Background:
Syringe exchange programs (SEP) reduce HIV incidence associated with injection drug use (IDU), but legislation often prohibits implementation. We examined the policy change impact allowing for SEP implementation on HIV diagnoses among people who inject drugs in 2 US cities.
Setting:
Philadelphia, PA, and Baltimore, MD.
Methods:
Using surveillance data from Philadelphia (1984–2015) and Baltimore (1985–2013) for IDU-associated HIV diagnoses, we used autoregressive integrated moving averages modeling to conduct 2 tests to measure policy change impact. We forecast the number of expected HIV diagnoses per city had policy not changed in the 10 years after implementation and compared it with the number of observed diagnoses postpolicy change, obtaining an estimate for averted HIV diagnoses. We then used interrupted time series analysis to assess the immediate step and trajectory impact of policy change implementation on IDU-attributable HIV diagnoses.
Results:
The Philadelphia (1993–2002) model predicted 15,248 new IDU-associated HIV diagnoses versus 4656 observed diagnoses, yielding 10,592 averted HIV diagnoses over 10 years. The Baltimore model (1995–2004) predicted 7263 IDU-associated HIV diagnoses versus 5372 observed diagnoses, yielding 1891 averted HIV diagnoses over 10 years. Considering program expenses and conservative estimates of public sector savings, the 1-year return on investment in SEPs remains high: $243.4 M (Philadelphia) and $62.4 M (Baltimore).
Conclusions:
Policy change is an effective structural intervention with substantial public health and societal benefits, including reduced HIV diagnoses among people who inject drugs and significant cost savings to publicly funded HIV care.
Key Words: injection drug use, HIV, syringe exchange, policy change, cost-effectiveness
INTRODUCTION
Syringe exchange programs (SEP), also known as needle exchange programs or syringe access programs, are an essential component to preventing HIV/AIDS among people who inject drugs (PWID). Studies have shown that SEP utilization is associated with reductions in bloodborne infections among PWID; research from Tacoma, WA,1 New Haven, CT,2 and New York City, NY,3 has demonstrated that SEP implementation was associated with significant reductions in hepatitis B and C incidence (80% reduction)1 and HIV incidence (estimated 33% reduction in New Haven and 70% reduction in New York).2,3 Despite evidence demonstrating the public health utility of SEPs,4–9 federal and state policies (eg, drug paraphernalia laws) may limit their implementation. Other policies more explicitly affect SEP operations. In 1988, Congress passed legislation prohibiting the use of federal monies to support SEPs. Aside from a brief period (2009–2012) during which the restriction was removed, the legislation remained until December 2015 when, largely in response to HIV outbreaks among suburban and rural populations injecting prescription opioids, the language was modified to allow federal monies to support SEP operations except for purchasing injection equipment.10
SEP Case Histories: Philadelphia and Baltimore
For Philadelphia and Baltimore in the 1990s, changing paraphernalia legislation was critical to creating SEPs.11
SEPs in Philadelphia originated when activists organized a community response to rising HIV infection rates among PWID.11 Pennsylvania's paraphernalia laws were conflicting: syringe possession and distribution were illegal in Philadelphia,12 but the Disease Prevention and Control Act of 1955 authorized syringe distribution as a disease prevention activity within individual cities. Seeing the AIDS epidemic as a public health emergency, activists felt that the Disease Prevention and Control Act authorized SEPs as a public health measure. In late 1991, Prevention Point Philadelphia (PPP) was created, operating illegally while working with and gaining support from the health commissioner, officials at the Department of Health's AIDS Office (now the AIDS Activities Coordinating Office), and the mayor.13 Despite state disapproval, the mayor signed Executive Order 4–92 on July 27, 1992, declaring HIV/AIDS a public health emergency in Philadelphia and authorizing SEPs as a measure to address it.14 On August 1, 1992, PPP held its first day of legal syringe exchange. Although data pertaining to syringe distribution from the initial years of PPP are not available, SEP operations' data from 1998 indicate that over 400,000 syringes were distributed during this year, with that amount almost doubling to 810,000 in 1999. In each of these years, over 2000 new clients were registered. PPP remains the sole officially recognized SEP in Philadelphia, providing syringe access and harm reduction services (including medical care, wound care, HIV/HCV testing, overdose prevention and reversal training, linkage to drug treatment, and medication-assisted treatment) through municipal and private support.15
Baltimore's legislative environment was also the biggest obstacle to SEP implementation. Maryland's Uniform Controlled Dangerous Substances Act of 1970 made drug paraphernalia possession illegal. For SEPs to open in Baltimore, state laws needed to change. The impetus for change came in 1992 from the city's own leadership: the mayor and the health commissioner. They lobbied the state legislature for exemptions to existing paraphernalia laws for Baltimore City so the Health Department could legally distribute sterile injection equipment. Early attempts at policy change were met with resistance from the Governor and other state legislators, but eventually, Senate Bill 402 was signed in to law on April 2, 1994,16 and went into effect on June 1, 1994. Since then, the Baltimore City Health Department has run the SEP.17 In addition to syringe access services, the SEP provides harm reduction education, linkage to addiction treatment services (including maintaining same day treatment openings), testing and counseling for HIV and syphilis, and opioid overdose response training.17
Why Policy Change Matters
These stories show 2 different mechanisms of policy change for SEP establishment; they also underscore the importance of policy change as a structural intervention for HIV prevention among PWID in that it changes individuals' risk environment without changing their behaviors or social interactions.18 Our study in the District of Columbia (DC) found that changing legislation to allow the use of municipal revenue for SEPs in 2008 was associated with an estimated 120 averted HIV diagnoses in PWID in the first 2 years after policy change and an estimated savings of $44.3 million in health care costs associated with HIV treatment.19
Given the historical contexts of Philadelphia and Baltimore, would one expect the same magnitude of epidemic impact that was observed in DC? We examined this question using similar methodologies used in the examination of policy change impact in DC.19 Although previous research has examined the association between presence of SEPs and HIV incidence, these studies were more ecological in nature. We build on these previous analyses by not only examining how SEP implementation affected the HIV epidemic in each city but also by attempting to isolate the epidemic impact of policy change specifically on the numbers of new HIV diagnoses.
METHODS
Using autoregressive integrated moving averages (ARIMA) modeling, forecasts were created to estimate the expected annual number of new HIV diagnoses that would have occurred in the 10 years had policies not changed. Forecasted numbers for each city were then compared with observed numbers of diagnoses to calculate the averted new HIV diagnoses that could be attributed to legal SEP implementation. We then used interrupted time series analysis (ITSA) to assess the impact that policy change (the “interruption”) had on injection drug use (IDU)–associated HIV diagnoses, both immediately and as a trend change. ITSA examines temporally ordered data to determine whether an experimental manipulation or intervention produced a reliable change in data20,21 while also allowing models to account for baseline levels and trends present in the data. Henceforth, we refer to observations before the interruption as the “preimplementation period” and those after the interruption as the “postimplementation period.”
The outcome measure for both cities was IDU-associated HIV diagnoses. In Philadelphia, data were extracted from Philadelphia's Enhanced HIV/AIDS Reporting System (eHARS), a population-based registry containing information on all HIV/AIDS diagnoses reported to the Philadelphia Department of Public Health AIDS Activities Coordinating Office Surveillance Unit since 1984. eHARS contains information abstracted from medical records, including HIV transmission risk (eg, IDU and MSM/IDU) and laboratory reporting of all CD4 cell counts and HIV-1 RNA levels. Thus, eHARS contains data from all people living with HIV (PLWH) diagnosed in Philadelphia.
Annual numbers of HIV diagnoses in Baltimore were extracted from the Baltimore City Annual HIV Epidemiological Profile,22 which contains data reported to the state through December 31, 2014. Annual percentages of diagnoses attributed to each exposure category are reported for the years 1985–2013. To determine the annual number of IDU-associated HIV cases, the percentages of new HIV diagnoses with a known exposure risk were multiplied by each IDU-associated exposure category (IDU and MSM/IDU). These 2 numbers were combined for each year to represent the total number of new IDU-associated HIV diagnoses.
ARIMA models were fit to the preimplementation data for each city using Box and Jenkins23 methods. Outlier detection was completed to identify significant (α < 0.01) shift and additive observations. Shift outliers were addressed in the model by entering dichotomous variables assigning 0 to observations prior to and 1 to all other observations including and following the identified shift outlier. For additive outliers, dichotomous variables were added to the model, assigning 1 to the additive outlier and 0 to all other observations.
ITSA models evaluated 2 types of impact—step change and slope change—on new HIV diagnoses. Step change tests for an immediate significant change between HIV diagnoses in the last preperiod and first postperiod observations. It was measured with a dichotomous variable that assigned 0 to all prepolicy and 1 to all postimplementation observations. Slope change tests for significant changes in trend and direction of the number of HIV diagnoses across the preperiod versus the postperiod. It was measured using a continuous variable that assigned 0 to all preimplementation observations and 1 to the first postinterruption observation, with subsequent observation values increasing by 1 (1, 2, …, n).
For the ITSA, the year ending after the date of policy change was used as the interruption. In the 6 months after each policy change, surveillance efforts and normal testing mechanisms would have been more likely to detect those who are already HIV-positive. Therefore, the real policy impact would have occurred after persons already living with HIV and previously undiagnosed were detected and after legal SEPs were operating and effectively reaching PWID. In Philadelphia, legal SEP began on August 1, 1992, so the data were divided into 2 periods: preimplementation (1984 through 1992) and postimplementation (1993 through 2015). Baltimore's legal SEP began on June 1, 1994, so data were similarly divided into preimplementation (1985 through 1994) and postimplementation (1995 through 2013) periods. In using annual data, our analyses attempt to conservatively account for the possible lag time between when the policy changed and when it started to have epidemic impact.
In addition, we replicated these methods in Baltimore using HIV diagnoses data among MSM without IDU exposure to examine the true impact of SEP utilization on new HIV diagnoses (ie, as a control). Notably, these analyses were only possible in Baltimore given HIV case-reporting data availability. Given that MSM in Baltimore would be similar to the PWID population in terms of exposure to public health efforts (eg, changes in HIV testing and access to HIV treatment) that would have been available during the same time period, these analyses allow us to control for other variables that may have affected new cases of HIV and better understand the true impact of SEP implementation on IDU-associated HIV diagnoses. All analyses were completed using SAS v9.4. This research was determined by the George Washington University's Institutional Review Board as exempt from oversight (IRB #051106).
RESULTS
Descriptive measures for the epidemiologic data are presented in Table 1. For Philadelphia, we observed a nonsignificant preimplementation to post implementation decrease in the mean number of new IDU-associated HIV diagnoses (419.9 and 291.6, respectively) as well as a significant preimplementation to post implementation decrease in the MSM/IDU exposure category (77.7 and 35.6, P < 0.05). For Baltimore, we observed a significant preimplementation to postimplementation decreases in both in the mean number of IDU-associated HIV diagnoses (607.9 and 357.0, P < 0.05) and the mean numbers of new diagnoses attributed to each IDU exposure category (IDU only: 542.8 and 332.2, P < 0.05, MSM/IDU: 65.0 and 24.8, P < 0.05).
TABLE 1.
Annual New Diagnoses of IDU-Associated HIV Infection: Baltimore and Philadelphia
Philadelphia
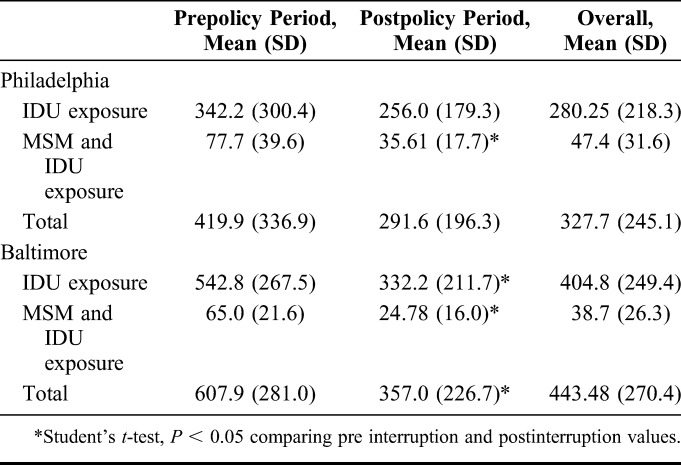
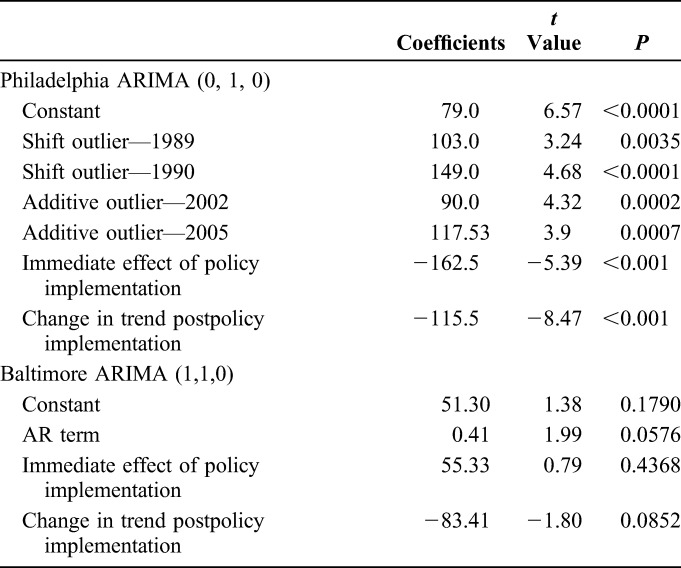
For Philadelphia, an ARIMA (0, 1, 0) model with 4 outliers—shift outliers at 1989 and 1990 and additive outliers at 2002 and 2005—was determined to be the best fit for the data. The 1989 and 1990 shift outliers were included in all analyses; the 2002 and 2005 additive outliers were only included in the ITSA. The model forecasts 15,248 new IDU-associated HIV diagnoses in Philadelphia between 1993 and 2002, whereas 4656 IDU-associated HIV diagnoses were reported (Fig. 1). This is a difference of 10,592 averted diagnoses of HIV over 10 years (approximately 1059 averted diagnoses annually). Using annual HIV case data from 1984 to 2015, ITSA was completed to determine whether SEP implementation resulted in an immediate step or trend change between the preimplementation and postimplementation periods. A significant negative step change (B = −162.5, P < 0.001) was identified, as was a significant decreasing slope change (B = −115.5, P < 0.001) (Table 2).
FIGURE 1.
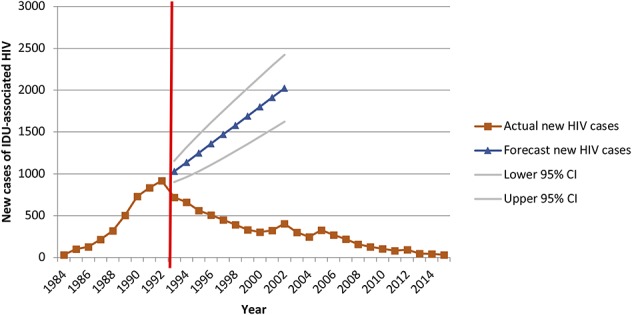
Forecasted versus actual diagnoses of IDU-associated HIV infection in Philadelphia during the 10 years after the change in syringe exchange policy.
TABLE 2.
Interrupted Time Series Analysis: Philadelphia and Baltimore
Baltimore
For Baltimore, an ARIMA (1, 1, 0) model with one order of nonseasonal differencing and a single autoregressive term was determined as the best fit for the data. The 10-year forecast predicted 7263 new IDU-associated HIV diagnoses in Baltimore between 1995 and 2004, whereas 5372 diagnoses were reported (Fig. 2). This is a difference of 1891 averted HIV diagnoses over 10 years, with 207 averted cases in the first 5 years (11% of total predicted averted cases) and 1684 in years 6 through 10 (89% of total predicted averted cases). Using surveillance data for 1985 to 2013, ITSA was completed to assess significant immediate and slope changes between the preimplementation and postimplementation periods. No significant immediate step (B = 55.34 P = 0.4368) or slope (B = −83.41, P = 0.0852) change was identified (Table 2).
FIGURE 2.
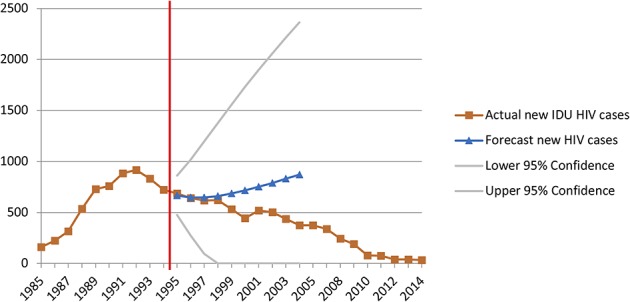
Forecasted versus actual diagnoses of IDU-associated HIV diagnoses in Baltimore during the 10 years after the change in syringe exchange policy.
With respect to the control model in Baltimore that used MSM-attributed HIV diagnoses, the ITSA found no significant immediate step change or slope change for new cases of HIV after SEP implementation (Fig. 3). In addition, the forecasting showed only a small difference between the observed (1,345) and expected (1,776) cases of MSM-attributed HIV in the 10 years after the SEP policy change.
FIGURE 3.
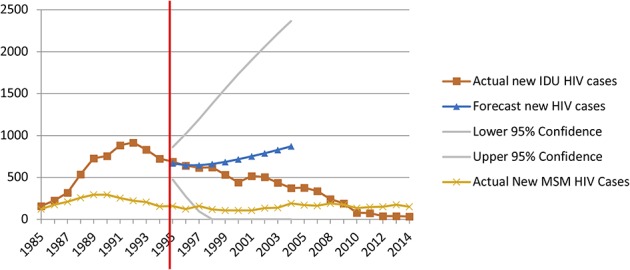
Forecasted versus actual diagnoses of IDU-associated and MSM-associated HIV diagnoses (control case scenario) in Baltimore during the 10 years after the change in syringe exchange policy.
DISCUSSION
These analyses demonstrate that SEP implementation in Philadelphia and Baltimore was associated with significant overall reductions in IDU-associated HIV diagnoses. Philadelphia's findings are particularly striking: the significant step and slope changes observed indicate that policy change and SEP implementation occurred in the same year as the number of HIV diagnoses was peaking. Although it cannot be stated with 100% certainty that the epidemic trajectory would have leveled after reaching that apex, the ARIMA forecast suggests that diagnoses would have continued to rise had SEP implementation not taken place. The significant level-shift outliers identified in 1989 and 1990 during the prepolicy change period indicate that Philadelphia had a significant increase in new HIV diagnoses during these 2 consecutive years that persisted for many years following. The additive outliers identified in 2002 and 2005 mark 2 years that had significantly higher numbers of reported new HIV cases; however, these changes did not persist or impact observations seen in the following years. Of interest and likely the explanation for these 2 outliers, Philadelphia moved to code-based reporting in 2002 and name-based reporting in 2005. These data support the possibility that the policy change in Philadelphia may have capped the peak of IDU-associated diagnoses in the city, explaining the rapid decrease in IDU-associated diagnoses after 1995.
The Baltimore data also showed a decrease in IDU-associated HIV diagnoses after the policy change. Although the surveillance data show that IDU-associated HIV diagnoses had begun to stabilize and decrease slightly before SEP implementation, the addition of SEPs contributed to a more rapid decline. The comparison of the more rapid decline in IDU-associated cases and the slower decline in the number of MSM-associated cases indicates that although other factors occurring within Baltimore likely played some role in the decrease in HIV seen among PWID, the vast majority of this decrease can be attributed to SEP implementation.
Limitations must be acknowledged in this research, the first pertaining to the quantity and quality of available surveillance data for each city. Although AIDS case surveillance in both cities began in the mid-1980s, HIV case reporting (either by name or code) was implemented much later; in Baltimore, code reporting began in 1994 and name reporting began in 2007, whereas in Philadelphia, code reporting began in 2002 and name reporting began in 2005. Since SEPs in both cities started in the early to mid-1990s, there were fewer observations of annual HIV diagnoses before the policy change event despite having sufficient numbers of observations to meet the minimum requirements for ARIMA modeling. More prepolicy change observations would have facilitated a more “finely tuned” model that better reflects longitudinal trends in IDU-associated HIV diagnoses. Also, our analyses modeled HIV diagnoses rather than incidence due to data limitations. Whether diagnoses are a good proxy for incidence depends on various factors, including stage of the local HIV epidemic, the number of PLWH unaware of their status, and the availability of local testing programs. Furthermore, data obtained from city health departments for these analyses may reflect issues present in disease surveillance systems, including inconsistencies in HIV/AIDS case reporting and variability in PWIDs' HIV testing patterns. Effects observed in both cities may actually underestimate impact given potential overlaps between diagnoses of IDU-associated and heterosexual transmission, as well as the availability and utilization of other interventions for PWID such as addiction treatment and access to antiretroviral medication.
A final point of consideration pertains to the determination of HIV epidemic impact of policy change within the PWID population when there are no precise size estimates of this population. Although estimates of the overall PWID population has remained stable from 1992 to 2002, there have been estimates of growing PWID populations in Baltimore (168–336 per 10,000 population) and the Philadelphia–New Jersey MSAs (from 151 to 173 per 10,000 population).24 Given that the time period for these estimates is similar to that of our analyses, we are confident that our estimates are measuring true population impact.
Our findings also demonstrate that averted HIV diagnoses translated to cost savings for cities where most PLWH are recipients of publicly funded healthcare. The forecasts estimated an average of 1059 HIV diagnoses in Philadelphia and 189 HIV diagnoses in Baltimore averted annually. Multiplying the lifetime costs of HIV treatment per person ($229,800)25 by the average number of diagnoses averted annually in both cities yields an estimated annual saving of $243.4 million for Philadelphia and $62.4 million for Baltimore. Considering diagnoses averted over the 10-year modeled period, the lifetime cost savings associated with averted HIV diagnoses stemming from policy change to support SEPs may be more than $2.4 billion and $624 million dollars for Philadelphia and Baltimore, respectively. Because SEPs are relatively inexpensive to operate,26 overall cost savings are substantial even when deducting program operational costs from the total amount. Considering annual program expense ($390,000 in 2011 for Philadelphia27 and $800,000 estimated in FY 2017 for Baltimore28) (Kathleen Goodwin, Baltimore City Health Department, personal communication, January 3, 2017) and cost savings in each city, and a conservative estimate that 75% of these savings would be experienced in the public sector, the 1-year return on investment in SEPs remains in the hundreds of millions of dollars ($182.5 M for Philadelphia, $46.8 M for Baltimore). Small investments in SEPs may yield large savings in HIV treatment costs, so implementing SEPs may liberate resources for other important interventions, such as expanded access to medication-assisted treatment, overdose prevention, and housing.
Another implication pertains to how variations in SEP implementation may have influenced intervention effectiveness. Policies governing SEPs affect not only the overall number of syringes distributed annually but also the ability of PWID to obtain sufficient coverage for all injection events. For example, PPP's clients may exchange syringes for themselves and others; recent data show that the mean number of syringes exchanged per exchange event increased from 1.53 in 1999 to 1.82 in 2014.13 In addition, PPP's annual syringe distribution has consistently increased from approximately 811,000 in 1999 to 1.2 million in 2014,13 allowing for greater coverage of injection events and more opportunities for disease prevention.
By contrast, Baltimore's SEP had a one-for-one (1:1) exchange policy from 1994 to 1999 but, in 2000, switched to a more restrictive policy, where clients were allowed 1:1 exchange for program-distributed syringes but could receive 1 sterile syringe in exchange for 2 nonprogram syringes. From 2005 to 2014, the SEP returned to the less restrictive 1:1 policy, after which they shifted to a need-based distribution model whereby PWID could access as many syringes as needed. Baltimore City's health commissioner estimated that moving from the 1:1 to the needs-based distribution policy could increase coverage of injection events from 42% to 61%.29 More flexible approaches to syringe access in Baltimore could have resulted in greater injection coverage and more dramatic declines in IDU-associated HIV diagnoses earlier. Regulations limiting clean needle and syringe distribution are important operational issues to consider if policy changes supporting harm reduction for PWID are to have optimal impact.
This research provides additional support for the effectiveness of policy change as a structural intervention for HIV prevention among PWID and the utility of syringe access as an effective, evidence-based approach to promote PWID health. The need for such approaches is particularly relevant given the current state of the opioid epidemic in the United States and the resurgence of IDU-associated HIV outbreaks in suburban and rural areas. A critical lesson learned from the Indiana HIV outbreak was that had the SEP been implemented earlier in the course of that outbreak, many infections could have been averted. Communities throughout the United States which are vulnerable to IDU-associated HIV outbreaks should consider the potential public health benefits, such as those experienced in Philadelphia and Baltimore; they may gain from implementing SEPs as they are one of our most powerful HIV prevention strategies for PWID populations.
ACKNOWLEDGMENTS
This work is part of a larger project—DC POINTE: Policy Impact on the Epidemic—whose main objective is to examine the epidemic impact of policy change as a structural intervention for HIV prevention for PWID in the District of Columbia. This research was supported by a grant from the National Institute on Drug Abuse (NIDA) to M.S.R. (R01DA031649). The authors of this paper have no conflicts of interest to declare.
We would also like to acknowledge the infrastructure, core, and administrative support provided by the District of Columbia Center for AIDS Research (CFAR; P30AI087714), the Penn Center for AIDS Research (CFAR; P30 AI045008), the Penn Mental Health AIDS Research Center (PMHARC; P30MH097488), and the Johns Hopkins University Center for AIDS Research (CFAR; P30AI094189). The CFARs are NIH funded programs which are supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, NIGMS, NIDDK, and OAR. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors would like to express gratitude to the Baltimore Department of Health and Mental Hygiene, the Philadelphia Department of Public Health, the Baltimore City Syringe Exchange program, and Prevention Point Philadelphia. We would also like to thank Danielle Fiore for her assistance with the abstraction of the Philadelphia surveillance data.
L.S.W. is currently a Visiting Professor of Health Policy and Management and Distinguished Fellow in the Fitzhugh Mullan Institute for Health Workforce Equity at the Milken Institute School of Public Health, George Washington university. D.R.H. is currently the Dean and SUNY Empire Innovation Professor at the University of Albany, State University of New York (SUNY). C.P.C. is currently retired from the Baltimore City Health Department.
Footnotes
This publication resulted in part from research supported by the Penn Center for AIDS Research (CFAR) (P30 AI 045008 - Ronald Collman, PI), the Penn Mental Health AIDS Research Center (PMHARC) (P30 MH 097488 - Dwight Evans, PI) and the CFAR Social & Behavioral Science Research Network National Scientific Meeting (SBSRN) (R13 HD 074468 - Michael Blank, PI).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hagan H, Des Jarlais DC, Friedman SR, et al. Reduced risk of hepatitis B and hepatitis C among injecting drug users participating in the Tacoma syringe exchange program. Am J Public Health. 1995;85:1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan E, Heimer R. HIV prevalence among intravenous drug users: model-based estimates from New Haven's legal needle exchange. J Acquir Immune Defic Syndr. 1992;5:163–169. [PubMed] [Google Scholar]
- 3.Des Jarlais DC, Marmor M, Paone D, et al. HIV incidence among injecting drug users in New York City syringe-exchange programmes. Lancet. 1996;348:987–991. [DOI] [PubMed] [Google Scholar]
- 4.Kerr T, Small W, Buchner C, et al. Syringe sharing and HIV incidence among injection drug users and increased access to sterile syringes. Am J Public Health. 2010;100:1449–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ksobiech K. A meta-analysis of needle sharing, lending, and borrowing behaviors of needle exchange program attenders. AIDS Educ Prev. 2003;15:257–268. [DOI] [PubMed] [Google Scholar]
- 6.Hurley SF, Jolley DJ, Kaldor JM. Effectiveness of needle exchange programmes for prevention of HIV infection. Lancet. 1997;21:1797–1800. [DOI] [PubMed] [Google Scholar]
- 7.Palmateer N, Kimber J, Hickman M, et al. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction. 2010;105:844–859. [DOI] [PubMed] [Google Scholar]
- 8.Wodak A, Cooney A. Do needle syringe programs reduce HIV infection among injecting drug users: a comprehensive review of the international evidence. Subst Use Misuse. 2006;41:777–816. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Preventing HIV Infection Among Injecting Drug Users in High-Risk Countries: An Assessment of the Evidence. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 10.PL 114-113: Consolidated Appropriations Act, 2016. Available at: https://www.congress.gov/114/plaws/publ113/PLAW-114publ113.pdf. Accessed June 8, 2016.
- 11.Allen ST, Ruiz MS, O'Rourke A. The evidence does not speak for itself: the role of research evidence in shaping policy change for the implementation of publicly funded syringe exchange programs in three US cities. Int J Drug Pol. 2015. 10.1016/j.drugpo.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennsylvania Drug Paraphernalia Act, 1980. P.L. 634 No. 186. Available at: http://www.palrb.us/pamphletlaws/19001999/1980/0/act/0186.pdf. Accessed June 8, 2016. [Google Scholar]
- 13.Maurer LA, Bass SB, Ye D, et al. Trend analyses of users of a syringe exchange program in Philadelphia, Pennsylvania: 1999-2014. AIDS Behav. 2016. 10.1007/s10461-016-1393-y. [DOI] [PubMed] [Google Scholar]
- 14.Executive order 4-92. Available at: http://www.phila.gov/ExecutiveOrders/Executive%20Orders/4-92.pdf. Accessed June 8, 2016.
- 15.Prevention Point Philadelphia website. Available at: http://ppponline.org. Accessed June 8, 2016.
- 16.Maryland General Assembly. Senate Bill 402: AIDS prevention sterile needle and syringe exchange pilot program. 1994. Available at: https://www.overdosepreventionstrategies.org/wp-content/uploads/2015/03/%C2%A7%C2%A724-801-to-%C2%A724-810-Aids-Prevention-Sterile-Needle-And-Syringe-Exchange-Pilot-Program.pdf. Accessed June 8, 2016. [Google Scholar]
- 17.Baltimore City Health Department. Community risk reduction: Baltimore city needle exchange program. Available at: http://health.baltimorecity.gov/hiv-std-services/community-risk-reduction. Accessed June 8, 2016.
- 18.Des Jarlais DC. Structural interventions to reduce HIV transmission among injecting drug users. AIDS. 2000;14(suppl 1):S41–S46. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz MS, O'Rourke A, Allen ST. Impact evaluation of a policy intervention for HIV prevention in Washington, DC. AIDS Behav. 2015. 10.1007/s10461-015-1143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann DP, Gottman JM, Jones RR, et al. Interrupted time series analyses and its application to behavioral data. J Appl Behav Anal. 1980;13:543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmour S, Degenhart L, Hall W, et al. Using intervention time series analyses to assess the effects of imperfectly identifiable natural events: a general method and example. BMC Med Res Tech. 2006;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baltimore City Annual HIV Epidemiological Profile 2013. Baltimore, MD: Center for HIV Surveillance, Epidemiology and Evaluation, Department of Health and Mental Hygiene; 2015. [Google Scholar]
- 23.Box GEP, Jenkins GM. Time Series Analysis: Forecasting and Control. San Francisco: Holden-Day; 1970. [Google Scholar]
- 24.Brady JE, Friedman SR, Cooper HLF, et al. Estimating the prevalence of injection drug users in the U.S. And in large U.S. Metropolitan areas from 1992 to 2002. J Urban Health 2008;85:323–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schackman BR, Fleishman JA, Su AE, et al. The lifetime medical cost savings from preventing HIV in the United States. Med Care. 2015;53:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Civil Liberties Union (ACLU). Needle exchange programs promote public safety: fact sheet on needle exchange programs. Available at: https://www.aclu.org/needle-exchange-programs-promote-public-safety. Accessed June 8, 2016.
- 27.Prevention point Philadelphia. Financial. Available at: http://ppponline.org/about/financial. Accessed June 8, 2016.
- 28.City of Baltimore. Open budget Baltimore. Available at: http://openbudget.baltimorecity.gov/#!/year/default. Accessed June 8, 2016.
- 29.Broadwater L. Baltimore wants to give out thousands more needles to drug users. Baltimore Sun. 2014. Available at: http://articles.baltimoresun.com/2014-01-17/news/bs-md-srb-legislative-agenda-20140117_1_clean-syringes-baltimore-county-addicts. Accessed June 8, 2016.


