Abstract
NTM infection demonstrates an increasing incidence and prevalence. We studied the impact of NTM in cardiovascular events. Using the Korean nationwide database, we included newly diagnosed 1,730 NTM patients between 2005 and 2008 and followed up for new-onset atrial fibrillation (AF), myocardial infarction (MI), heart failure (HF), ischemic stroke (IS), and death. Covariates-matched non-NTM subjects (1:5, n = 8,650) were selected and analyzed. Also, NTM infection was classified into indolent or progressive NTM for risk stratification. During 4.16 ± 1.15 years of the follow-up period, AF, MI, HF, IS, and death were newly diagnosed in 87, 125, 121, 162, and 468 patients. In multivariate analysis, NTM group showed an increased risk of AF (hazard ratio [HR] 2.307, 95% confidence interval [CI] 1.560–3.412) and all-cause death (HR 1.751, 95% CI 1.412–2.172) compared to non-NTM subjects, whereas no significant difference in MI (HR 0.868, 95% CI 0.461–1.634), HF (HR 1.259, 95% CI 0.896–2.016), and IS (HR 1.429, 95% CI 0.981–2.080). After stratification, 1,730 NTM patients were stratified into 1,375 (79.5%) indolent NTM group and 355 (20.5%) progressive NTM group. Progressive NTM showed an increased risk of AF and mortality than indolent NTM group. Screening for AF and IS prevention would be appropriate in these high-risk patients.
Subject terms: Interventional cardiology, Risk factors
Introduction
Nontuberculous mycobacteria (NTM) are a widespread pathogen in the environment and consist of various species1. While NTM can cause lymphadenitis, skin infections, and catheter-related infections, pulmonary infection with NTM is the most common clinical presentation1–3. The worldwide incidence of pulmonary infection with NTM has significantly increased3–6, and the burden now exceeds that of Mycobacterium tuberculosis in some countries5,7. Also, NTM-related mortality and incidence rates are increasing8.
Cardiovascular (CV) events including myocardial infarction (MI), atrial fibrillation (AF), heart failure (HF), and ischemic stroke (IS) are associated with substantial morbidity and mortality worldwide9–12. Efforts have been made to identify and manage potential risk factors to improve clinical outcomes and reduce disease burden. Infection and inflammation are well-known causes of CV disease13–15, and various inflammatory diseases have shown an association with CV events16,17. In spite of the increasing importance of NTM infection, however, few studies have examined the relationship between NTM infection and CV events.
NTM infection can be either indolent or aggressively progressive1,18. Progressive NTM lung disease should be treated with medication while indolent NTM infection can be observed over time19. It is unclear whether this difference in severity is associated with the occurrence of CV events.
This study investigated the prognostic significance of NTM infection for CV events and all-cause mortality. We hypothesized that the association between NTM and CV events would be more prominent in patients with progressive NTM infection than in those with indolent NTM infection.
Results
Incidence and prevalence of NTM infection
The incidence of NTM infection in Korea consistently increased. Between 2005 and 2015, 24,413 patients were newly diagnosed with NTM infection. The annual incidence of NTM infection gradually increased and peaked in 2015 (20.884 per 100,000 person-years). The incidence was greater in females than in males, and in older groups than in younger counterparts for all study periods. Owing to the gradual increase in NTM incidence, the prevalence of NTM infection also consistently increased. The prevalence of NTM infection increased from 0.906 per 100,000 in 2005 to 26.483 per 100,000 in 2015. When analyzed according to sex and age group, NTM prevalence increased in both sexes, with a female preponderance, and across all age groups.
Characteristics of the study population
During the enrolment period, 1,730 patients without a previous history of NTM, MI, AF, or HF were newly diagnosed with NTM infection. Among patients with NTM, 1,375 (79.5%) had indolent infection and 355 (20.5%) had progressive infection. The characteristics of the baseline population according to NTM infection are shown in Supplemental Table 1. The mean age in the NTM infection group was 52.8 ± 14.4 years, and 628 (36.3%) were males. Among NTM patients, 7.2% had diabetes mellitus, 25.3% had hypertension, and 13.3% had dyslipidemia; the differences were not significant between those with and without NTM infection. However, chronic obstructive lung disease (30.5% vs. 10.5%, P < 0.001) and peripheral artery disease (9.8% vs. 5.7%, P < 0.001) were more frequently observed in those with NTM infection than in those without infection.
After 1:5 matching, 8,650 subjects were classified in the non-NTM infection group. As shown in Table 1, all clinical variables were well balanced between the non-NTM and NTM group after matching. Successful matching was validated by estimating standardized differences in baseline characteristics.
Table 1.
Baseline characteristics of the matched cohort.
| Without NTM infection (n = 8,650) |
NTM infection (n = 1,730) |
p-value | Absolute standardized difference | |
|---|---|---|---|---|
| Demographic data | ||||
| Age | 52.8 ± 14.4 | 52.8 ± 14.4 | 1.000 | <0.001 |
| 20–39 | 1,535 (17.8) | 307 (17.8) | 1.000 | <0.001 |
| 40–59 | 4,015 (46.4) | 803 (46.4) | ||
| 60- | 3,100 (35.8) | 620 (35.8) | ||
| Male | 3,140 (36.3) | 628 (36.3) | ||
| Past medical history | ||||
| Diabetes mellitus | 625 (7.2) | 125 (7.2) | 1.000 | <0.001 |
| Hypertension | 2,185 (25.3) | 437 (25.3) | 1.000 | <0.001 |
| Dyslipidemia | 1,150 (13.3) | 230 (13.3) | 1.000 | <0.001 |
| Chronic obstructive pulmonary disease | 2,635 (30.5) | 527 (30.5) | 1.000 | <0.001 |
| Peripheral artery disease | 850 (9.8) | 170 (9.8) | 1.000 | <0.001 |
| End stage renal disease | 10 (0.1) | 2 (0.1) | 1.000 | <0.001 |
| Social history | ||||
| Low income level | 1,615 (18.7) | 323 (18.7) | 1.000 | <0.001 |
| Smoking history | 1.000 | <0.001 | ||
| Never smoking | 6,930 (80.1) | 1,386 (80.1) | ||
| Ex-smoking | 700 (8.1) | 140 (8.1) | ||
| Current smoking | 1,020 (11.8) | 204 (11.8) | ||
| Follow-up | ||||
| Duration of follow-up (year) | 4.1 ± 1.2 | 3.6 ± 1.2 | <0.001 | 0.407 |
NTM, nontuberculous mycobacteria.
Association between NTM infection and CV events
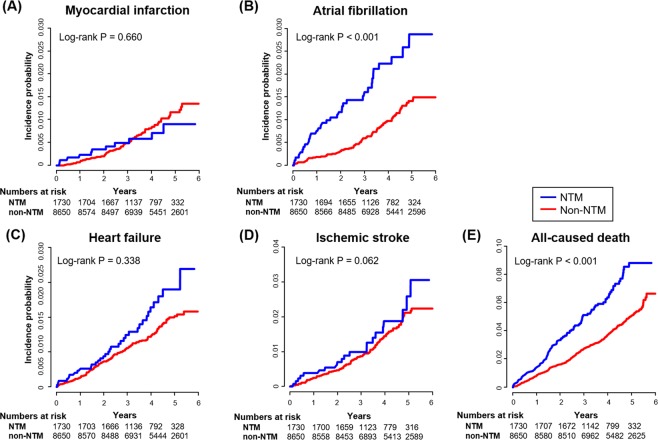
In Kaplan-Meier analysis, increases in AF and all-cause death (both, log-rank test P < 0.001) were frequently observed in patients with NTM. However, there was no significant difference in the incidence of MI (log-rank P = 0.660), and HF (log-rank P = 0.338) (Fig. 1A–D).
Figure 1.
Cardiovascular outcomes according to NTM infection. The Kaplan-Meier survival curves for myocardial infarction, atrial fibrillation, heart failure, ischemic stroke, and all-cause death are presented. Patients with NTM infection showed a higher risk of atrial fibrillation and mortality than those without infection, but not myocardial infarction or heart failure. NTM, nontuberculous mycobacteria.
During 4.16 ± 1.15 years of follow-up, 11 cases of MI (IR 1.76 per 1,000 person-years), 35 cases of AF (IR 5.65 per 1,000 person-years), 21 cases of HF (IR 3.36 per 1,000 person-years), 34 cases of IS (IR 5.49 per 1,000 person-years), and 108 cases of death from any cause (IR 17.24 per 1,000 person-years) were observed in the NTM group. In contrast, 76 cases of MI (IR 2.14 per 1,000 person-years), 90 cases of AF (IR 2.54 per 1,000 person-years), 100 cases of HF (IR 2.82 per 1,000 person-years), 138 cases of IS (IR 3.91 per 1,000 person-years), and 360 cases of death from any cause (IR 10.13 per 1,000 person-years) were observed in the non-NTM group. Table 2 shows the number of endpoints, estimated IRs, and HR for CV events. In matched cohorts, the NTM infection group showed an increased risk of AF (HR 2.307, 95% CI 1.560–3.412, P < 0.001), and all-cause death (HR 1.751, 95% CI 1.412–2.172), while statistical significance was not observed for differences in MI (HR 0.868, 95% CI 0.461–1.634), HF (HR 1.259, 95% CI 0.896–2.016), and IS (HR 1.429, 95% CI 0.981–2.080). Supplemental Table 2 shows multivariate-adjusted analyses for the baseline population, and the results are consistent with those in Table 2.
Table 2.
Risk of cardiovascular events according to NTM infection in the matched cohort.
| NTM infection | Number | Events | Duration | Incidence rate* | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Myocardial infarction | ||||||
| No | 8,650 | 76 | 35,453 | 2.144 | 1 (reference) | 0.661 |
| Yes | 1,730 | 11 | 6,250 | 1.760 | 0.868 (0.461–1.634) | |
| Atrial fibrillation | ||||||
| No | 8,650 | 90 | 35,415 | 1.609 | 1 (reference) | <0.001 |
| Yes | 1,730 | 35 | 6,197 | 5.648 | 2.307 (1.560–3.412) | |
| Heart failure | ||||||
| No | 8,650 | 100 | 35,429 | 1.582 | 1 (reference) | 0.339 |
| Yes | 1,730 | 21 | 6,240 | 3.365 | 1.259 (0.896–2.016) | |
| Ischemic stroke | ||||||
| No | 8,650 | 138 | 35,290 | 3.910 | 1 (reference) | 0.063 |
| Yes | 1,730 | 24 | 6,195 | 5.488 | 1.429 (0.981–2.080) | |
| Death | ||||||
| No | 8,650 | 360 | 35,550 | 4.968 | 1 (reference) | <0.001 |
| Yes | 1,730 | 108 | 6,266 | 17.235 | 1.751 (1.412–2.172) | |
CI, confidence interval; HR, hazard ratio; NTM, nontuberculous mycobacteria.
*Incidence rates were calculated per 1,000 patient-years from matched cohort.
Risk of CV events according to the severity of NTM infection
We also investigated the risk of CV events according to infection severity stratification. The demographic features of non-NTM, indolent NTM, and progressive NTM groups are shown in Supplemental Table 3. Patients in the progressive NTM group were older and more likely to have a previous history of chronic obstructive pulmonary artery disease and peripheral artery disease. The prevalence of diabetes mellitus, hypertension, and dyslipidemia were not different across groups.
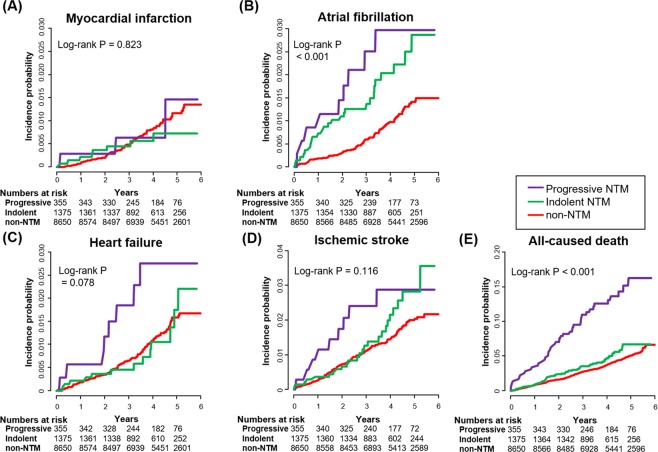
In Kaplan-Meier analysis (Fig. 2A–E), indolent and progressive NTM both showed an increased risk of AF (log-rank P < 0.001, respectively) and death (log-rank P < 0.001, respectively). Interestingly, the progressive NTM group showed a higher risk of mortality than the indolent NTM group (log-rank P = 0.003). The progressive NTM group showed a nonsignificant trend toward increased risk of HF and IS compared to that in the indolent NTM or control (log-rank P = 0.078 and log-rank P = 0.116) groups. However, there was no association between indolent or progressive NTM and the risk of MI. These results were also observed when analyzed with Cox regression models, as shown in Supplemental Table 4. After adjusting for covariates in the baseline population, the indolent and progressive NTM groups demonstrated significant associations with AF (HR 2.207, 95% CI 1.502–3.241 and HR 2.217, 95% CI 1.154–4.261, respectively) and all-cause death (HR 3.248, 95% CI 2.433–4.336 and HR 1.486, 95% CI 1.159–1.906), but not with MI or HF.
Figure 2.
Cardiovascular outcomes according to indolent and progressive NTM infection. Kaplan-Meier survival analyses were performed to evaluate the prognostic impact on myocardial infarction, atrial fibrillation, heart failure, ischemic stroke, and all-cause death according to indolent and progressive NTM infection. NTM, nontuberculous mycobacteria.
Subgroup analysis
We performed a subgroup analysis based on CV risk factors: age, sex, diabetes mellitus, hypertension, dyslipidemia, chronic obstructive pulmonary disease, income level, and smoking history. Analysis of those with NTM infection demonstrated higher risks of AF and mortality in most of the exploratory subgroups and similar risks of MI, HF, and IS (Supplemental Fig. A–E). There was no observed significant interaction between subgroups of patients.
Discussion
The major findings of our study were as follows: (1) the annual incidence and prevalence of NTM infection gradually increased between 2005 and 2015; (2) those with NTM infection showed an increased risk of AF and death but not MI, HF, and IS, compared to those without NTM infection; (3) about 20% of NTM patients were classified as having progressive NTM, which requires antibiotic treatment; and (4) the progressive NTM group showed an increased risk of AF and death compared to that in the indolent NTM group, but there was no significant difference in the risk of MI, HF, and IS between the groups.
Although we did not explore the mechanism of the relationship between infection and CV events, there are several possible explanations for the increased risk in patients with NTM. Previous studies reported that CV events were significantly associated with various infectious or inflammatory disease such as pneumonia and psoriasis13–17,20–22. Inflammatory cytokines such as C-reactive protein, interleukin-6, and tumor necrosis factor-alpha (TNF-α) showed significant associations with AF23. Cytokines and proinflammatory factors also have an impact on the pathophysiology of HF24. NTM infection causes chronic pulmonary inflammation, which is difficult to eradicate and requires long-term and sometimes life-long treatment1,25. NTM infection may induce changes in inflammatory cells and cytokines that sustain prolonged inflammation26,27 and may be associated with an increased risk of CV events.
Additionally, some reports indicated that patients with NTM infection had combined risk factors such as old age, smoking, and chronic obstructive lung disease1,4. Given that these are conventional CV risk factors that have consistently shown prognostic value, we could postulate that NTM patients might have a higher risk of CV events. Interestingly, NTM infection showed a significant association with AF, while there was no association between NTM infection and MI, and only a marginal association between NTM infection, HF, and IS. Prognostic differences among CV diseases should be investigated in future studies.
In this study, we not only investigated the association between NTM and CV events but also stratified NTM infection according to severity. As NTM is characterized by a variety of manifestations, we postulated prognostic differences according to the severity of NTM infection and found that progressive NTM infection had a stronger association with CV events than indolent NTM infection.
The prevalence and incidence of NTM infection have surged in both Western and Asian populations3–6,28. As urbanization and piped water have been suggested as causes for this global epidemiologic trend, it is conceivable that NTM infection will become an important theme in public health25,29,30. Because of the high social burden of CV events in spite of recent advances in treatment9, there is a need to identify high-risk subjects and to perform tailored management. However, there is a paucity of data on prognostic associations between NTM infection and CV events. To the best of our knowledge, this study is the first to report that NTM patients – especially those with progressive NTM – had an increased risk of CV events.
Our study had several strengths. First, we performed this analysis using a large, nationwide cohort. Therefore, we had access to many NTM patient records and a follow-up duration adequate for investigation of discrepancies in CV prognosis. Second, because of the variety of clinical manifestations, we further analyzed the association between NTM infection and CV events after stratification according to NTM severity.
Our study had several limitations. First, this was an observational study, and there may have been unmeasured confounders that we did not adjust for. Second, as we used diagnostic codes from claims data, our results might be skewed due to misclassification or underestimation bias. However, the insurance database has been well managed under national supervision. Also, we performed a validation test, which showed a 98.6% positive predictive value. Third, sophisticated research is needed to explain the mechanism of the association between NTM infection and the increased risk of CV events. Fourth, there might be a bias that patients with NTM might visit medical institutes more frequently which could cause increased diagnosis of AF. However, patients with AF usually have significant symptoms, and those symptomatic patients would be finally diagnosed with AF irrespective of routine check-up for other complaints. In addition, 12-lead ECG is not routinely recommended in patients with NTM infection. At last, the risks of CV and non-CV deaths were not presented because the cause of death was not available in our database. In accordance with previous studies based on real-world databases, we only report the results of all-cause deaths as one of the end points31,32.
Conclusions
Using the Korean nationwide health insurance database for this cohort study, we found that NTM infection was associated with AF and mortality. We also found that progressive NTM infection was strongly associated with increased CV risks. These findings suggest the potential benefit of CV surveillance in NTM patients.
Methods
Data source and database contents
The Korean National Health Insurance System (NHIS) covers health care services for the entire Korean population of approximately 50 million. The NHIS sponsors a program of regular check-ups for the public at least biennially. Details on the NHIS system have been reported elsewhere33. The NHIS maintains claims, health check-up, qualification, and death information databases. This study analyzed information from the claims, health check-up, and death information databases, and was conducted according to the Declaration of Helsinki and approved by the Seoul National University Hospital Institutional Review Board (IRB No. 1801-005-911). The institutional review board waived the need for a written informed consent.
Establishment of the study cohort
We initially recruited 17,750,973 Koreans who underwent a baseline health check-up through the NHIS between 2005 and 2008 (Fig. 3). Of these, 17,721,976 were older than 20 years. From this population, we excluded 197 who were diagnosed with NTM infection before the initial health check-up. We then excluded those with a history of more than 1 episode of MI, AF, HF, or IS within the first 5 years after the initial health check-up after the inception of the database, leaving 16,455,469 subjects in the baseline cohort. This policy guaranteed an adequate washout period for previously diagnosed clinical endpoints. Finally, 1,730 patients newly diagnosed with NTM infection were identified during the 4 years of the enrolment period.
Figure 3.
Study flow chart. NTM, nontuberculous mycobacteria.
Using the baseline cohort, we performed 1:5 matching to adjust for possible confounders, as discrepancies in baseline characteristics could contribute to CV events and biased results. Variables that could affect clinical outcomes included: age, sex, previous history of diabetes mellitus, hypertension, dyslipidemia, chronic obstructive pulmonary disease, peripheral artery disease, end-stage renal disease, income, and smoking history. Matching was conducted for a comparison between patients with and without NTM infection.
Definition of NTM infection, CV events, and other variables
NTM infection was defined using the ICD-10 codes (A318) registered in the NHIS database by the physician responsible for the treatment. To evaluate the accuracy of our definition of NTM infection, we performed a validation study in our institution for 295 adult patients with ICD-10 code A318. By reviewing the comprehensive medical record, the positive predictive value was found to be 98.6%. Patients with progressive NTM infection were defined as those with both NTM diagnosis and anti-NTM antibiotic treatment, and other patients were defined as those with indolent NTM infection (Supplemental Table 5).
Clinical endpoints were defined as the occurrence of CV events including MI, AF, HF, IS, or death during follow-up. The definition of each CV event was described in our previous reports, and Supplemental Table 5 17,34. Comorbidities such as hypertension, diabetes mellitus, dyslipidemia, chronic obstructive lung disease, peripheral artery disease, and end-stage renal disease were also defined using ICD-10 codes with additional information (Supplemental Table 5). Smoking history was obtained by questionnaire.
Statistical analysis
Data are presented as numbers and relative frequencies for categorical variables and as a mean ± standard deviation for continuous variables. For comparisons between groups, the chi-square test (or Fisher’s exact test when any expected cell count was <5 for a 2 × 2 table) was adopted for categorical variables, and an unpaired Student’s t-test was performed for continuous variables. The temporal trend of the outcomes was studied by using Kaplan-Meier estimates and compared according to NTM infection. Cox proportional hazard models were used to estimate the hazard ratio (HR) and corresponding 95% confidence interval (CI) for the association between CV endpoints and NTM infection. Each CV event was followed and performed analysis independently except death.
Two-sided P-values < 0.05 were considered statistically significant. Statistical tests were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R programming version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
Supplementary information
Acknowledgements
All authors received no financial support for the research, authorship, and/or publication of this manuscript.
Author contributions
C.S.P., E.-K.C., S.-R.L., M.-J.C. and S.O. contributed to the conception of the study. C.S.P., E.-K.C., B.K., K.-D.H., S.-R.L., M.-J.C. and S.O. contributed to the study design. C.S.P., E.-K.C., B.K. and K.-D.H. contributed to the data acquision and analysis of the work. C.S.P., E.-K.C., S.-R.L., M.-J.C. and S.O. contributed to interpretation of data for the work. C.S.P., E.-K.C. drafted the manuscript, and the rest of authors critically revised it. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-51801-w.
References
- 1.Griffith DE, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Saleeb P, Olivier KN. Pulmonary nontuberculous mycobacterial disease: new insights into risk factors for susceptibility, epidemiology, and approaches to management in immunocompetent and immunocompromised patients. Curr Infect Dis Rep. 2010;12:198–203. doi: 10.1007/s11908-010-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim N, Yi J, Chang CL. Recovery Rates of Non-Tuberculous Mycobacteria from Clinical Specimens Are Increasing in Korean Tertiary-Care Hospitals. J Korean Med Sci. 2017;32:1263–1267. doi: 10.3346/jkms.2017.32.8.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C, et al. Increasing Prevalence of Nontuberculous Mycobacteria in Respiratory Specimens from US-Affiliated Pacific Island Jurisdictions(1) Emerg Infect Dis. 2018;24:485–491. doi: 10.3201/eid2403.171301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien JY, Lai CC, Sheng WH, Yu CJ, Hsueh PR. Pulmonary infection and colonization with nontuberculous mycobacteria, Taiwan, 2000–2012. Emerg Infect Dis. 2014;20:1382–1385. doi: 10.3201/eid2008.131673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namkoong H, et al. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan(1) Emerg Infect Dis. 2016;22:1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iseman MD, Marras TK. The importance of nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2008;178:999–1000. doi: 10.1164/rccm.200808-1258ED. [DOI] [PubMed] [Google Scholar]
- 8.Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999-2010: a population-based comparative study. PLoS One. 2014;9:e91879. doi: 10.1371/journal.pone.0091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin EJ, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 10.Son MK, Lim NK, Cho MC, Park HY. Incidence and Risk Factors for Atrial Fibrillation in Korea: the National Health Insurance Service Database (2002–2010). Korean. Circ J. 2016;46:515–521. doi: 10.4070/kcj.2016.46.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SR, Choi EK, Han K, Cha MJ, Oh S. Prevalence of Non-valvular Atrial Fibrillation Based on Geographical Distribution and Socioeconomic Status in the Entire Korean Population. Korean Circ J. 2018;48:622–634. doi: 10.4070/kcj.2017.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, et al. The Trends of Atrial Fibrillation-Related Hospital Visit and Cost, Treatment Pattern and Mortality in Korea: 10-Year Nationwide Sample Cohort Data. Korean. Circ J. 2017;47:56–64. doi: 10.4070/kcj.2016.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieminen MS, Mattila K, Valtonen V. Infection and inflammation as risk factors for myocardial infarction. Eur Heart J. 1993;14(Suppl K):12–16. [PubMed] [Google Scholar]
- 14.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 15.Briasoulis A, Androulakis E, Christophides T, Tousoulis D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev. 2016;21:169–176. doi: 10.1007/s10741-016-9533-z. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 17.Rhee TM, et al. Increased Risk of Atrial Fibrillation and Thromboembolism in Patients with Severe Psoriasis: a Nationwide Population-based Study. Sci Rep. 2017;7:9973. doi: 10.1038/s41598-017-10556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook JL. Nontuberculous mycobacteria: opportunistic environmental pathogens for predisposed hosts. Br Med Bull. 2010;96:45–59. doi: 10.1093/bmb/ldq035. [DOI] [PubMed] [Google Scholar]
- 19.Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108:417–425. doi: 10.1016/j.rmed.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Smeeth L, et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 21.Cha MJ, et al. Increased Risk of Atrial Fibrillation in the Early Period after Herpes Zoster Infection: a Nationwide Population-based Case-control Study. J Korean Med Sci. 2018;33:e160. doi: 10.3346/jkms.2018.33.e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon I, et al. Ankylosing spondylitis: A novel risk factor for atrial fibrillation - A nationwide population-based study. Int J Cardiol. 2019;275:77–82. doi: 10.1016/j.ijcard.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Sata N, et al. C-reactive protein and atrial fibrillation. Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J. 2004;45:441–445. doi: 10.1536/jhj.45.441. [DOI] [PubMed] [Google Scholar]
- 24.Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 25.Thomson RM, Centre, N.T.M.w.g.a.Q.T.C. & Queensland Mycobacterial Reference, L. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16:1576–1583. doi: 10.3201/eid1610.091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helguera-Repetto AC, et al. Differential macrophage response to slow- and fast-growing pathogenic mycobacteria. Biomed Res Int. 2014;2014:916521. doi: 10.1155/2014/916521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuyama M, et al. Transcriptional Response of Respiratory Epithelium to Nontuberculous Mycobacteria. Am J Respir Cell Mol Biol. 2018;58:241–252. doi: 10.1165/rcmb.2017-0218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax. 2007;62:661–666. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feazel LM, et al. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA. 2009;106:16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falkinham JO, III, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl Environ Microbiol. 2001;67:1225–1231. doi: 10.1128/AEM.67.3.1225-1231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham DJ, et al. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Beneficiaries Treated With Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA. Intern Med. 2016;176:1662–1671. doi: 10.1001/jamainternmed.2016.5954. [DOI] [PubMed] [Google Scholar]
- 32.Lee SR, et al. Edoxaban in Asian Patients With Atrial Fibrillation: Effectiveness and Safety. J Am Coll Cardiol. 2018;72:838–853. doi: 10.1016/j.jacc.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 33.Song SO, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park CS, et al. Association between adult height, myocardial infarction, heart failure, stroke and death: a Korean nationwide population-based study. Int J Epidemiol. 2018;47:289–298. doi: 10.1093/ije/dyx175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.