen 10, consisting of 1.5 mg/kg once daily in Week 1, followed by an increase to 3 mg/kg twice daily in Weeks 2 to 4, and to 6 mg/kg twice daily in Weeks 5 and 6. This regimen incorporated an 8-fold increase in the total daily dose between the initial and week 4 doses. The pharmacokinetic profile for a typical neonate treated by this regimen shown in Figure 1 demonstrates how the changes in dose at the end of week 1 and week 4 were predicated to maintain adequate and safe raltegravir exposures from birth through age 6 weeks. The changes in Ctrough, AUC0–24 (once daily) and AUC00–12 (twice daily) over the first 6 weeks of life are shown in Figure 2. The 1.5 mg/kg once a day dosing regimen in the first week of life resulted in plasma raltegravir concentrations above 0.033 mg/L, a target concentration associated with suppression of viral replication, while AUC0–24 remained below the 40 mg*hr/L safety threshold. The dose change at the end of week 1, to 3 mg/kg twice a day involved a 4-fold increase in total raltegravir daily dose and maintained raltegravir plasma concentrations above the Ctrough target but below the AUC0–12 safety threshold. The second dose change at the end of week 4, to 6 mg/kg twice daily, was a doubling of the previous dose and resulted in a dose regimen that aligns with the raltegravir FDA label approved in 2013 for treatment of infants 4 weeks of age and older.
Figure 1.
Simulated pharmacokinetic profile of raltegravir for typical individual treated by dose regimen scenario 10: 1.5 mg/kg once daily from day-1 to day-7, 3 mg/kg twice daily from day-8 to day-28 and 6 mg/kg twice daily from day-29 to day-42.
Figure 2.
Simulated Ctrough and AUC profiles for dosing regimen no. 10. Ctrough is plotted against the left axis and AUC against the right axis. Black dots represent Ctrough values, red squares represent AUC0–24 for days 1–7 and red triangles represent AUC0–12 for days 8–42. Dotted lines present the Ctrough criterion ≥0.033 mg/L, AUC0–24 criterion ≤ 40 mg*hr/L and AUC 0–12 criterion ≤ 20 mg*hr/L.
Discussion
Determination of appropriate neonatal dosing regimens is crucial to the safe and efficacious use of medications in neonates. Neonates have a unique physiology as they make the transition from the fetal to the extrauterine environment and these changes may have a significant impact on drug disposition. Maturation of gastrointestinal function with development of regular oral intake, changes in gastric pH, and motility of the gastrointestinal tract function may have a large effect on drug absorption.15 Neonates lose weight over the first days of life due to loss of total body water and then enter a period of rapid weight gain with increasing lean body mass and body fat, resulting in changes in drug distribution.15 Drug elimination will be affected by maturation of renal and hepatic function, resulting in large increases in drug metabolism and excretion over the first weeks of life.15 Dosing regimens during this period of rapid growth and development must be carefully designed to avoid the risks of over-dosing and under-dosing of various medications used in this patient population.
The need for safe and effective antiretroviral regimens to treat and prevent HIV infection in neonates has become evident in recent years due to the development and availability of rapid HIV nucleic acid tests allowing diagnosis of HIV infection in the first days and weeks of life. In addition, neonates born to mothers at high risk of transmitting HIV to their infants may benefit from receipt of empiric treatment with a fully suppressive antiretroviral regimen to reduce the risk of infant infection and to begin treatment as early as possible in those infants who become infected.2 Despite this need for provision of effective combination antiretroviral therapy in neonates, few antiretrovirals have sufficient neonatal pharmacokinetic and safety data to allow their safe and effective use in neonates. When using antiretrovirals in neonates to treat or prevent HIV, it is critical to avoid both under-dosing, which may allow ongoing viral replication and development of antiretroviral resistance, and overdosing, which may expose the infant to greater risk of drug related toxicity.
We have described a step wise progression of studies to allow the safe and efficient development of a neonatal dosing regimen for raltegravir, the first HIV integrase strand inhibitor to be licensed. The first step was initial assessment of neonatal clearance by study of washout elimination of raltegravir acquired across the placenta in neonates whose mothers received raltegravir during pregnancy. This information helped with the determination of the initial doses to be studied. Next step was administration of 2 individual doses during the first weeks of life to allow assessment of changes in raltegravir absorption, distribution and drug elimination over the first weeks of life. The pharmacokinetic data from 6 neonates receiving 2 individual doses were incorporated into a population model along with existing raltegravir pharmacokinetic data from infants over 4 weeks of age. This model was used to run simulations predicting neonatal exposure over the first 4 weeks of life with proposed neonatal dosing regimens.
During model development special attention was paid to a number of expected characteristics of raltegravir pharmacokinetics in neonates. Raltegravir is primarily metabolized by UGT 1A1, a glucuronosyltransferase which was already known to have very low activity at birth. Consistent with literature reports of UGT 1A1 enzyme activity, our model incorporated low UGT 1A1 immediately after birth with a dramatic increase over the first weeks of life to nearly adult levels.11,16 The body weight changes of neonates are also significant in the first six weeks of life, so the combination of maturation of enzyme activity and growth will determine the actual increase of neonatal clearance capacity.
The absorption rate constant for raltegravir could not be estimated precisely due to limited pharmacokinetic data characterizing the absorption phase. The modelling suggests that the absorption rate constant increased quickly to a maximum from birth up to age 2 weeks, possibly reflecting important changes in neonatal gastrointestinal activity shortly after birth. Feeding was not restricted in the neonates enrolled in P1110 so the potential effect of food intake on absorption could not be evaluated. It was assumed that raltegravir free fraction in plasma is similar between adults and neonates, so that pharmacokinetic endpoints based on total concentration are predictive of the free concentration, which is considered relevant to both efficacy and safety.
The simulations indicated that two dose changes would be required to meet all the efficacy and safety endpoint criteria during a daily treatment course of raltegravir during the first 6 weeks of life. While frequent dose changes pose challenges for parents in the first weeks of life, they were necessary to avoid periods of either undertreatment or overtreatment. Avoiding extremely high raltegravir plasma concentrations is very important during the first week of life, when displacement of unconjugated bilirubin from albumin could pose a risk for bilirubin induced neurologic dysfunction. A rapid increase in clearance capacity as a result of UGT-1A1 enzyme maturation during the first week of life appeared to be the major factor underlying the need for these dose changes. This maturation has an exponential profile, requiring the first dose regimen change from 1.5 mg/kg once daily to 3 mg/kg twice daily at one week after birth and the next dose change from 3 mg/kg twice daily to 6 mg/kg twice daily 3 weeks later at 4 weeks of age. After 4 weeks, the model suggested that 72% of full enzyme maturation is achieved and that the dose required to maintain adequate and safe raltegravir exposures is 8-fold higher compared to birth.
The study design for IMPAACT P1110 involves an adaptive design using two cohorts. Infants in the first cohort received 2 single doses one week apart. The dose size evaluated in these infants was 3 mg/kg, selected based on results from the P1097 protocol describing washout elimination of raltegravir in neonates whose mothers received raltegravir prior to delivery. 3 The first dose was administered within 48 hours of delivery to full-term neonates weighing at least 2 kg. A repeat dose was then given at one week of age. The raltegravir concentration data from these first 6 infants were combined with raltegravir concentration data from older children enrolled in the P1066 protocol for use in population modelling and simulations to select a daily dosing regimen for evaluation in a subsequent cohort of neonates. Pharmacokinetic and safety results from both cohorts of P1110 were submitted by Merck & Co. for a label extension for use in neonates. This raltegravir dosing regimen was approved by the FDA for use in neonates in November 2017. The modelling strategy described above played a critical role in facilitating the neonatal studies that resulted in raltegravir becoming the first new antiretroviral to be approved in neonates since emtricitabine in 2006.
Raltegravir readily crosses the placenta, with a median cord blood/maternal delivery plasma concentration ratio of 1.48.3 Neonates born to mothers who received raltegravir 2 hours to 24 hours prior to delivery should have their first dose of raltegravir delayed until 24 hours to 48 hours after birth. This recommendation was based on modeling and simulations submitted to the FDA by Merck & Co. when the label was expanded to include neonates. Other integrase inhibitors such as dolutegravir and elvitegravir also readily cross the placenta and significant plasma concentrations have been seen in infants born to mothers receiving these medications prior to delivery.17,18
An estimated 20% of pregnancies in women living with HIV are associated with preterm deliveries.19 Few antiretrovirals have been adequately studied in preterm, low birth weight infants. A new version of IMPAACT P1110 is under development for preterm, low birth weight infants. The IMPAACT P1097 protocol was expanded to include low birth weight neonates and provide washout raltegravir concentration data from trans-placentally acquired raltegravir in infants with birth weight less than 2500 grams whose mothers received raltegravir during pregnancy. The raltegravir concentration data from full-term neonates combined with low birth weight infant washout raltegravir concentration data have been included in an updated model for preterm infants and will be used to provide guidance for appropriate dosing of premature neonates.
Conclusion
Neonates undergo significant physiologic changes as part of their adaptation to extrauterine life, which may result in dramatic changes in drug disposition. As a result, pharmacokinetic parameters cannot be extrapolated from older infants but must be obtained directly from neonates. While ethical, safety and practical considerations may make neonatal studies difficult to perform, such studies may be facilitated by using an adaptive design with population pharmacokinetic modelling and simulations as described here for raltegravir.
Acknowledgments
Conflicts of Interest and Source of Funding
AC and HT are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) [license holder Isentress®]. JL is a paid consultant for Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). MM has research support from Gilead Sciences, Merck and Co., and ViiV Healthcare. DC and EA have research support from Merck and Co. and ViiV Healthcare.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed (October 8, 2018).
- 2.Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. Accessed (November 18, 2018).
- 3.Clarke DF, Acosta EP, Rizk ML, et al. Raltegravir pharmacokinetics in neonates following maternal dosing. Journal of Acquired Immune Deficiency Syndromes. November 1 2014;67(3):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J. April 15 1981;196(1):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krekels EH, Danhof M, Tibboel D, Knibbe CA. Ontogeny of hepatic glucuronidation; methods and results. Curr Drug Metab. July 2012;13(6):728–743. [DOI] [PubMed] [Google Scholar]
- 6.Clarke DF, Wong RJ, Wenning L, Stevenson DK, Mirochnick M. Raltegravir in vitro effect on bilirubin binding. Pediatr Infect Dis J. September 2013;32(9):978–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen DH, Blanc WA, Crozier DN, Silverman WA. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics. October 1956;18(4):614–625. [PubMed] [Google Scholar]
- 8.Long MC, Bennetto-Hood C, Acosta EP. A sensitive HPLC-MS-MS method for the determination of raltegravir in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. May 15 2008;867(2):165–171. [DOI] [PubMed] [Google Scholar]
- 9.Rizk ML, Du L, Bennetto-Hood C, et al. Population pharmacokinetic analysis of raltegravir pediatric formulations in HIV-infected children 4 weeks to 18 years of age. J Clin Pharmacol. July 2015;55(7):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachman S, Alvero C, Acosta EP, et al. Pharmacokinetics and 48-Week Safety and Efficacy of Raltegravir for Oral Suspension in Human Immunodeficiency Virus Type-1-Infected Children 4 Weeks to 2 Years of Age. Journal of the Pediatric Infectious Diseases Society. December 2015;4(4):e76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyagi SJ, Collier AC. The development of UDP-glucuronosyltransferases 1A1 and 1A6 in the pediatric liver. Drug Metab Dispos. May 2011;39(5):912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chirch LM, Steigbigel RT. Raltegravir in combination with other antiretroviral agents for the treatment of HIV infection. Infect Drug Resist. 2010;3:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachman S, Zheng N, Acosta EP, et al. Pharmacokinetics, safety, and 48-week efficacy of oral raltegravir in HIV-1-infected children aged 2 through 18 years. Clin Infect Dis. February 2014;58(3):413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lommerse J, Clarke D, Chain A, Witjes H, Teppler H, Capparelli E, Acosta EP, Rizk M, Wenning L, Kerbusch, Spector S, Smith B and Mirochnick M Raltegravir dosing in neonates (IMPAACT P1110) - Use of allometry and maturation in PK modeling to develop a daily dosing regimen for investigation during the first weeks of life. Population Approach Group Europe (PAGE) Conference Hersonissos, Crete, Greece2015. [Google Scholar]
- 15.van Donge T, Evers K, Koch G, van den Anker J, Pfister M. Clinical Pharmacology and Pharmacometrics to Better Understand Physiological Changes During Pregnancy and Neonatal Life. Handb Exp Pharmacol. April 10 2019. [DOI] [PubMed] [Google Scholar]
- 16.Onishi S, Kawade N, Itoh S, Isobe K, Sugiyama S. Postnatal development of uridine diphosphate glucuronyltransferase activity towards bilirubin and 2-aminophenol in human liver. Biochem J. December 15 1979;184(3):705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulligan N, Best BM, Wang J, et al. Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. Aids. March 27 2018;32(6):729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Momper JD, Best BM, Wang J, et al. Elvitegravir/cobicistat pharmacokinetics in pregnant and postpartum women with HIV. Aids. November 13 2018;32(17):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez M, Figueras F, Hernandez S, et al. Association of HIV infection with spontaneous and iatrogenic preterm delivery: effect of HAART. Aids. January 2 2012;26(1):37–43. [DOI] [PubMed] [Google Scholar]