Abstract
Many studies have been conducted to examine whether Caesarean Section (CS) or vaginal birth (VB) was optimal for better maternal and neonatal outcomes in preterm births. However, findings remain unclear. Therefore, this secondary analysis of World Health Organization Global Survey (GS) and Multi-country Survey (MCS) databases was conducted to investigate outcomes of preterm birth by mode of delivery. Our sample were women with singleton neonates (15,471 of 237 facilities from 21 countries in GS; and 15,053 of 239 facilities from 21 countries in MCS) delivered between 22 and <37 weeks of gestation. We assessed association between mode of delivery and pregnancy outcomes in singleton preterm births by multilevel logistic regression adjusted for hierarchical data. The prevalences of women with preterm birth delivered by CS were 31.0% and 36.7% in GS and MCS, respectively. Compared with VB, CS was associated with significantly increased odds of maternal intensive care unit admission, maternal near miss, and neonatal intensive care unit admission but significantly decreased odds of fresh stillbirth, and perinatal death. However, since the information on justification for mode of delivery (MOD) were not available, our results of the potential benefits and harms of CS should be carefully considered when deciding MOD in preterm births.
Subject terms: Epidemiology, Outcomes research
Introduction
Preterm birth defined as birth before 37 completed weeks of gestation or fewer than 259 days since the first day of a woman’s last menstrual period1. A global report in 2015 indicated that preterm birth complications accounted for 17.8% (uncertainty range 15.4 to 19.0%) of all deaths in children under five years old2. Due to immature organ systems, newborns who survive are prone to develop both short- and long-term complications, i.e. neurodevelopmental disability, respiratory illnesses, chronic disease in adulthood compared to children born at term3. Preterm birth rates appear to increase in many countries. Data from 65 developed countries, Latin America and Caribbean regions showed that its rates has risen from 7.5% in 1990 to 8.6% in 20104.
Many studies have been conducted to assess whether Caesearean Section (CS) or vaginal birth (VB) confers benefits to preterm newborn with minimal harms to mother in preterm birth, but mostly in high-income contries and limited in low- and middle-income countries. However, findings remains unclear. Some observational studies showed that CS could improve outcomes of preterm neonates5–7, while other studies suggested that VB was protective against neonatal death8. Yet others demonstrated that there was no significant difference in neonatal mortality between both groups9–14. Moreover, impact of mode of delivery (MOD) may vary by fetal presentation. For non-vertex-presenting fetuses, CS has been reported to reduce risk of neonatal mortality15–18, but evidence was less clear for vertex-presenting fetuses15,17,19–22. For mothers, CS seems to cause more severe adverse outcomes13,23. Randomized controlled trials (RCTs) had also been conducted to address this question, but planned sample sizes could not be met due to difficulties in recruiting pregnant women24–28. A systematic review that assess effects of planned immediate CS versus planned VB for women in preterm labour could include only six RCTs, and involved a total of only 122 women9. Consequently, there is little high-quality trial data regarding effects of MOD on outcomes for preterm neonates to guide clinical practice. The World Health Organization (WHO) has indicated that there is insufficient evidence to inform which MOD is optimal for preterm infants29.
A clear understanding of maternal and newborn outcomes for different modes of preterm birth could guide clinicians and mothers in making appropriate decisions. This analysis aimed to investigate the relationship between MOD and pregnancy outcomes among women giving preterm birth in two large WHO multi-country surveys.
Results
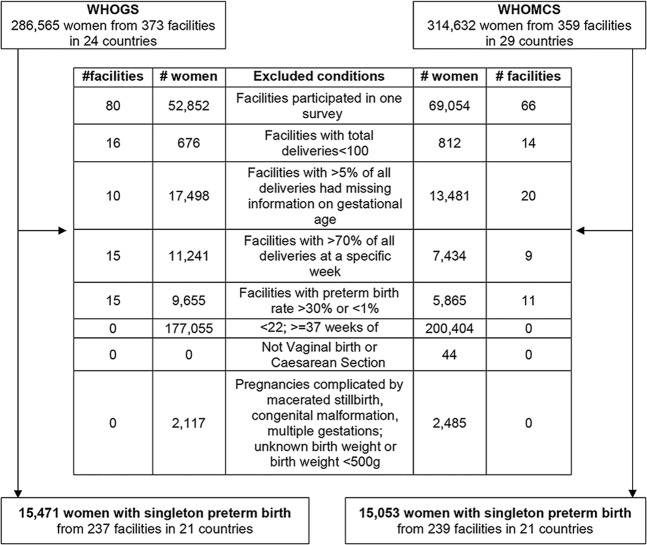
A total of 286,565 and 314,623 pregnant women were available from the WHOGS and WHOMCS datasets, respectively. From these two databases, 15,471 and 15,053 women with a singleton delivery from 22 to <37 weeks of gestation were eligible and included in the analysis (Fig. 1). Of the eligible women, the prevalence of CS in these preterm births in WHOGS and WHOMCS were 31.0% and 36.7%, respectively.
Figure 1.
Study population.
Table 1 presented the maternal and perinatal characteristics of the CS and VB groups in WHOGS and WHOMCS. We found that maternal age, maternal education attainment, parity, underlying disease, preeclampsia and eclampsia, gestational age, fetal presentation, corticosteroids administration, newborn’s sex and birthweight were significantly different between CS and VB in both WHOGS and WHOMCS; but marital status was significantly different only in WHOMCS.
Table 1.
Characteristics of mothers, fetuses and neonates by mode of delivery. *Number of mothers/neonates for each characteristic were not the same due to missing data; NA: Data was not available.
| GS (N = 15,471) | p value | MCS (N = 15,053) | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VB (N = 10,669) n/N(%) | CS (N = 4,802) n/N (%) | VB (N = 9,524) n/N (%) | CS (N = 5,529) n/N (%) | |||||||
| Maternal characteristics* | ||||||||||
| Marital status | ||||||||||
| Single | 1,408/10,629 | (13.2) | 596/4,792 | (12.4) | 0.17 | 1,205/9,458 | (12.7) | 624/5,491 | (11.4) | 0.013 |
| Married/cohabiting | 9,221/10,629 | (86.8) | 4,196/4,792 | (87.6) | 8,253/9458 | (87.3) | 4,867/5,491 | (88.6) | ||
| Maternal age (years) | ||||||||||
| <20 | 1,785/10,660 | (16.7) | 481/4,799 | (10.0) | <0.001 | 1,456/9,501 | (15.3) | 541/5,513 | (9.8) | <0.001 |
| 20–34 | 7,962/10,660 | (74.7) | 3,437/4,799 | (71.6) | 7,235/9,501 | (76.1) | 3,941/5,513 | (71.5) | ||
| ≥35 | 913/10,660 | (8.6) | 881/4,799 | (18.4) | 810/9,501 | (8.5) | 1,031/5,513 | (18.7) | ||
| Education attainment (years) | ||||||||||
| <=5 | 2,250/10,005 | (22.5) | 640/4,506 | (14.2) | <0.001 | 2,045/8,807 | (23.2) | 696/5,019 | (13.9) | <0.001 |
| 6–9 | 3,629/10,005 | (36.3) | 1,516/4,506 | (33.6) | 2,985/8,807 | (33.9) | 1,475/5,019 | (29.4) | ||
| 10–12 | 3,123/10,005 | (31.2) | 1,543/4,506 | (34.2) | 2,783/8,807 | (31.6) | 1,693/5,019 | (33.7) | ||
| >12 | 1,003/10,005 | (10.0) | 807/4,506 | (17.9) | 994/8,807 | (11.3) | 1,152/5,019 | (23.0) | ||
| Parity | ||||||||||
| Nulliparous | 5,098/10,615 | (48.0) | 1,899/4,792 | (39.6) | <0.001 | 4,739/9,516 | (49.8) | 2,487/5,521 | (45.0) | <0.001 |
| Multiparous | 5,517/10,615 | (52.0) | 2,893/4,792 | (60.4) | 4,777/9,516 | (50.2) | 3,034/5,521 | (55.0) | ||
| Underlying disease | ||||||||||
| Yes | 699/10,595 | (6.6) | 488/4,768 | (10.2) | <0.001 | 444/9,524 | (4.7) | 633/5,526 | (11.5) | <0.001 |
| No | 9,896/10595 | (93.4) | 4,280/4,768 | (89.8) | 9,080/9,524 | (95.3) | 4,893/5,526 | (88.5) | ||
| Preeclampsia | ||||||||||
| Yes | 323/10,653 | (3.0) | 758/4,788 | (15.8) | <0.001 | 350/9,524 | (3.7) | 933/5,527 | (16.9) | <0.001 |
| No | 10,330/10,653 | (97.0) | 4,030/4,788 | (84.2) | 9,174/9524 | (96.3) | 4,594/5,527 | (83.1) | ||
| Eclampsia | ||||||||||
| Yes | 93/10,652 | (0.9) | 107/4,788 | (2.2) | <0.001 | 83/9,524 | (0.9) | 129/5,527 | (2.3) | <0.001 |
| No | 10,559/10,652 | (99.1) | 4,681/4,788 | (97.8) | 9,441/9,524 | (99.1) | 5,398/5527 | (97.7) | ||
| Perinatal characteristics | ||||||||||
| Gestational age | ||||||||||
| Extremely preterm | 419/10,669 | (3.9) | 113/4,802 | (2.4) | <0.001 | 480/9,524 | (5.0) | 135/5,529 | (2.4) | <0.001 |
| Very preterm | 1,052/10,669 | (9.9) | 532/4,802 | (11.1) | 1,191/9,524 | (12.5) | 653/5,529 | (11.8) | ||
| Moderate preterm | 9,198/10,669 | (86.2) | 4,157/4,802 | (86.6) | 7,853/9,524 | (82.5) | 4,714/5,529 | (85.7) | ||
| Fetal presentation* | ||||||||||
| Vertex | 10,117/10,664 | (94.9) | 3,887/4,784 | (81.2) | <0.001 | 8,990/9,513 | (94.5) | 4,581/5,510 | (83.1) | <0.001 |
| Non-vertex | 547/10,664 | (5.1) | 897/4,784 | (18.8) | 523/9,513 | (5.5) | 929/5,510 | (16.9) | ||
| Corticosteroids* | ||||||||||
| No | NA | NA | 6,562/9,308 | (70.5) | 3,172/5,284 | (60.0) | <0.001 | |||
| Yes | NA | NA | 2,746/9,308 | (29.5) | 2,112/5,284 | (40.0) | ||||
| Sex* | ||||||||||
| Female | 5,199/10,661 | (48.8) | 2,222/4,800 | (46.3) | 0.005 | 4,644/9,514 | (48.8) | 2,580/5,522 | (46.7) | 0.014 |
| Male | 5,462/10,661 | (51.2) | 2,578/4,800 | (53.7) | 4,870/9,514 | (51.2) | 2,939/5,522 | (53.3) | ||
| Birth weight | ||||||||||
| ≤1,000 g | 433/10,669 | (4.1) | 170/4,802 | (3.5) | <0.001 | 654/9,524 | (6.9) | 226/5,529 | (4.1) | <0.001 |
| >1,000–1,500 g | 757/10,669 | (7.1) | 447/4,802 | (9.3) | 982/9,524 | (10.3) | 681/5,529 | (12.3) | ||
| >1,500–2,500 g | 4,536/10,669 | (42.5) | 2,210/4,802 | (46.0) | 4,780/9,524 | (50.2) | 2,878/5,529 | (52.1) | ||
| >2500 g | 4,943/10,669 | (46.3) | 1,975/4,802 | (41.1) | 3,108/9,524 | (32.6) | 1,744/5,529 | (31.5) | ||
Adverse pregnancy outcomes by modes of delivery
For women with a singleton preterm birth, the prevalence of MICU admission were 3.7% in GS and 1.9% in MCS, maternal near miss was 1.7% in MCS (no information on maternal near miss in GS) and maternal death were 0.2% in GS and 0.3% in MCS. Women with CS was significantly associated with increased odds of MICU admission (aORs (95% CIs): 3.7 (1.6–8.5) in GS and 5.0 (3.3–7.7) in MCS), and maternal near miss (aOR (95% CI): 3.7 (2.6–5.4) in MCS). However, it was not significantly different in term of maternal death (aORs (95% CIs): 1.0 (0.4–2.4) in GS and 1.0 (0.5–2.2) in MCS) (Table 2).
Table 2.
Adverse maternal outcomes by modes of delivery.
| Maternal outcomes | GS | MCS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N (%) | Crude OR (95% CI) | aOR (95% CI) | n/N (%) | Crude OR (95% CI) | aOR (95% CI) | |||||||||
| MICU admission | 578/ | 15,464 | (3.7) | * | 287/ | 15,035 | (1.9) | ‡ | ||||||
| VB | 128/ | 10,665 | (1.2) | 1 | 1 | 42/ | 9,518 | (0.4) | 1 | 1 | ||||
| CS | 450/ | 4,799 | (9.4) | 8.5 | (7.0–10.4) | 3.7 | (1.6–8.5) | 245/ | 5,517 | (4.4) | 10.5 | (7.6–14.6) | 5.0 | (3.3–7.7) |
| Maternal near miss | NA | NA | NA | 257/ | 15,053 | (1.7) | * | |||||||
| VB | NA | NA | NA | 61/ | 9,524 | (0.6) | 1 | 1 | ||||||
| CS | NA | NA | NA | 196/ | 5,529 | (3.5) | 5.7 | (4.3–7.6) | 3.7 | (2.6–5.4) | ||||
| Maternal death | 34/ | 15,465 | (0.2) | † | 44/ | 15,050 | (0.3) | § | ||||||
| VB | 20/ | 10,666 | (0.2) | 1 | 1 | 23/ | 9,524 | (0.2) | 1 | |||||
| CS | 14/ | 4,799 | (0.3) | 1.6 | (0.8–3.1) | 1.0 | (0.4–2.4) | 21/ | 5,529 | (0.4) | 1.6 | (0.9–2.9) | 1.0 | (0.5–2.2) |
*Adjusted for maternal age, maternal education, parity,underlying disease, preeclampsia, eclampsia, mode of delivery, fetal presentation, and FCI; facility was adjusted as a random effect; †adjusted for maternal education, underlying disease, preeclampsia, eclampsia, mode of delivery, fetal presentation, and FCI; facility was adjusted as a random effect; ‡ adjusted for maternal age, maternal education, underlying disease, preeclampsia, eclampsia, mode of delivery, fetal presentation, and FCI; facility was adjusted as a random effect; § adjusted for maternal education, parity, underlying disease, preeclampsia, eclampsia, mode of delivery, fetal presentation, and FCI; facility was adjusted as a random effect; || adjusted for maternal education, parity, underlying disease, preeclampsia, eclampsia, mode of delivery, and FCI; facility was adjusted as a random effect.
NA: Data was not available.
The prevalences of Apgar score <7 at 5 minutes, NICU admission, fresh stillbirth, early neonatal and perinatal death were 11.9% and 8.3%, 33.1% and 36.4%, 4.6% and 6.3%, 4.5% and 5.9%, and 8.6% and 11.9%, in GS and MCS, respectively. Delivery by CS was associated with significantly increased odds of NICU admission (aORs (95% CIs): 2.5 (2.1–2.9) in GS and 1.7 (1.4–2.0) in MCS), but decreased odds of fresh stillbirth (aORs (95% CIs): 0.4 (0.2–0.6) in GS and 0.4 (0.3–0.6) in MCS) and perinatal death (aORs (95% CIs): 0.6 (0.5–0.8) in GS and 0.6 (0.5–0.8) in MCS) compared to those delivered by VB. The odds of early neonatal death was not significantly different between CS and VB (aORs (95% CIs): 1.1 (0.8–1.6) in GS and 1.1 (0.9–1.5) in MCS). Neonates delivered by CS was found to be associated with significantly decreased odds of having APGAR score <7 at 5 minutes but only in GS (aOR (95% CI): 0.8 (0.6–0.9)) compared to those delivered by VB (Table 3).
Table 3.
Adverse perinatal outcomes by modes of delivery.
| Perinatal outcomes | GS | MCS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N (%) | Crude OR (95% CI) | aOR (95% CI) | n/N (%) | Crude OR (95% CI) | aOR (95% CI) | |||||||||
| APGAR score <7 at 5 minutes | 1,833/ | 15,401 | (11.9) | * | 1,165/ | 14,072 | (8.3) | ‡ | ||||||
| VB | 1,299/ | 10,610 | (12.2) | 1 | 1 | 742/ | 8,738 | (8.5) | 1 | 1 | ||||
| CS | 534/ | 4,791 | (11.1) | 0.9 | (0.8–1.0) | 0.8 | (0.6–0.9) | 423/ | 5,334 | (7.9) | 0.9 | (0.8–1.1) | 1.1 | (0.8–1.4) |
| NICU admission | 4,888/ | 14,746 | (33.1) | * | 5,133/ | 14,117 | (36.4) | § | ||||||
| VB | 2,640/ | 10,089 | (26.2) | 1 | 1 | 2,685/ | 8,774 | (30.6) | 1 | 1 | ||||
| CS | 2,248/ | 4,657 | (48.3) | 2.6 | (2.5–2.8) | 2.5 | (2.1–2.9) | 2,448/ | 5,343 | (45.8) | 1.9 | (1.8–2.1) | 1.7 | (1.4–2.0) |
| Fresh stillbirth | 719/ | 15,471 | (4.6) | * | 954/ | 15,053 | (6.3) | § | ||||||
| VB | 576/ | 10,669 | (5.4) | 1 | 1 | 766/ | 9,524 | (8.0) | 1 | 1 | ||||
| CS | 143/ | 4,802 | (3.0) | 0.5 | (0.4–0.7) | 0.4 | (0.2–0.6) | 188/ | 5.529 | (3.4) | 0.4 | (0.3–0.5) | 0.4 | (0.3–0.6) |
| Early neonatal death | 616/ | 4,648 | (4.5) | † | 836/ | 14,061 | (5.9) | || | ||||||
| VB | 406/ | 10,077 | (4.0) | 1 | 1 | 549/ | 8,739 | (6.3) | 1 | 1 | ||||
| CS | 210/ | 4,648 | (4.5) | 1.1 | (0.9–1.3) | 1.1 | (0.8–1.6) | 287/ | 5,322 | (5.4) | 0.9 | (0.7–0.9) | 1.1 | (0.9–1.5) |
| Perinatal death | 1,335/ | 15,444 | (8.6) | * | 1,790/ | 15,015 | (11.9) | § | ||||||
| VB | 982/ | 10,653 | (9.2) | 1 | 1 | 1,315/ | 9,505 | (13.8) | 1 | 1 | ||||
| CS | 353/ | 4,791 | (7.4) | 0.8 | (0.7–0.9) | 0.6 | (0.5–0.8) | 475/ | 5,510 | (8.6) | 0.6 | (0.5–0.7) | 0.6 | (0.5–0.8) |
*Adjusted for maternal age, maternal education, marital status, parity, underlying disease, preeclampsia, eclampsia, mode of delivery, severity of preterm, fetal presentation, birth weight, sex, and FCI; facility was adjusted as a random effect; †adjusted for maternal education, marital status, parity, underlying disease, preeclampsia, eclampsia, mode of delivery, severity of preterm, fetal presentation, birth weight, sex, and FCI; facility was adjusted as a random effect; ‡adjusted for maternal age, maternal education, marital status, underlying disease, preeclampsia, eclampsia, mode of delivery, severity of preterm, fetal presentation, birth weight, sex, corticosteroids, and FCI; facility was adjusted as a random effect; §adjusted for maternal age, maternal education, marital status, parity, underlying disease, preeclampsia, eclampsia, mode of delivery, severity of preterm, fetal presentation, birth weight, sex, corticosteroids, and FCI; facility was adjusted as a random effect; ||adjusted for maternal age, maternal education, marital status, parity, preeclampsia, eclampsia, mode of delivery, severity of preterm, fetal presentation, birth weight, sex, corticosteroids, and FCI; facility was adjusted as a random effect.
There was a broadly consistent pattern for newborn outcomes, when stratified by vertex and non-vertex presentation. Significant reductions of fresh stillbirth and perinatal death were noticed in non-vertex presenting neonates delivered by CS than those with vertex presenting neonates (Tables S1 and S2).
Similar patterns with the primary analysis of perinatal outcomes were seen across all gestational age subgroups, as well as the regions with consistent reductions in fresh stillbirth associated with CS compared to VB (Tables S3–S7).
Discussion
Our analysis indicated that in women with preterm singletons, CS was associated with increased odds of MICU admission, maternal near miss (but not maternal death) compared to those delivered by VB. It was also associated with increased odds of NICU admission, but decreased odds of fresh stillbirth and perinatal death. The study also found that the odds of Apgar score <7 at 5 minutes and early neonatal death were not significantly different between CS and VB.
Our major strength was that we utilised two large WHO multi-country databases to evaluate association between MOD and outcomes of preterm birth using standardized data collection methods. This study had a large sample size to detect the association of MOD on relatively rare maternal outcomes (i.e. maternal death and maternal near miss). Although a RCT might be feasible, it would be very difficult to obtain enough sample size to evaluate the association between MOD and important (but statistically rarer) outcomes in preterm birth24–28. Therefore, observational designs could be a more practical approach to assess possible associations.
Nonetheless, some limitations need to be considered. First, we had information on mortality, morbidities only up to hospital discharge or seven days after delivery, and no information of long-term pregnancy outcomes, we therefore could not evaluate overall risks and benefits of MOD. However, death and severe morbidities occuring after discharge from hospital should be comparatively rare. Second, despite adjusting for potential confounding factors, there might be some other factors that we could not account for. For example, the decision regarding selection of MOD could be due to specific circumstances, i.e., obstetricians might be unwilling to perform CS for fetuses with little chance of survival in order to avoid any risks to the mothers. Alternatively, a CS might be performed because the fetus had fetal distress. Unfortunately, we didn’t have information about justification for MOD. We also performed subgroup analyses by severity of preterm birth, and excluded those with birth weight <500 g and congenital malformation. The results of subgroup analyses confirmed the primary analysed results. Third, since data were collected from patients’ records, some information might be missing. Fourth, admission criteria to ICU was likely to be different between settings, and women or newborns may not be admitted due to unavailibility of beds. Fifth, data of gestational age was recorded in completed weeks based on the best available obstetric estimate but method of estimation was not recorded. As poor gestational age data can potentially confound our findings, we therefore tried to maximise the validity of this information by excluding facilities with poorer gestational age data. Lastly, our findings can be generalized only to hospitals with more than 1,000 births per year and those able to perform.
Our study found that CS was associated with increased odds of adverse maternal outcomes. The explanation is that CS is an invasive surgical procedure, which puts a mother at higher risks of morbidities and mortality. It is possible that some maternal underlying diseases as well as obstetric complications might lead to CS, and affect maternal outcomes, which complicates the assessment of the risk of CS. However, these factors were already taken into account in our analyses. Like our study, others also illustrated that CS (either for preterm or all births) was associated with increased risks of adverse maternal outcomes13,30,31. However, there was no significant difference in the odds of maternal death between CS and VB. This finding was similar to a prospective four-year observational study, involving 3,119 women with singleton preterm pregnacy of 24 to 36 weeks of gestation at 19 academic medical centers in United States32.
Among preterm birth, neonates delivered by CS was associated with increased odds of NICU admission. One explanation might be that neonates delivered by CS was due to fetal compromise (i.e. fetal distress). Therefore, they were more likely to be admitted to NICU for further specialized care. Sangkomkamhang et al. showed a significantly shorter length of hospital stay in infants delivered vaginally than those delivered by CS13.
In vertex-presenting fetuses, MOD was not associated with Apgar score <7 at 5 minutes. This was also found in previous reports9,13,21. Likewise, CS was not associated with the odds of early neonatal death. This finding was consistent with previous studies9,13,21,22,33,34. However, it was contradicted with another study in United States20. This might be due to difference in study population. Werner et al. recruited preterm births from 24 to 34 weeks of gestation but only those with appropriate birthweight for gestational age. In vertex presentation, we also found lower odds of fresh stillbirth in CS group than VB group. One explanation could be that CS prevented neonates from a potentially stressful labour encountered in VB. CS also offered vertex-presenting neonates no significant difference for the odds of perinatal death compared to VB. This finding was in line with the work of Wallace et al.27, a RCT which involved 38 vertex-presenting singleton preterm pregnancies of 26 to 33 weeks of gestation in the United States.
Similar findings were found for moderate preterm birth: CS was associated with decreased odds of perinatal death. Different with our findings, Malloy et al. found that CS was associated with increased odds of early neonatal death8. The explanation might be that they included a much more restricted group of preterm fetuses with birthweight ranges that varied for specific gestational ages. Different findings were found for very preterm births. The odds of perinatal death was decreased in those delivered by CS in MCS, but was not different in GS. One explanation might be due to improvement of health care services over time, which increased the chance of survival for these neontates. The WHOMCS was conducted more recently (2010–2011) than WHOGS (from 2004–2008) so improvements in quality of care were not unlikely. In MCS, for extremely preterm fetuses, CS was associated with reduced odds of fresh stillbirth, but there were no differences in other outcomes, i.e. Apgar score <7 at 5 minutes, early neonatal death and perinatal death. These findings were consistent with others15,17. However, in GS, for extremely preterm birth, CS was protective for fetuses from the odds of perinatal death.
Additionally, our study upheld the hypothesis that for non-vertex fetuses, CS was associated with reduced odds of Apgar score <7 at 5 minutes, fresh stillbirth and perinatal deaths. These might be the results of avoiding difficult labour and delivery which allowed a less stressful or traumatic birth in CS than VB. Previous studies were also in accordance with our findings15,17,18. They were retrospective studies of Effer et al. (involving 860 singleton live-births at 24 and 25 weeks gestational age in 13 of 17 Canadian tertiary centres)15, Lodha et al. (3,552 preterm neonates of ≤32 weeks of gestation)16, and Reddy et al. (768 preterm births of 24 to <32 weeks of gestation)17. Another systematic review by Bergenhenegouwen et al. also illustrated decreased odds of neonatal death in non-vertex-presenting fetuses delivered by CS18.
Our findings suggest that there might be benefit if carrying out CS for preterm birth with non-vertex presentation. For vertex-presenting fetuses, health care providers should counsel pregnant women and their families about benefits and harms before selecting MOD. Further well-designed prospective observational studies are needed to assess the effect of MOD on pregnancy outcomes in preterm births.
Methods
Study design and settings
This was a secondary analysis of two facility-based, cross-sectional surveys led by the WHO, the WHO Global Survey (GS) on Maternal and Perinatal Health (2004–2008), and the WHO Multi-Country Survey (MCS) on Maternal and Newborn Health (2010–2011). Details of these surveys have been published elsewhere35,36. Briefly, the WHOGS captured data on all women who gave birth in 373 randomly selected health facilities in 24 countries; and the WHOMCS captured data on births and women with severe maternal outcomes in 359 health facilities from 29 countries. For the WHOGS, data collection was conducted from 2004 to 2005 in Africa and Latin America, from 2007 to 2008 in Asia. WHOMCS was conducted from 2010 to 2011 in Africa, Asia, Latin America, and the Middle East. The period of data collection was two or three months depending on the institutional number of annual births. Data for all women (and their babies) were collected from medical records and could not be linked to participants. The technical content of both protocols was reviewed by specialist panels at the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction. The Specialist Panel on Epidemiological Research reviewed and approved the WHOGS study protocol for technical content; the Research Project Review Panel (name of panel was changed in 2010) reviewed and approved the technical content of the WHOMCS. The WHOGS and WHOMCS were approved by the WHO Ethical Review Committee and the relevant ethical clearance bodies in participating countries and facilities. Written consent from individual women was not needed because there was no contact between the data collectors (who extracted routine medical record data) and individual women.
Study population
For this analysis, we included only facilities that participated in both surveys, with at least 100 deliveries in total and with 5% or less missing information on gestational age. We also excluded facilities with unreliable gestational age data, defined as facilities with more than 70% of total deliveries at a specific week of gestational age, or with preterm birth rate more than 30% or less than 1%. Thus, there were 237 and 239 health facilities in WHOGS and WHOMCS, respectively, from 21 countries included in this analysis. We included singleton pregnant women who delivered between 22 and <37 weeks of gestation and their newborns (regardless of vital status at birth). Exclusion criteria included women with ectopic pregnancies or abortion; pregnancies with a documented congenital malformation, or where birth weight was unknown or <500 g (Fig. 1). Pregnancies ending with macerated stillbirth were also excluded, since these likely occurred prior to the onset of labour.
Definitions of variables
Our main independent variable was MOD, which was categorized as VB or CS. Adverse pregnancy outcomes included adverse maternal and perinatal outcomes. For maternal outcomes, we used maternal intensive care unit (MICU) admission, maternal near miss, and maternal death up to hospital discharge. Maternal near miss was defined as a woman who presented with any life-threatening condition and survived a complication during pregnancy, childbirth or within 7 days of termination of pregnancy (this outcome was captured only for the MCS database). For perinatal outcomes, we used APGAR score <7 at 5 minutes after birth, neonatal intensive care unit (NICU) admission, fresh stillbirth (fetal death, with no signs of maceration), early neonatal death (death of a live born neonate at discharge or within 7 days after birth), and perinatal death (fresh stillbirth or early neonatal death).
Potential confounding variables that were available in WHOGS and WHOMCS databases included both individual and facility characteristics. Individual characteristics were maternal sociodemographic and obstetric characteristics (i.e marital status, maternal age, maternal education, parity); maternal underlying disease (HIV/chronic hypertension/malaria/dengue fever/heart/lung/renal disease/anaemia); obstetric complications (i.e preeclampsia, eclampsia); and fetal and neonatal characteristics (i.e fetal presentation, severity of preterm birth, birth weight, sex). We used the facility complexity index (FCI) to determine the level of services available in each of the facilities and to summarize a facility’s capacity to provide obstetric care (which has been used in previous WHOMCS analyses)37. Since there were differences in some composite variables of FCI available in MCS compared to GS, for consistency, we used the FCI score calculated from MCS in both datasets. Scores for the sampled facilities varied from 12 to 57 points.
Statistical analysis
Characteristics of the participants were described using frequency and percentage for categorical data. To investigate the association between MOD and adverse pregnancy outcomes, odds ratio (OR) and 95% confidence intervals (95% CI) were estimated using generalized linear mixed model with forward stepwise procedure. We also performed sub-group analyses of the risks for adverse perinatal outcomes according to fetal presentation. We expected poorer perinatal outcomes for newborns at earlier gestational ages, hence we also conducted a stratified analysis by severity of preterm birth: extremely preterm birth (<28 weeks), very preterm birth (28- < 32 weeks) and moderately preterm birth (32- < 37 weeks). These models were adjusted for potential confounding factors; facility was also adjusted as a random effect. VB was treated as a reference group. The Akaike’s information criterion38 was used to assess the goodness of fit of the model at p < 0.05. All analyses were performed using R program39, and the lme4 package40 was used for generalized linear mixed model.
Ethics approval
The GS and MCS were approved by the WHO Ethical Review Committee and the relevant ethical clearance mechanisms in all facilities.
Supplementary information
Acknowledgements
We thank all members of the WHO Global Survey on Maternal and Perinatal Health Research Network and the WHO Multi-Country Survey on Maternal and Newborn Health Research Network, including regional and country coordinators, data collection coordinators, facility coordinators, data collectors, and all staff of the participating facilities who made the surveys possible. Our thanks are also expressed to Dr. Cameron Hurst for his helpful suggestions in data analysis. We would also like to thank the Thailand Research Fund (Distinguished Professor Award) for supporting this secondary analysis. This article represents the views of the named authors only, and does not represent the views of the World Health Organization. The WHO Global Survey on Maternal and Perinatal Health was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction; WHO; the Governments of China, India, and Japan; and the United States Agency for International Development (USAID). The WHO Multi-Country Survey on Maternal and Newborn Health was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction; WHO; USAID; the Ministry of Health, Labour and Welfare of Japan; and Gynuity Health Projects.
Author contributions
B.Y.L.T., P.L., P.P., M.L. conceptualized the research question and participated in the analysis plan. B.Y.L.T. performed the data analysis and drafted the manuscript. J.P.V., O.T.O., C.P.C., R.M., K.J., Z.Q. and J.P.S. advised about details of the contents. All authors read and approved the final draft of the manuscript.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to they belonged to Department of Reproductive Health and Research, The World Health Organization but could be available from WHO on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-52015-w.
References
- 1.WHO . Acta Obstet Gynecol Scand. 1977. recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976; pp. 247–253. [PubMed] [Google Scholar]
- 2.Liu L, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 4.Blencowe H, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 5.Demol S, et al. Breech presentation is a risk factor for intrapartum and neonatal death in preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2000;93:47–51. doi: 10.1016/S0301-2115(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 6.Hogberg U, Hakansson S, Serenius F, Holmgren PA. Extremely preterm cesarean delivery: a clinical study. Acta Obstet Gynecol Scand. 2006;85:1442–1447. doi: 10.1080/00016340600969366. [DOI] [PubMed] [Google Scholar]
- 7.Hogberg U, Holmgren PA. Infant mortality of very preterm infants by mode of delivery, institutional policies and maternal diagnosis. Acta Obstet Gynecol Scand. 2007;86:693–700. doi: 10.1080/00016340701371306. [DOI] [PubMed] [Google Scholar]
- 8.Malloy MH. Impact of cesarean section on intermediate and late preterm births: United States, 2000–2003. Birth. 2009;36:26–33. doi: 10.1111/j.1523-536X.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- 9.Alfirevic, Z., Milan, S. J. & Livio, S. Caesarean section versus vaginal delivery for preterm birth in singletons. Cochrane Database Syst Rev, CD000078 (2013). [DOI] [PMC free article] [PubMed]
- 10.Ghi T, et al. Mode of delivery in the preterm gestation and maternal and neonatal outcome. J Matern Fetal Neonatal Med. 2010;23:1424–1428. doi: 10.3109/14767051003678259. [DOI] [PubMed] [Google Scholar]
- 11.Haque KN, Hayes AM, Ahmed Z, Wilde R, Fong CY. Caesarean or vaginal delivery for preterm very-low-birth weight (<or =1,250 g) infant: experience from a district general hospital in UK. Arch Gynecol Obstet. 2008;277:207–212. doi: 10.1007/s00404-007-0438-x. [DOI] [PubMed] [Google Scholar]
- 12.Malloy MH, Onstad L, Wright E. The effect of cesarean delivery on birth outcome in very low birth weight infants. National Institute of Child Health and Human Development Neonatal Research Network. Obstet Gynecol. 1991;77:498–503. [PubMed] [Google Scholar]
- 13.Sangkomkamhang U, Pattanittum P, Laopaiboon M, Lumbiganon P. Mode of delivery and outcomes in preterm births. J Med Assoc Thai. 2011;94:415–420. [PubMed] [Google Scholar]
- 14.Sonkusare S, Rai L, Naik P. Preterm birth: mode of delivery and neonatal outcome. Med J Malaysia. 2009;64:303–306. [PubMed] [Google Scholar]
- 15.Effer SB, et al. Neonatal survival rates in 860 singleton live births at 24 and 25 weeks gestational age. A Canadian multicentre study. BJOG. 2002;109:740–745. doi: 10.1111/j.1471-0528.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 16.Lodha A, Zhu Q, Lee SK, Shah PS, Canadian Neonatal Network Neonatal outcomes of preterm infants in breech presentation according to mode of birth in Canadian NICUs. Postgrad Med J. 2011;87:175–179. doi: 10.1136/pgmj.2010.107532. [DOI] [PubMed] [Google Scholar]
- 17.Reddy UM, et al. Neonatal mortality by attempted route of delivery in early preterm birth. Am J Obstet Gynecol. 2012;207:117.e111–118. doi: 10.1016/j.ajog.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergenhenegouwen LA, et al. Vaginal delivery versus caesarean section in preterm breech delivery: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2014;172:1–6. doi: 10.1016/j.ejogrb.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Lee HC, Gould JB. Survival rates and mode of delivery for vertex preterm neonates according to small- or appropriate-for-gestational-age status. Pediatrics. 2006;118:e1836–1844. doi: 10.1542/peds.2006-1327. [DOI] [PubMed] [Google Scholar]
- 20.Werner EF, Han CS, Savitz DA, Goldshore M, Lipkind HS. Health outcomes for vaginal compared with cesarean delivery of appropriately grown preterm neonates. Obstet Gynecol. 2013;121:1195–1200. doi: 10.1097/AOG.0b013e3182918a7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werner EF, et al. Mode of delivery and neonatal outcomes in preterm, small-for-gestational-age newborns. Obstet Gynecol. 2012;120:560–564. doi: 10.1097/AOG.0b013e318265b16c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wylie, B. J., Davidson, L. L., Batra, M. & Reed, S. D. Method of delivery and neonatal outcome in very low-birthweight vertex-presenting fetuses. Am J Obstet Gynecol198, e1-7; discussion e1-4 (2008). [DOI] [PubMed]
- 23.Souza JP, et al. Caesarean section without medical indications is associated with an increased risk of adverse short-term maternal outcomes: the 2004–2008 WHO Global Survey on Maternal and Perinatal Health. BMC Med. 2010;8:71. doi: 10.1186/1741-7015-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumley J, Lester A, Renou P, Wood C. A failed RCT to determine the best method of delivery for very low birth weight infants. Control Clin Trials. 1985;6:120–127. doi: 10.1016/0197-2456(85)90117-5. [DOI] [PubMed] [Google Scholar]
- 25.Penn ZJ, Steer PJ, Grant A. A multicentre randomised controlled trial comparing elective and selective caesarean section for the delivery of the preterm breech infant. Br J Obstet Gynaecol. 1996;103:684–689. doi: 10.1111/j.1471-0528.1996.tb09838.x. [DOI] [PubMed] [Google Scholar]
- 26.Viegas OA, et al. Collaborative study on preterm breeches: vaginal delivery versus caesarean section. Asia Oceania J Obstet Gynaecol. 1985;11:349–355. doi: 10.1111/j.1447-0756.1985.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 27.Wallace Rl, Schifrin BS, Paul RH. The delivery route for very-low-birth-weight infants. A preliminary report of a randomized, prospective study. J Reprod Med. 1984;29:736–740. [PubMed] [Google Scholar]
- 28.Zlatnik FJ. The Iowa premature breech trial. Am J Perinatol. 1993;10:60–63. doi: 10.1055/s-2007-994704. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes (2015). [PubMed]
- 30.Villar J, et al. Maternal and neonatal individual risks and benefits associated with caesarean delivery: multicentre prospective study. BMJ. 2007;335:1025. doi: 10.1136/bmj.39363.706956.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumbiganon P, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007–08. The Lancet. 2010;375:490–499. doi: 10.1016/S0140-6736(09)61870-5. [DOI] [PubMed] [Google Scholar]
- 32.Durnwald CP, et al. The Maternal-Fetal Medicine Units Cesarean Registry: safety and efficacy of a trial of labor in preterm pregnancy after a prior cesarean delivery. Am J Obstet Gynecol. 2006;195:1119–1126. doi: 10.1016/j.ajog.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 33.Durie DE, Sciscione AC, Hoffman MK, Mackley AB, Paul DA. Mode of delivery and outcomes in very low-birth-weight infants in the vertex presentation. Am J Perinatol. 2011;28:195–200. doi: 10.1055/s-0030-1266156. [DOI] [PubMed] [Google Scholar]
- 34.Riskin A, Riskin-Mashiah S, Lusky A, Reichman B. & Israel Neonatal Network. The relationship between delivery mode and mortality in very low birthweight singleton vertex-presenting infants. BJOG. 2004;111:1365–1371. doi: 10.1111/j.1471-0528.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- 35.Shah A, et al. Methodological considerations in implementing the WHO Global Survey for Monitoring Maternal and Perinatal Health. Bull World Health Organ. 2008;86:126–131. doi: 10.2471/BLT.06.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souza JP, et al. The world health organization multicountry survey on maternal and newborn health: study protocol. BMC Health Serv Res. 2011;11:286. doi: 10.1186/1472-6963-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel JP, et al. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121:76–88. doi: 10.1111/1471-0528.12633. [DOI] [PubMed] [Google Scholar]
- 38.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 39.R: A language and enviroment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2014).
- 40.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to they belonged to Department of Reproductive Health and Research, The World Health Organization but could be available from WHO on reasonable request.