Abstract
Purpose This is an official guideline, published and coordinated by the Gynecological Oncology Working Group (AGO) of the German Cancer Society (DKG) and the German Society for Gynecology and Obstetrics (DGGG). Vaginal cancers are rare tumors, which is why there is very little evidence on these tumors. Knowledge about the optimal clinical management is limited. This first German S2k guideline on vaginal cancer has aimed to compile the most current expert knowledge and offer new recommendations on the appropriate treatment as well as providing pointers about individually adapted therapies with lower morbidity rates than were previously generally available. The purpose of this guideline is also to set up a register to record data on treatment data and the course of disease as a means of obtaining evidence in future.
Methods The present S2k guideline was developed by members of the Vulvar und Vaginal Tumors Commission of the AGO in an independently moderated, structured, formal consensus process and the contents were agreed with the mandate holders of the participating scientific societies and organizations.
Recommendations To optimize the daily care of patients with vaginal cancer: 1. Monitor the spread pattern; 2. Follow the step-by-step diagnostic workup based on initial stage at detection; 3. As part of individualized clinical therapeutic management of vaginal cancer, follow the sentinel lymph node protocol described here, where possible; 4. Participate in the register study on vaginal cancer.
Key words: vaginal cancer, VaIN, sentinel lymph node biopsy
I Guideline Information
Guidelines program of the DGGG, OEGGG and SGGG
For information on the guidelines program, please refer to the end of the guideline.
Citation format
Diagnosis, Therapy and Follow-up of Vaginal Cancer and Its Precursors. Guideline of the DGGG and the DKG (S2k-Level, AWMF Registry No. 032/042, October 2018). Geburtsh Frauenheilk 2019: 79: 1060–1078
Guideline documents
The complete long version in German, the short version and a German-language PDF slide version for PowerPoint presentations are available on the homepage of the AWMF: https://www.awmf.org/leitlinien/detail/ll/032-042.html
Numbering
This text version of the guideline is a concise abridged version which has omitted generally applicable chapters (12 Supportive Therapy, 13 Psycho-oncology and Quality of Life, 14 Rehabilitation, 15 Integrative Medicine, 18 Palliative Medical Support). The numbering of the chapters after “IV Guideline” and the numbering of the recommendations and statements in this short version corresponds to the numbering used in the long version, despite the obvious gaps in numbering. This was done to make it easier to find the text in the long version if further information is desired.
Guideline group
The members of the guideline group are listed under “Authors”. The group is composed of authors and mandate holders. The latter are representatives of the medical societies, working groups, organizations, and associations who stated their interest in being involved in the compilation of the guideline text and participated in the consensus conference. The authors and mandate holders and their functions are listed in Table 1 .
Table 1 Guideline authors and mandate holders (listed alphabetically).
| Author, mandate holder | Function* | Group, body |
|---|---|---|
| * A = author, M = mandate holder | ||
| Ackermann, PD Dr. med. Sven | A | German Society of Gynecology and Obstetrics [Deutsche Gesellschaft für Gynäkologie und Geburtshilfe] (DGGG), German Cancer Society [Deutsche Krebsgesellschaft] (DKG), Gynecological Oncology Working Group [Arbeitsgemeinschaft Gynäkologische Onkologie] (AGO) |
| Alt-Radtke, Dr. med. Celine Desirée | A, M | German Roentgen Society [Deutsche Röntgengesellschaft] (DRG), Uroradiology Working Group [AG Uroradiologie] |
| Angleitner, Prof. Dr. Lukas | M | Austrian Society of Gynecology and Obstetrics [Österreichische Gesellschaft für Gynäkologie und Geburtshilfe] (OEGGG) |
| Barinoff, Dr. med. Jana | A | DGGG, DKG, AGO |
| Beckmann, Prof. Dr. med. Matthias W. | A | DGGG, DKG, German Cancer Aid [Deutsche Krebshilfe] (DKH), German Society for Senology [Deutsche Gesellschaft für Senologie] (DGS), AGO, Comprehensive Cancer Center Erlangen EMN (CCC ER-EMN) |
| Böing, Dr. med. Carsten | A | DGGG, DKG, AGO |
| Dannecker, Prof. Dr. med. Christian | A | DGGG, DKG, AGO |
| Fehm, Prof. Dr. med. Tanja | M | AGO Executive Board |
| Gaase, Dr. med. Rüdiger | M | Professional Association of Gynecologists [Berufsverband der Frauenärzte e. V.] (BVF) |
| Gaß, Dr. med. Paul | A | DGGG-Leitliniensekretariat |
| Gebhardt, Marion | M | Federal Association of Womenʼs Self-help Groups After Cancer [Bundesverband Frauenselbsthilfe nach Krebs e. V.] (FSH) |
| Gieseking, Dr. med. Friederike | A, M | DGGG, DKG, AGO, Cervical Pathology and Colposcopy Working Group [AG Cervix-Pathologie und Colposcopie] (AGCPC) |
| Günthert, Prof. Dr. med. Andreas | A | DGGG, DKG, AGO |
| Hack, PD Dr. med. Caroline C. | A, M | DGGG, DKG, AGO |
| Hampl, Prof. Dr. med. Monika | A, M | DGGG, DKG, AGO |
| Hantschmann, Dr. med. Peer | A | DGGG, DKG, AGO |
| Horn, Prof. Dr. med. Lars Christian | A, M | German Society of Pathology [Deutsche Gesellschaft für Pathologie] (DGP), DKG, AGO |
| Koch, Dr. med. Martin C. | A | DGGG, DKG, AGO |
| Letsch, Dr. med. Anne | A, M | German Society for Hematology and Medical Oncology [Deutsche Gesellschaft für Hämatologie und medizinische Onkologie] (DGHO), German Society for Palliative Medicine [Deutsche Gesellschaft für Palliativmedizin] (DGPm) |
| Mallmann, Prof. Dr. med. Peter | A, M | DGGG, DKG, AGO |
| Mangold, Dr. med. Bernhard | A, M | German Society for Cytology [Deutsche Gesellschaft für Zytologie] (DGZ) |
| Marnitz, Prof. Dr. med. Simone | A, M | German Society for Radio-oncology [Deutsche Gesellschaft für Radioonkologie] (DEGRO), DKG, AGO |
| Mehlhorn, PD Dr. med. Grit | A | DGGG, DKG, AGO |
| Paradies, Kerstin | M | Conference for Oncological Nursing Care and Nursing Care of Children [Konferenz onkologischer Kranken- und Kinderkrankenpflege e. V.] (KOK) |
| Reinhardt, Prof. Dr. med. Michael J. | M | German Society of Nuclear Medicine [Deutsche Gesellschaft für Nuklearmedizin e. V.] (DGN) |
| Schnürch, Prof. Dr. med. Hans-Georg | A, M | DGGG, DKG, AGO |
| Tholen, Reina | M | German Association for Physiotherapy [Deutscher Verband für Physiotherapie] |
| Torsten, PD Dr. med. Uwe | A | DGGG, DKG, AGO |
| Weikel, Prof. Dr. med. Wolfgang | A | DGGG, DKG, AGO |
| Wetzig, PD Dr. med. Tino | M | German Dermatological Society [Deutsche Dermatologische Gesellschaft] (DDG) |
| Wölber, Prof. Dr. med. Linn | A | DGGG, DKG, AGO |
| Zraik, Dr. med. Isabella | M | German Society for Urology [Deutsche Gesellschaft für Urologie] (DGU) |
Dr. M. Nothacker (an AWMF-certified guidelines advisor/moderator) kindly agreed to moderate the guideline. Dr. P. Gaß (DGGG Guidelines Office, Erlangen) contributed substantially to the compilation of the long and short versions of this guideline.
Abbreviations
- AG
working group [Arbeitsgemeinschaft]
- AG-CPC
Cervical Pathology and Colposcopy Working Group [Arbeitsgemeinschaft für Cervixpathologie und Colposkopie]
- AGO
Gynecological Oncology Working Group [Arbeitsgemeinschaft Gynäkologische Onkologie]
- AWMF
Association of the Scientific Medical Societies in Germany [Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften]
- CAM
complementary and alternative medicine
- CCC
comprehensive cancer center
- CIN
cervical intraepithelial neoplasia
- CT
computed tomography
- DHCD
data-sparing homogeneous cancer documentation
- DGGG
German Society of Gynecology and Obstetrics [Deutsche Gesellschaft für Gynäkologie und Geburtshilfe]
- DKG
German Cancer Society [Deutsche Krebsgesellschaft]
- EORTC
European Organisation for Research and Treatment of Cancer
- FDG-PET
fluorodeoxyglucose-positron emission tomography
- FIGO
Fédération Internationale de Gynécologie et dʼObstétrique
- H&E
hematoxylin and eosin
- HPV
human papillomaviruses
- HSIL
high-grade squamous intraepithelial lesion
- LDA
lymph drainage area
- LN
lymph node
- LNE
lymph node excision
- LN status
lymph node status
- LSIL
low grade squamous intraepithelial lesion
- MRI
magnetic resonance imaging
- NCCAM
National Center of Complementary and Alternative Medicine
- NCDB
National Cancer Data Base
- OEGGG
Austrian Society of Gynecology and Obstetrics [Österreichische Gesellschaft für Gynäkologie und Geburtshilfe]
- OL
Oncology Guidelines Program [Leitlinienprogramm Onkologie]
- PE
biopsy [Probe-Exzision]
- PET
positron emission tomography
- PNI status
perineural invasion status
- QI
quality indicator
- QLQ
quality of life questionnaire
- R1
microscopic residual disease
- R2
macroscopic residual disease
- RCTX
radio(chemo)therapy
- SGB
(German) Social Security Statute Book [Sozialgesetzbuch]
- SGGG
Swiss Society of Gynecology and Obstetrics [Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe]
- SLN
sentinel lymph node
- SLNB
sentinel lymph node biopsy
- SPECT
single-photon emission computed tomography
- TNM system
tumor-node-metastasis staging system
- UICC
Union internationale contre le cancer
- VaIN
vaginal intraepithelial neoplasia
- VIN
vulvar intraepithelial neoplasia
- V status
vessel status
- WHO
World Health Organization
- WLE
wide local excision
II Guideline Application
Purpose and objectives
Although vaginal cancer is a rare tumor entity, there is no summary of recent experience or list of appropriate recommendations for the medical staff treating these rare cases. This guideline completes the range of gynecological oncology guidelines of the DGGG and DKG. As radiotherapy also plays an important role in the treatment of vaginal cancer, the interdisciplinary compilation of this guideline ensures that all treatment modalities – surgery, radiotherapy and systemic therapy – were given appropriate consideration. This guideline aims to make the specialist knowledge about the diagnosis and treatment of vaginal cancer available to all treatment teams in the form of recommendations, statements and explanatory text.
Targeted areas of patient care
The guideline covers the full spectrum of diagnosis, treatment and follow-up of patients with vaginal cancer, including patients with microinvasive lesions and high-grade precursors. This guidelineʼs area of application is cross-sectional and includes both outpatient and inpatient care. All relevant scientific medical societies, self-help groups and professional associations in Germany were therefore involved in the compilation of this guideline.
Target user groups/target audience
This S2k guideline is aimed at all physicians and healthcare professionals involved in the outpatient and/or inpatient care of patients with vaginal cancer. This guideline also aims to serve as an important source of information for affected patients and their relatives.
Adoption and period of validity
The validity of this guideline was confirmed by the executive boards of the participating medical societies, working groups, organizations and associations as well as by the executive board of the DGGG, the DGGG guidelines commission and the DKG in October 2018 and was thus confirmed in its entirety. This guideline is valid from 1 October 2018 through to 30 September 2023. Because of the contents of this guideline, this period of validity is only an estimate.
III Method
The method used to prepare this guideline was determined by the class to which this guideline was assigned. The AWMF Guidance Manual (version 1.0) has set out the respective rules and requirements for different classes of guidelines. Guidelines are differentiated into lowest (S1), intermediate (S2) and highest (S3) class. The lowest class is defined as a set of recommendations for action compiled by a non-representative group of experts. In 2004, the S2 class was divided into two subclasses: a systematic evidence-based subclass (S2e) and a structural consensus-based subclass (S2k). The highest S3 class combines both approaches.
This guideline is classified as: S2k
Grading of recommendations
The grading of evidence and of recommendations is not envisaged for S2k-level guidelines. The individual Statements and Recommendations are differentiated by syntax, not by symbols ( Table 2 ).
Table 2 Grading of recommendations.
| Level of recommendation | Syntax |
|---|---|
| Strong recommendation, highly binding | must/must not |
| Simple recommendation, moderately binding | should/should not |
| Open recommendation, not binding | may/may not |
Statements
Scientific statements given in this guideline which do not consist of any direct recommendations for action but are simple statements of fact are referred to as “Statements”. It is not possible to provide any information about the grading of evidence for these Statements.
Achieving consensus and strength of consensus
As part of the structured process to achieve consensus (S2k/S3 level), authorized participants attending the consensus conference vote on draft Statements and Recommendations. This can lead to significant changes in the wording, etc. Finally, the extent of consensus is determined based on the number of participants who voted and the number of participants who voted in favor ( Table 3 ).
Table 3 Grading of strength of consensus.
| Symbol | Strength of consensus | Extent of agreement in percent |
|---|---|---|
| +++ | Strong consensus | > 95% of participants agree |
| ++ | Consensus | > 75 – 95% of participants agree |
| + | Majority agreement | > 50 – 75% of participants agree |
| – | No consensus | < 51% of participants agree |
Expert consensus
As the name already implies, this term refers particularly to consensus decisions on Recommendations/Statements where the decision was taken without a prior systematic search of the literature (S2k) or for which evidence is lacking (S2e/S3). The term “expert consensus” (EC) used here is synonymous with terms used in other guidelines such as “good clinical practice” (GCP) or “clinical consensus point” (CCP). The strength of the recommendation is graded as previously described in the chapter “Grading of recommendations”, i.e., purely semantically (“must”/“must not” or “should”/“should not” or “may”/“may not”) without the use of symbols.
Quality indicator
The QI working group of the Vulva and Vagina Commission has proposed that histological confirmation of the primary vaginal cancer prior to planning therapy in a tumor conference should be the only quality indicator for this rare entity.
IV Guideline
1 Epidemiology
Vaginal cancer is a rare tumor with only around 500 new cases reported annually in Germany 1 . The majority of all invasive vaginal cancers are squamous cell carcinomas (> 95%), followed by adenocarcinomas (< 5%) and melanomas (< 1%) 2 . The majority of clear cell adenocarcinomas are associated with maternal intake of the drug diethylstilbestrol (DES) during pregnancy 3 . Vaginal metastasis and recurrence and vaginal infiltration caused by direct expansion of an adjacent malignancy of the cervix, vulva, endometrium or rectum are more common than primary vaginal cancer, and primary vaginal carcinoma must be differentiated from all of these entities.
1.1 Age distribution
In more than 50% of cases, the age at onset of primary vaginal cancer is 70 years and above. Only 15% of these carcinomas are diagnosed in patients between the ages of 20 and 49 years 4 .
1.2 Survival rate
The absolute 5-year survival rate correlates with the histological subtype: it is 54% for squamous cell carcinoma, 60% for adenocarcinoma, and 13% for patients with malignant melanoma 4 . The 5-year survival rate also correlates with the tumor stage at primary diagnosis: it is 20% for stage IV.
1.3 Risk factors
| Consensus-based Statement 1.S1 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The risk factors for developing vaginal cancer are similar to those for cervical cancer. | |
| Consensus-based Statement 1.S2 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Infection with human high-risk papillomavirus (HR-HPV) is one of the most important risk factors. | |
| Consensus-based Statement 1.S3 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Vaginal cancer has been reported to develop in up to 5% of cases with VaIN. A VaIN 2 – 3/HSIL is a precancerous lesion. | |
The risk factors for developing squamous cell tumors of the vagina are the same as those for cervical cancer:
infection with high-risk human papillomavirus
previous treatment for preinvasive or invasive HPV-induced lesions
multiple sexual partners
early age at first intercourse
smoking 5
1.3.1 HPV
HPV DNA was detected in 74% of vaginal carcinomas and in 96% of VaIN 2/3 lesions. HPV 16 is the most commonly detected subtype both for VaIN and invasive tumors (59%).
1.3.3 VaIN
A vaginal intraepithelial neoplasia (VaIN) may be found sequentially or simultaneously with vaginal cancer. The precise incidence of VaIN ist not clear. It is often reported as 0.2 to 0.3 cases per 100 000 women and year 6 . There is a coincidence with other neoplasias of the lower anogenital tract 7 , 14 . The data show that long-term follow-up is necessary in all cases with VaIN. The rate of progression for VaIN developing into vaginal carcinoma is 2 – 5%. The same chapter in the long version of this guideline provides more details on the above data on the rate of progression.
2 Prevention and screening
2.1 Primary prevention
| Consensus-based Statement 2.S4 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Because of the heterogeneity of the data, it is not possible to provide a final statement on whether targeted sexual hygiene, which is recommended for other sexually transmissible diseases, has a preventative effect on vaginal neoplasia. | |
Based on the distribution of HPV types, up to 60% of all vaginal carcinomas could theoretically be prevented by HPV vaccination 8 . According to the current recommendations of the STIKO, HPV vaccination is also useful with respect to the primary prevention of vaginal cancer and its precursors. As smoking increases the risk of women who test positive for HPV (high-risk types) developing a VaIN 9 , women who smoke should always be provided with appropriate information in the context of preventing VaIN. The long version of this chapter includes details on the role of specific protective measures.
2.2 Screening (secondary prevention)
| Consensus-based Statement 2.S5 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The legally mandated screening program for cervical cancer offers the opportunity to diagnose vaginal cancers and their precursors at an early stage. | |
3 Healthcare structures
3.2 Treatment in oncology centers
| Consensus-based Recommendation 3.E1 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Patients with vaginal cancer should be treated by an interdisciplinary, interprofessional team. The team should have access to all necessary medical specialties and occupations in a cross-sectoral network. A certified center is most likely to be able to provide this extensive access. | |
3.2.2 Centers – interdisciplinary tumor board
| Consensus-based Recommendation 3.E2 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| All patients with vaginal cancer must be presented to an interdisciplinary tumor board. | |
4 Pathology
4.1 Classification of precancerous lesions ( Table 4 )
| Description | Condylomatous lesion | Low-grade dysplasia | Intermediate-grade dysplasia | High-grade dysplasia | Carcinoma in situ |
|---|---|---|---|---|---|
| * LAST = Lower Anogenital Squamous Terminology Standardization project of the College of American Pathologists and the American Society of Colposcopy and Cervical Pathology 13 . | |||||
| WHO (2003) | VaIN 1 | VaIN 2 | VaIN 3 | ||
| LAST project* | Low-grade squamous intraepithelial lesion (LSIL) | High-grade squamous intraepithelial lesion (HSIL) | |||
| WHO (2014) | Low-grade squamous intraepithelial lesion (LSIL) syn.: VaIN 1 |
High-grade squamous intraepithelial lesion (HSIL) syn.: VaIN 2 and VaIN 3 |
|||
4.2 Morphology of precancerous vaginal lesions
| Consensus-based Recommendation 4.E3 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The clinical terminology and morphological workup of a precancerous vaginal lesion (vaginal intraepithelial neoplasia; VaIN) must be based on the most recent valid version of the WHO classification. In addition to its classification as LSIL or HSIL, HPV-associated changes (e.g. condylomas) should be specified and the lesion should be graded (VaIN 1 to 3). | |
4.3 Vaginal Pagetʼs disease
| Consensus-based Recommendation 4.E4 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| If histological examination detects pagetoid changes in a vaginal biopsy specimen, locoregional cancer and vulvar Pagetʼs disease with vaginal involvement must be clinically excluded. | |
4.4 Morphology of malignant vaginal tumors
4.4.1 Tumor typing
| Consensus-based Recommendation 4.E5 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The histological classification of malignant vaginal tumors must be based on the most current version of the WHO classification. | |
4.4.2 Staging of vaginal carcinoma
Postoperative staging is done using the pTNM classification system 14 ; citing the FIGO stage 15 is optional (s. Chapter 5.7).
From a morphological standpoint, the WHO definition of vaginal cancer is problematic. According to this definition, squamous cell cancer of the vagina is defined as a vaginal carcinoma without a previous cervical or vulvar carcinoma in the 10 years before the current diagnosis 11 , 16 , 17 . Otherwise the vaginal cancer is considered to be a recurrence of a previously treated carcinoma. Equally problematic is the statement by FIGO that squamous cell cancer of the vagina with involvement of the cervix should be classified as a cervical carcinoma 17 .
As with cervical and vulvar carcinomas, the depth of invasion should be defined as the extent of stromal invasion measured from the epithelial-stromal border at the most superficial dermal papilla adjacent to invasion to the deepest point of invasion 13 , 14 , 18 . Lymph node infiltration which extends beyond the deepest point of invasion must not be included in the measured depth of invasion or the tumor thickness but must be classified as L1 13 , 14 , 18 . Tumor thickness is measured from the surface of the tumor or, in the case of (strongly) keratinized squamous cell carcinomas, from the granular cell layer to the deepest point of invasion 13 , 19 .
4.4.4 Evaluation of tissue specimens
| Consensus-based Recommendation 4.E6 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Biopsy specimens obtained from areas suspicious for VaIN must be processed and sliced into sections for further evaluation. | |
4.4.4.1 Diagnostic biopsies
| Consensus-based Recommendation 4.E7 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The biopsy findings report must provide information about the confirmation and type of VaIN, the presence of any dermatological disease, any virus-associated changes, and possible invasion. | |
4.4.4.2 Specimens after local excision, colpectomy, hysterocolpectomy and lymph node status
| Consensus-based Recommendation 4.E8 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Morphological processing of colpectomy specimens in vaginal cancer must be carried out in such a way that all therapeutic and prognostically relevant parameters can be evaluated. The findings report must use the most recent valid WHO classification of tumor types and the most current TNM classification for staging. | |
| Consensus-based Recommendation 4.E9 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
The report detailing the histological findings of (radical) colpectomy specimens resected for vaginal cancer must include the following information:
| |
| Consensus-based Recommendation 4.E10 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| All resected lymph nodes obtained by lymphadenectomy during the surgical treatment of vaginal cancer must be examined histologically. | |
| Consensus-based Recommendation 4.E11 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Lymph nodes up to a diameter of approx. 0.3 cm should be entirely embedded; larger lymph nodes should be cut in half along their longitudinal axis and also completely embedded. | |
According to the UICC and TNM classification systems, micrometastasis is defined as the histological confirmation of tumor cells measuring ≥ 0.2 mm and no larger than 0.2 cm in lymph nodes 20 , 21 . Tumor cells with a total length of < 0.2 mm are defined as isolated tumor cells in lymph nodes 20 , 21 . The detection of isolated tumor cells and evidence of micrometastasis are not relevant for the staging of vaginal cancer.
| Consensus-based Recommendation 4.E12 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
The report on the findings for lymphadenectomy specimens resected for vaginal cancer must include the following information:
| |
4.4.4.3 Sentinel lymph nodes
| Consensus-based Recommendation 4.E13 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| In cases with vaginal cancer, the sentinel lymph nodes must be completely embedded and cut into slides for examination. Sentinel lymph nodes which are found to be negative based on an examination of their morphology using H & E staining, must additionally be investigated by immunohistochemistry (ultrastaging). | |
4.4.5 Morphological prognostic factors
The prognostic contribution of individual morphological tumor characteristics is summarized in Table 5 .
Table 5 Prognostic factors for vaginal cancer.
| Name | Standard factor | Risk/prognostic factor | Relevant for treatment |
|---|---|---|---|
| Tumor stage | Yes | Yes | Yes |
| Lymph node status | Yes | Yes | Yes |
| Size of respective lymph node metastasis | Yes | Unclear | Yes |
| Number of inguinal lymph nodes with metastatic disease | Yes | Unclear | Yes |
| Extracapsular extension of inguinal lymph node metastasis | Yes | Unclear | Yes |
| Perineural infiltration (PNI status) | Yes | Unclear | No |
| Lymph node infiltration (LN status) | Yes | Unclear | No |
| Venous invasion (V status) | Yes | Unclear | No |
| Resection margins (residual tumor status; R classification) | Yes | Yes | Yes |
| Depth of invasion in mm | Yes | Unclear | No |
| Grading | Yes | Unclear | No |
| Three-dimensional tumor size in cm | Yes | Unclear | Yes |
| Location of the cancer in the vagina | Yes | Unclear | Yes (poss. surgical approach) |
| Macroscopic type of growth of the carcinoma | Yes | Unclear | No |
| Multifocal carcinoma | Yes | Unclear | Yes (surgical approach) |
| Peritumoral VaIN | Yes | Unclear | Yes (surgical approach) |
| Histological tumor type | Yes | Unclear | No (pure adenocarcinoma, poss. not radiosensitive) |
| HPV confirmation in the carcinoma | No | Unclear | No |
| Pattern of invasion | No | Unclear | No |
| Immunohistochemical ultrastaging of lymph nodes for metastasis | No | Unclear | Unclear |
| Molecular markers | No | Unclear | Unclear |
5 Diagnostic workup
5.2 Clinical workup
| Consensus-based Statement 5.S6 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The diagnostic workup of a suspicious vaginal lesion must consist of clinical, cytological and colposcopic examinations depending on the manifestation of the lesion. The lesion must be biopsied if the findings are suspicious. | |
5.3 Histological workup
All suspicious lesions must be examined histologically.
5.4 Pretreatment staging of a carcinoma
| Consensus-based Recommendation 5.E14 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
Prior to treatment of a confirmed carcinoma, the following examinations must be carried out:
| |
5.5 Diagnostic workup of advanced tumors
Because of the close proximity of neighboring organs and because surgical treatment often requires extensive excision to obtain healthy margins, the indications for exploration using endoscopy and imaging should be interpreted liberally.
For this reason, screening for distant metastasis in cases with large tumors must be done prior to starting treatment (see 5.6).
5.6 Imaging workup for vaginal cancer
5.6.1 Diagnostic workup of the primary tumor
| Consensus-based Statement 5.S7 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Pelvic MRI is the imaging modality of choice for the local staging of histologically confirmed vaginal carcinoma from FIGO stage II onwards. | |
| Consensus-based Recommendation 5.E15 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The current recommendations (protocol) for gynecological pelvic tumors must be followed when carrying out a functional pelvic MRI to achieve the best possible diagnostic accuracy. | |
| Consensus-based Statement 5.S8 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The value of FDG-PET/CT for the staging of vaginal cancer has not been confirmed in the primary setting. | |
5.6.2 Diagnostic workup of lymph nodes
| Consensus-based Statement 5.S9 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The amount of information which can be obtained from imaging procedures to assess pelvic lymphatic drainage in cases with primary vaginal carcinoma is higher with functional MRI than with CT or standard MRI. | |
5.6.3 Distant metastasis
| Consensus-based Recommendation 5.E16 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| For complete staging, a CT scan of the abdomen and chest using contrast medium should be carried out in cases with advanced vaginal carcinoma (≥ FIGO II) or if there is a reasonable suspicion of metastasis. | |
| Consensus-based Recommendation 5.E17 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Prior to any planned pelvic exenteration, PET/CT may be used in patients with primary vaginal cancer to exclude distant metastasis insofar as this is possible. | |
5.6.4 Imaging in radiation therapy
| Consensus-based Recommendation 5.E18 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Patients who are treated with percutaneous radiotherapy, combined radiochemotherapy or brachytherapy must have an MRI scan at the start of planning treatment and to monitor therapy; where possible, the scan should be a functional MRI scan. | |
5.6.5 Diagnostic workup for recurrence
| Consensus-based Recommendation 5.E19 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Patients with a suspicion of local recurrence of vaginal cancer should have a functional pelvic MRI scan to assess the extent of local spread in the pelvis. | |
| Consensus-based Recommendation 5.E20 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Prior to planning treatment, PET/CT may be indicated in patients with a suspicion of extensive recurrence of vaginal carcinoma extending beyond the pelvic area. | |
5.7 Staging
Staging is carried out using the FIGO and TNM classification systems ( Table 6 ). The findings at surgery together with the results of the histopathological examination of the surgical specimens will determine the staging.
| UICC | FIGO | Tumor spread |
|---|---|---|
|
1)
The FIGO classification does not envisage a stage 0 (Tis).
2) The presence of a bullous edema in the bladder mucosa is not enough to classify a tumor as T4. | ||
| TX | Primary tumor cannot be assessed due to lack of information | |
| T0 | No evidence of a primary tumor | |
| Tis | 1) | Carcinoma in situ, vaginal intraepithelial neoplasia (VaIN) grade 3 |
| T1a | I | Tumor is limited to the vagina and no larger than 2 cm, it has not spread (N0, M0) |
| T1b | I | Tumor is limited to the vagina and larger than 2 cm, it has not spread (N0, M0) |
| T2a | II | Tumor has infiltrated paravaginal tissue (paracolpium), is not larger than 2 cm, has not reached the pelvic wall, it has not spread (N0, M0) |
| T2b | II | Tumor has infiltrated paravaginal tissue (paracolpium), is larger than 2 cm but has not reached the pelvic wall, it has not spread (N0, M0) |
| T1 bis T3 N1 T3 N0 M0 |
III III |
Tumor is growing into the pelvic wall and/or the lower third of the vagina and/or blocks the flow of urine, which is causing kidney problems Tumor has spread into regional pelvic or inguinal LNs, no distant metastasis Tumor is growing into the pelvic wall and/or the lower third of the vagina and/or blocks the flow of urine Tumor has not spread (N0, M0) |
| T4 Any N M0 |
IVA |
Tumor has infiltrated the bladder mucosa and/or rectum and/or has spread beyond the pelvis
2)
Tumor may or may not have spread to regional LNs, no distant metastasis |
| Any T Any N M1 |
IVB | Tumor has spread to distant organs (incl. pelvic LN metastasis), may or may not be infiltrating adjacent structures Tumor may or may not have spread to regional LNs |
The pelvic lymph nodes (obturator, internal iliac, external iliac and other unspecified pelvic LNs) are the regional lymph nodes for tumors in the upper two-thirds of the vagina. The inguinofemoral LNs are the regional LNs for tumors in the lower third of the vagina.
7 Treatment of vaginal intraepithelial neoplasia (VaIN)
7.1 Local surgical therapy
| Consensus-based Statement 7.S10 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Before starting any superficial treatment for VaIN, one or more biopsies must be carried out to exclude invasive carcinoma where possible. If there is a cytological suspicion of invasion, destructive therapy must not be carried out and treatment must consist of excision instead. | |
Table 7 shows the optimal treatment approaches for high-grade VaIN according to their HPV status and vaginal location.
Table 7 Treatment options for high-grade VaIN depending on the location of the lesion.
| VaIN 2 – 3 (HSIL) |
Lower third | Intermediate third | Vaginal vault | Entire vagina |
|---|---|---|---|---|
| HPV-induced | Excision, PE/laser vaporization, local drug therapy | Excision, PE/laser vaporization, local drug therapy | Excision, PE/laser vaporization, upper colpectomy | Skinning resection of vaginal epithelium, colpectomy, local drug therapy |
| HPV-negative | Excision | Excision | Excision, upper colpectomy | Colpectomy, skinning resection of vaginal epithelium |
| Consensus-based Recommendation 7.E21 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The decision which of the available guideline-based treatment options for VaIN to apply must be made on an individual basis. | |
7.2 Local drug therapy
Local treatment may be used as an alternative to surgical treatment. This includes therapy with 5-FU or imiquimod (both are off-label uses). There are also reports of using brachytherapy.
The choice of treatment is based on the grade of the VaIN lesion ( Table 8 ).
Table 8 Recommendations for treatment according to VaIN grade.
| VaIN 1 (condylomatous lesion) Low-grade squamous intraepithelial lesion LGSIL, flat condyloma |
VaIN 2 – 3 High-grade intraepithelial neoplasia HGSIL |
|---|---|
| Regular monitoring, in exceptional cases destruction, excision, local application of imiquimod (off-label use) | Extensive PEs, followed by destruction or surgical removal (excision, skinning colpectomy, skinning excision, partial/total colpectomy) or radiotherapy |
7.3 Recurrence and progression rates
| Consensus-based Recommendation 7.E22 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Patients treated for VaIN must be offered regular follow-up examinations for the rest of their lives because the rate of recurrence for VaIN is high. | |
More information on the rates of recurrence and progression is available in the long version of the guideline.
8 Surgical treatment of invasive carcinoma
The majority of invasive vaginal cancers are treated with radio(chemo)therapy 8 , 23 , 24 , 25 . Local surgical treatment ranges from local excision to partial or total colpectomy to anterior and/or posterior exenteration 26 .
8.1 FIGO stage I
| Consensus-based Statement 8.S11 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Surgery may be used to treat FIGO stage I vaginal carcinoma. | |
| Consensus-based Recommendation 8.E23 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Circumscribed FIGO stage I tumors should be excised locally with tumor-free margins; the surgical treatment of larger tumors should consist of colpectomy or hysterectomy, if necessary. | |
8.2 FIGO stage II
| Consensus-based Statement 8.S12 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Radio(chemo)therapy is the standard therapy for tumor stages II to IV. Exenteration to treat stage IV vaginal carcinoma is an individual therapy decision. | |
8.3 Stage III/IV
The standard therapy consists of radio(chemo)therapy. In cases with infiltration through the wall into adjacent organs (bladder and/or rectum), anterior and/or posterior exenteration with creation of a neobladder and/or a colostomy is a surgical option which has to be decided on in the context of an individual therapy decision.
8.4 Plastic reconstruction
| Consensus-based Recommendation 8.E24 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Before undergoing colpectomy, the patient should be informed about the possibility of vaginal plastic reconstruction. | |
9 Surgery of lymph drainage areas
9.1 Introduction
To date, sentinel lymph node imaging has not played any significant role in the diagnosis and treatment of vaginal cancer. The lack of information about lymphatic drainage in vaginal cancer is why lymphatic imaging with sentinel node analysis is necessary. The current practice of treating all regional lymph node stations either surgically or with radiotherapy is probably overtreatment and could be reduced in individual cases with targeted imaging and biopsies of sentinel lymph nodes.
9.2 Indication
The issue whether to lymph node (LN) staging should be carried out in cases with vaginal carcinoma or not is a question which affects both patients for whom primary surgery is indicated and patients for whom primary radio(chemo)therapy is indicated. If surgical examination of the lymph node areas is not possible or the patient does not want it, then primary radio(chemo)therapy is indicated and should potentially also include the site of the primary tumor (see Chapter 10).
9.3 Lymphatic drainage from the vagina
| Consensus-based Statement 9.S13 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Because the correlation between tumor location and the direction of lymphatic flow is not clear due to extensive lymphatic anastomosis in the vagina and lesser pelvis, it is not possible in individual cases to clearly link the vaginal tumor site with the direction of lymphatic flow. | |
The literature on this issue is discussed in the long version of the guideline.
9.4 Vaginal cancer and risk of lymphatic metastasis
| Consensus-based Statement 9.S14 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| There are no evidence-based parameters which show a close correlation between vaginal cancer and the risk of lymphatic metastasis. As with vulvar cancer, an infiltration depth of > 1 mm may be an indication of an incipient risk of lymphatic metastasis. | |
9.5 Sentinel node procedures
9.5.1 Benefit of sentinel node procedures
Because of the lack of studies on the benefits of sentinel node procedures in vaginal cancer, the use of these procedures in vaginal cancer is still experimental. Studies of adjacent primary tumors of the uterine cervix and vulva provide some positive evidence for the use of sentinel node procedures in vaginal cancer. The flow chart in Fig. 1 shows the conventional and the experimental sequence for diagnosis and treatment; the sequence to be followed is described in Chapter 9.6.
Fig. 1.
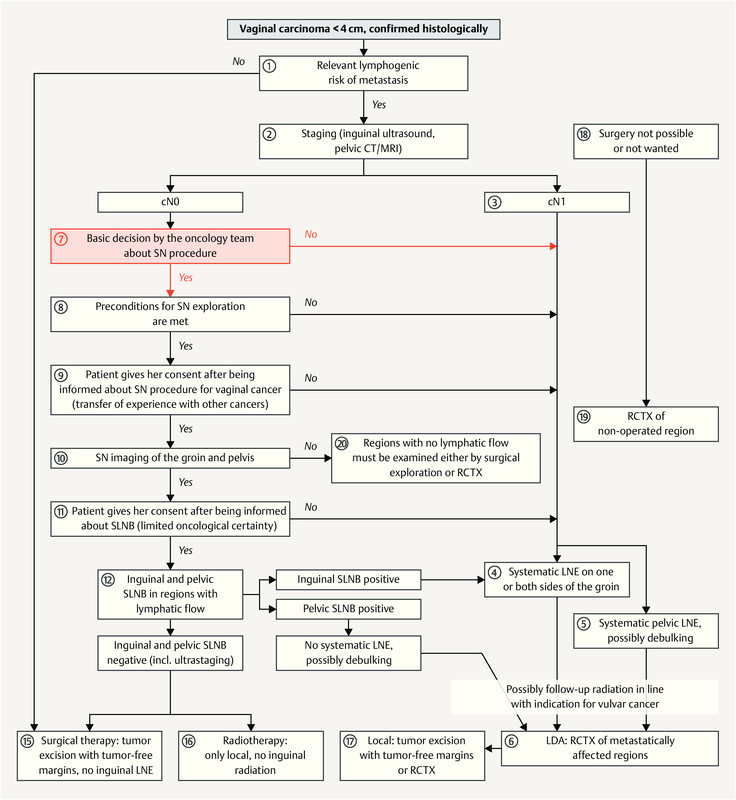
Flowchart for the diagnostic workup and treatment of vaginal carcinoma with the option of carrying out a sentinel LN procedure or not. [rerif]
9.5.2 Lymph node mapping using sentinel lymph node technique
| Consensus-based Statement 9.S15 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Sentinel lymph node mapping and tracing the lymphatic drainage pathway in cases with vaginal cancer is the precondition for carrying out SLNB and for planning any subsequent surgical and/or radiotherapeutic treatment. | |
| Consensus-based Recommendation 9.E25 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
The following preconditions must be adhered to when using the sentinel lymph node technique in cases with vaginal cancer:
| |
9.5.3 Validity of sentinel lymph node biopsy
| Consensus-based Recommendation 9.E26 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The sentinel lymph node technique may be used if the patient has consented after being informed in detail about the limited validity of the method in vaginal cancer because of the current lack of data. | |
| Consensus-based Statement 9.S16 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Because of the rarity of these tumors, no valid data on the diagnostic certainty of SLNB in vaginal cancer will be available in the foreseeable future. | |
Systematic inguinofemoral or pelvic lymphadenectomy or radio(chemo)therapy of the respective areas probably offers greater oncological safety but patients will have to accept a higher rate of side effects.
To evaluate the usefulness of sentinel lymph node biopsy in vaginal cancer, a new central register has been set up to collect treatment data and the course of disease of all patients with vaginal cancer in Germany (“knowledge-generating healthcare research” by the DKG). This national interdisciplinary vaginal carcinoma register is supported by the medical societies DGGG, AGO and DEGRO. All cases of vaginal cancer must be reported to the Radiotherapy Department of Cologne University (email: martina.illig@uk-koeln.de ). All hospitals with a tumor board which discusses and treats cases of vaginal carcinoma are requested to contact the registration office.
9.5.5 Planning primary radio(chemo)therapy
| Consensus-based Recommendation 9.E27 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| If SLN imaging is carried out preoperatively followed by intraoperative SLN biopsy, the findings should be taken into consideration when planning the radiation fields for primary radiotherapy. | |
Please refer to this chapter in the long version of the guideline to read how staging results from sentinel lymph node procedures can improve the situation.
9.6 Diagnosis and therapy with and without sentinel lymph node procedures
9.6.1 Process description of diagnostic workup and treatment
This chapter describes the recommended treatment approach for primary vaginal carcinoma with and without a sentinel lymph node procedure; the circled numbers correspond to the respective steps shown in Fig. 1 .
9.7 Systematic lymphanedectomy
Lymphatic drainage areas relevant for vaginal cancer include the pelvic, anorectal and inguinofemoral lymph nodes. In addition to these LNs, very advanced lymphogenic metastatic vaginal cancer may also affect the paraaortic LNs. As with SLN procedures, pretherapeutic systematic LNE in the above-mentioned lymphatic drainage areas may be useful to determine the extent of the required radiation for subsequent primary radio(chemo)therapy based on staging findings. Lymph node regions shown to have no metastatic involvement should not be irradiated.
If metastatic involvement is found to be present in pelvic lymph nodes on one side, it is important to ensure that both sides are treated with radiotherapy in accordance with the uniform approach used to treat pelvic lymph node metastasis from other primary tumors (carcinomas of the uterine cervix, prostate, endometrium, rectum, and anus), There are no data available on hemipelvis radiotherapy for vaginal cancer.
9.8 Surgical tumor reduction (debulking) prior to radio(chemo)therapy
In cases with advanced vaginal carcinoma and large circumscribed lymph node metastases in the groin or pelvis, the option to carry out preparatory surgical debulking as a means of improving the response to radio(chemo)therapy should be considered. The overall oncological situation, the patientʼs general operability, and local resectability as well as the expected morbidity following therapy must all be taken into consideration.
| Consensus-based Recommendation 9.E28 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Treatment planning for vaginal carcinoma should be based on the flowchart showing the diagnostic workup and treatment of vaginal carcinoma ( Fig. 1 ). | |
10 Radio(chemo)therapy for vaginal carcinoma
10.1 Introduction
Radiotherapy alone has been used since many decades to treat both local and advanced squamous cell carcinoma and adenocarcinoma of the vagina.
Depending on the tumor stage, radiotherapy alone can achieve high local control rates, ranging from 85 – 95% for FIGO stage I, to 70 – 80% for FIGO stage II and around 50 – 70% for FIGO stages III–IVA 27 , 28 , 29 , 30 , 31 .
Please refer to this chapter in the long version of the guideline for details on the dose-response relationship, the different radiotherapy techniques and the target volume definitions.
10.5 Radiotherapy of the tumor region
A second resection should be considered if the tumor was not resected in its entirety. If this is not feasible or if the expected functional results of this second resection are expected to be unsatisfactory, then local radiotherapy should be administered to improve local control.
| Consensus-based Recommendation 10.E29 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| In primary radio(chemo)therapy, the target volume should cover the primary tumor including the safety margin with a dose of > 70 Gy. | |
| Consensus-based Statement 10.S17 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| There is no evidence which specifies a minimal histological safety margin between the tumor and the edge of the resected specimen nor that resection margins below such a minimal histological safety margin represent an indication for administering radiotherapy to the tumor region postoperatively. | |
| Consensus-based Recommendation 10.E30 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Postoperative radiotherapy of the tumor region must be recommended after R1 and R2 resection if a second resection is not feasible. | |
10.6 Radiotherapy of the lymphatic drainage pathways
10.6.1 Inguinal lymph nodes
If inguinal lymph node metastasis has been confirmed histologically, the recommendations to administer postoperative radiotherapy will be based on the recommendations used to treat vulvar cancer. Radiotherapy planning should opt for modern proton therapy in preference to electron therapy.
10.6.2 Pelvic lymph nodes
Radiotherapy with bilateral radiation of the pelvic lymph drainage pathways is recommended if pelvic lymph node metastasis has been confirmed histologically. Even if metastasis is only confirmed on one side, radiotherapy should always be administered to both sides.
10.7 Radio(chemo)therapy
| Consensus-based Recommendation 10.E31 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Primary radiotherapy should also be offered in the form of simultaneous radiochemotherapy. | |
| Consensus-based Recommendation 10.E32 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The inguinofemoral and pelvic lymphatic drainage areas on both sides should also be irradiated if surgical lymph node staging was indicated but surgical staging was not carried out. | |
| Consensus-based Recommendation 10.E33 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The patientʼs comorbidities, own wishes, and clinical situation need to be considered before administering cisplatin ± 5-FU or mitomycin ± 5-FU. | |
| Consensus-based Statement 10.S18 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| In primary radiotherapy, achieving sufficiently high tumor doses is decisive for the success of therapy. Brachytherapy, possibly in combination with percutaneous radiotherapy, plays an important role in this context. | |
| Consensus-based Recommendation 10.E34 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| As with vulvar cancer, adjuvant radio(chemo)therapy after R1/R2 resection should be administered to the tumor area and the affected groin area if inguinal metastasis is present and bilaterally if there is pelvic metastasis. | |
The recommendation to administer simultaneous radiochemotherapy to treat vaginal cancer is a transfer of the experience with this approach in other tumors. Because of the rarity of vaginal carcinoma, we recommend that vaginal cancer should be treated in centers which deal with larger numbers of patients 32 , 33 , 34 .
10.9 Side effects of therapy, their prophylaxis and treatment
10.9.2 Late radiogenic side effects
Please refer to this chapter in the long version of the guideline for details on therapy-related toxicity, the choice of radiation technique and its impact on toxicity.
Symptomatic measures such as treatment for diarrhea, sitz baths, vaginal douching and the intravaginal application of a topical ointment (poss. using a tampon) to prevent adhesions are useful. Acute reactions are common but usually heal after the end of radiotherapy with no further effects. Important prophylactic measures against chronic consequences of scarring such as vaginal stenosis include the above-listed therapies applied consistently to treat acute reactions and the early and regular use of vaginal dilators, particularly in young and sexually active patients 35 , 36 , 37 , 38 .
10.9.3 Preservation of ovarian function and/or fertility
Young patients should be informed about the possibility of preserving ovarian function using ovariopexy with clip marking and the use of the latest radio-oncology techniques 39 , 40 , 41 , 42 , 43 . Please consult the German-language AWMF S2k guideline “Fertilitätserhalt bei onkologischen Erkrankungen” on preserving fertility in patients with oncologic disease for more information about indications and techniques 44 .
11 Systemic therapy
The current data on the use of systemic therapy to treat vaginal cancer is based on single case reports and small series which were analyzed retrospectively. Most therapeutic concepts were adapted from the treatment concepts used for cervical cancer.
11.2 Neoadjuvant chemotherapy
| Consensus-based Statement 11.S19 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The use of neoadjuvant chemotherapy to treat vaginal cancer is currently still an experimental concept. | |
11.3 Radio(chemo)therapy
| Consensus-based Statement 11.S20 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Using radio(chemo)therapy to treat vaginal cancer is analogous to using radio(chemo)therapy to treat cervical and vulvar cancer. | |
11.4 Chemotherapy in the palliative setting (recurrence, secondary metastasis, primary metastasizing vaginal carcinoma)
The references for the few available reports and studies on vaginal cancer are given in the long version of the guideline.
For the general chapters 12 Supportive Therapy, 13 Psycho-oncology and Quality of Life, 14 Rehabilitation and 15 Integrative Medicine , please refer to the long version of the guideline on the homepage of the AWMF: https://www.awmf.org/leitlinien/detail/ll/032-042.html
16 Follow-up
| Consensus-based Recommendation 16.E35 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| Patients who were treated for vaginal HSIL should have regular risk-adapted follow-up appointments for the rest of their lives. | |
| Consensus-based Recommendation 16.E36 | |||
|---|---|---|---|
| Expert consensus | Strength of consensus +++ | ||
| References: Adapted from Salani R et al. 2011 45 , Dannecker C et al. 2016 46 , Oonk MH et al. 2003 47 | |||
| Follow-up care of vaginal cancer should include the following routine examinations: | |||
| Examination | 1st–3rd year | 4th–5th year | From 6th year |
| Medical history | every 3 months | every 6 months | annually |
| Clinical examination | every 3 months | every 6 months | annually |
| Speculum examination, colposcopy (cervix/vagina/ vulva/anus), cytology | every 3 months | every 6 months | annually |
The long version of this guideline includes additional information on the clinical follow-up, the short and long-term impact of the disease and its therapy, and the effects of lifestyle choices and psychosexual and psychosocial follow-up care.
17 Therapy for locoregional recurrence and distant metastasis
Around 90% of recurrences occur within the first five years 8 , 48 , 49 . About 25% of all recurrences are local or locoregional 48 .
A palliative concept is used to treat distant metastasis; it is important to exclude any distant metastasis before deciding on the appropriate therapy to treat locoregional recurrence.
17.1 Diagnostic workup
The following diagnostic workup should be carried out if there is a suspicion of locoregional recurrence:
gynecological examination and histological examination
vaginal ultrasound imaging
pelvic MRI 48
cystoscopy and rectoscopy, exclusion of fistula
CT scan of the thorax and abdomen to exclude distant metastasis
It should be noted in this context that it is not uncommon for primary therapy to create long-term complications which can mimic recurrence, particularly fistula formation 49 .
17.2 Treatment of locoregional recurrence
| Consensus-based Statement 17.S21 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The decision to treat local or locoregional recurrence depends on the previous therapy, the extent of recurrence, and the patientʼs general condition. The decision is made an individual basis; treatment options include surgery, radiotherapy, radio(chemo)therapy and best supportive care. | |
| Consensus-based Recommendation 17.E37 | |
|---|---|
| Expert consensus | Strength of consensus +++ |
| The decision about the appropriate treatment approach for a patient with local or locoregional recurrence must be made by an interdisciplinary tumor board. | |
The long version of the guideline has drafted some cornerstones for treating recurrence which must be followed. The limited options to treat distant metastasis are explained in the long version.
To read the general chapter 18 Palliative Medical Care, please refer to the long version on the homepage of the AWMF: https://www.awmf.org/leitlinien/detail/ll/032-042.html
Footnotes
Conflict of Interest/Interessenkonflikt The conflicts of interests of the authors are listed in the long version of the guideline./Die Interessenkonflikte der Autoren sind in der Langfassung der Leitlinie aufgelistet.
Guideline Program. Editors.



Leading Professional Medical Associations
German Society of Gynecology and Obstetrics (Deutsche Gesellschaft für Gynäkologie und Geburtshilfe e. V. [DGGG]) Head Office of DGGG and Professional Societies Hausvogteiplatz 12, DE-10117 Berlin mailto:info@dggg.de http://www.dggg.de/
President of DGGG
Prof. Dr. med. Anton Scharl Direktor der Frauenkliniken Klinikum St. Marien Amberg Mariahilfbergweg 7, DE-92224 Amberg Kliniken Nordoberpfalz AG Söllnerstraße 16, DE-92637 Weiden
DGGG Guidelines Representatives
Prof. Dr. med. Matthias W. Beckmann Universitätsklinikum Erlangen, Frauenklinik Universitätsstraße 21–23, DE-91054 Erlangen
Prof. Dr. med. Erich-Franz Solomayer Universitätsklinikum des Saarlandes Geburtshilfe und Reproduktionsmedizin Kirrberger Straße, Gebäude 9, DE-66421 Homburg
Guidelines Coordination
Dr. med. Paul Gaß, Dr. med. Gregor Olmes, Christina Meixner Universitätsklinikum Erlangen, Frauenklinik Universitätsstraße 21–23, DE-91054 Erlangen fk-dggg-leitlinien@uk-erlangen.de http://www.dggg.de/leitlinienstellungnahmen
Austrian Society of Gynecology and Obstetrics (Österreichische Gesellschaft für Gynäkologie und Geburtshilfe [OEGGG])
Frankgasse 8, AT-1090 Wien stephanie.leutgeb@oeggg.at http://www.oeggg.at
President of OEGGG
Prof. Dr. med. Petra Kohlberger Universitätsklinik für Frauenheilkunde Wien Währinger Gürtel 18–20, AT-1090 Wien
OEGGG Guidelines Representatives
Prof. Dr. med. Karl Tamussino Universitätsklinik für Frauenheilkunde und Geburtshilfe Graz Auenbruggerplatz 14, AT-8036 Graz
Prof. Dr. med. Hanns Helmer Universitätsklinik für Frauenheilkunde Wien Währinger Gürtel 18–20, AT-1090 Wien
Swiss Society of Gynecology and Obstetrics (Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe [SGGG]) Gynécologie Suisse SGGG Altenbergstraße 29, Postfach 6, CH-3000 Bern 8 sekretariat@sggg.ch http://www.sggg.ch/
President in SGGG
Dr. med. Irène Dingeldein Längmatt 32, CH-3280 Murten
SGGG Guidelines Representatives
Prof. Dr. med. Daniel Surbek Universitätsklinik für Frauenheilkunde Geburtshilfe und feto-maternale Medizin Inselspital Bern Effingerstraße 102, CH-3010 Bern
Prof. Dr. med. René Hornung Kantonsspital St. Gallen, Frauenklinik Rorschacher Straße 95, CH-9007 St. Gallen
References/Literatur
- 1.Schubert-Fritschle G, Schlesinger-Raab A, Engel J. 2. Aufl. München: Zuckschwerdt Verlag; 2011. Malignome der Vulva und Vagina. [Google Scholar]
- 2.Lilic V, Lilic G, Filipovic S. Primary carcinoma of the vagina. J BUON. 2010;15:241–247. [PubMed] [Google Scholar]
- 3.Melnick S, Cole P, Anderson D. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. An update. N Engl J Med. 1987;316:514–516. doi: 10.1056/NEJM198702263160905. [DOI] [PubMed] [Google Scholar]
- 4.Ries L AG, Young J L, Keel G E, Eisner M P, Lin Y D, Horner M J. SEER Survival Monograph: Cancer Survival among Adults: U.S. SEER Program, 1988 – 2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD: NIH. 2007.
- 5.Madsen B S, Jensen H L, van den Brule A J. Risk factors for invasive squamous cell carcinoma of the vulva and vagina–population-based case-control study in Denmark. Int J Cancer. 2008;122:2827–2834. doi: 10.1002/ijc.23446. [DOI] [PubMed] [Google Scholar]
- 6.Henson D, Tarone R. An epidemiologic study of cancer of the cervix, vagina, and vulva based on the Third National Cancer Survey in the United States. Am J Obstet Gynecol. 1977;129:525–532. [PubMed] [Google Scholar]
- 7.Aho M, Vesterinen E, Meyer B. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195–197. doi: 10.1002/1097-0142(19910701)68:1<195::aid-cncr2820680135>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Gadducci A, Fabrini M G, Lanfredini N. Squamous cell carcinoma of the vagina: natural history, treatment modalities and prognostic factors. Crit Rev Oncol Hematol. 2015;93:211–224. doi: 10.1016/j.critrevonc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Strander B, Andersson-Ellstrom A, Milsom I. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: population based cohort study. BMJ. 2007;335:1077. doi: 10.1136/bmj.39363.471806.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn L C, Schierle K. Pathologie der Präkanzerosen und der Karzinome von Vulva und Vagina sowie morphologische Prognosefaktoren. Onkologe. 2009;15:15–27. [Google Scholar]
- 11.Ferenzcy A S, Colgan T J, Herrington C S, Hirschowitz L, Löning T, Park K J, Stoler M, Wells M, Wilbur D C, Wright T. Lyon: IARC Press; 2014. Epithelial Tumors of the Vagina; pp. 210–217. [Google Scholar]
- 12.Horn L C, Schierle K, Schmidt D. [Current TNM/FIGO classification for cervical and endometrial cancer as well as malignant mixed mullerian tumors. Facts and background] Pathologe. 2011;32:239–243. doi: 10.1007/s00292-010-1273-6. [DOI] [PubMed] [Google Scholar]
- 13.Darragh T M, Colgan T J, Thomas Cox J. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32:76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 14.Wittekind C. Weinheim: Wiley-VHC; 2017. TNM-Klassifikation maligner Tumoren. 8th ed; pp. 213–215. [Google Scholar]
- 15.Adams T S, Cuello M A. Cancer of the vagina. Int J Gynaecol Obstet. 2018;143 02:14–21. doi: 10.1002/ijgo.12610. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Matanoski G, Chen V W. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer. 2008;113:2873–2882. doi: 10.1002/cncr.23757. [DOI] [PubMed] [Google Scholar]
- 17.Kurman R J, Ronnett B M, Sherman M E, Wilkinson E J. Silver Spring: ARP Press; 2010. Tumors of the Vagina. Tumors of the Cervix, Vagina and Vulva; pp. 255–291. [Google Scholar]
- 18.Wilkinson E J, Rico M J, Pierson K K. Microinvasive carcinoma of the vulva. Int J Gynecol Pathol. 1982;1:29–39. doi: 10.1097/00004347-198201000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Crum C P, Herrington C S, McCluggage W G, Regauer S, Wilkinson E J. Lynon: IARC Press; 2014. Epithelial Tumors of the Vulva; pp. 233–242. [Google Scholar]
- 20.Hermanek P, Hutter R V, Sobin L H. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 21.Wittekind C, Compton C, Brirley J, Sobin L. London: Wiley-Blackwell; 2012. TNM Supplement: a Commentary on Uniform Use. [Google Scholar]
- 22.American Joint Committee on Cancer . New York: Spinger; 2017. Vagina; pp. 641–647. [Google Scholar]
- 23.Luo L M, Huang H F, Pan L Y. [Clinical analysis of 42 cases of primary malignant tumor in vagina] Zhonghua Fu Chan Ke Za Zhi. 2008;43:923–927. [PubMed] [Google Scholar]
- 24.Stock R G, Chen A S, Seski J. A 30-year experience in the management of primary carcinoma of the vagina: analysis of prognostic factors and treatment modalities. Gynecol Oncol. 1995;56:45–52. doi: 10.1006/gyno.1995.1008. [DOI] [PubMed] [Google Scholar]
- 25.Manetta A, Gutrecht E L, Berman M L. Primary invasive carcinoma of the vagina. Obstet Gynecol. 1990;76:639–642. [PubMed] [Google Scholar]
- 26.Tjalma W A, Monaghan J M, de Barros Lopes A. The role of surgery in invasive squamous carcinoma of the vagina. Gynecol Oncol. 2001;81:360–365. doi: 10.1006/gyno.2001.6171. [DOI] [PubMed] [Google Scholar]
- 27.Frank S J, Jhingran A, Levenback C. Definitive radiation therapy for squamous cell carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 2005;62:138–147. doi: 10.1016/j.ijrobp.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 28.de Crevoisier R, Sanfilippo N, Gerbaulet A. Exclusive radiotherapy for primary squamous cell carcinoma of the vagina. Radiother Oncol. 2007;85:362–370. doi: 10.1016/j.radonc.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Greenwalt J C, Amdur R J, Morris C G. Outcomes of Definitive Radiation Therapy for Primary Vaginal Carcinoma. Am J Clin Oncol. 2015;38:583–587. doi: 10.1097/COC.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 30.Lian J, Dundas G, Carlone M. Twenty-year review of radiotherapy for vaginal cancer: an institutional experience. Gynecol Oncol. 2008;111:298–306. doi: 10.1016/j.ygyno.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Jang W I, Wu H G, Ha S W. Definitive radiotherapy for treatment of primary vaginal cancer: effectiveness and prognostic factors. Int J Gynecol Cancer. 2012;22:521–527. doi: 10.1097/IGC.0b013e31823fd621. [DOI] [PubMed] [Google Scholar]
- 32.Smith G L, Jiang J, Giordano S H. Trends in the Quality of Treatment for Patients With Intact Cervical Cancer in the United States, 1999 Through 2011. Int J Radiat Oncol. 2015;92:260–267. doi: 10.1016/j.ijrobp.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Showalter T N, Camacho F, Cantrell L A. Determinants of Quality Care and Mortality for Patients With Locally Advanced Cervical Cancer in Virginia. Medicine (Baltimore) 2016;95:e2913. doi: 10.1097/MD.0000000000002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J F, Berger J L, Krivak T C. Impact of facility volume on therapy and survival for locally advanced cervical cancer. Gynecol Oncol. 2014;132:416–422. doi: 10.1016/j.ygyno.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Bakker R M, ter Kuile M M, Vermeer W M. Sexual rehabilitation after pelvic radiotherapy and vaginal dilator use: consensus using the Delphi method. Int J Gynecol Cancer. 2014;24:1499–1506. doi: 10.1097/IGC.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 36.Bakker R M, Mens J W, de Groot H E. A nurse-led sexual rehabilitation intervention after radiotherapy for gynecological cancer. Support Care Cancer. 2016 doi: 10.1007/s00520-016-3453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakker R M, Vermeer W M, Creutzberg C L. Qualitative accounts of patientsʼ determinants of vaginal dilator use after pelvic radiotherapy. J Sex Med. 2015;12:764–773. doi: 10.1111/jsm.12776. [DOI] [PubMed] [Google Scholar]
- 38.Lethaby A, Ayeleke R O, Roberts H.Local oestrogen for vaginal atrophy in postmenopausal women Cochrane Database Syst Rev 201608CD001500 10.1002/14651858.CD001500.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perri T, Ben-Baruch G, Davidson T. Use of Titanium Spiral Tacks for Long-term Oophoropexy Before Pelvic Irradiation. Int J Gynecol Cancer. 2014;24:1133–1136. doi: 10.1097/IGC.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 40.Han S S, Kim Y H, Lee S H. Underuse of ovarian transposition in reproductive-aged cancer patients treated by primary or adjuvant pelvic irradiation. J Obstet Gynaecol Res. 2011;37:825–829. doi: 10.1111/j.1447-0756.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- 41.Morice P, Castaigne D, Haie-Meder C. Laparoscopic ovarian transposition for pelvic malignancies: indications and functional outcomes. Fertil Steril. 1998;70:956–960. doi: 10.1016/s0015-0282(98)00284-2. [DOI] [PubMed] [Google Scholar]
- 42.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 43.Ghadjar P, Budach V, Kohler C. Modern radiation therapy and potential fertility preservation strategies in patients with cervical cancer undergoing chemoradiation. Radiat Oncol. 2015;10:50. doi: 10.1186/s13014-015-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DGGG; DGU BGRM Fertility preservation for patients with malignant disease. Guideline of the DGGG, DRU and BGRM (S2k – Level, AWMF Registry No. 015/082, November 2017). 2017Online:http://www.ammf.org/leitlinien/detail/II/015-082.htmllast access: 15.02.2018 [DOI] [PMC free article] [PubMed]
- 45.Salani R, Backes F J, Fung M F. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204:466–478. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Dannecker C, Strand V, Hegewisch-Becker S. Nachsorge beim Vulva- und Vaginalkarzinom. Der Onkologe. 2014;20:355–357. [Google Scholar]
- 47.Oonk M H, de Hullu J A, Hollema H. The value of routine follow-up in patients treated for carcinoma of the vulva. Cancer. 2003;98:2624–2629. doi: 10.1002/cncr.11837. [DOI] [PubMed] [Google Scholar]
- 48.Gardner C S, Sunil J, Klopp A H. Primary vaginal cancer: role of MRI in diagnosis, staging and treatment. Br J Radiol. 2015;88:2.0150033E7. doi: 10.1259/bjr.20150033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunderson C C, Nugent E K, Yunker A C. Vaginal cancer: the experience from 2 large academic centers during a 15-year period. J Low Genit Tract Dis. 2013;17:409–413. doi: 10.1097/LGT.0b013e3182800ee2. [DOI] [PubMed] [Google Scholar]