Abstract
For over a half-century the anti-malarial drug chloroquine (CQ) has been used as a therapeutic agent, alone or in combination, to treat autoimmune diseases. However, neither the underlying mechanism(s) of action nor their molecular target(s) are well defined. The orphan nuclear receptor Nurr1 (also known as NR4A2) is an essential transcription factor affecting the development and maintenance of midbrain dopaminergic neurons. In this study, using in vitro T cell differentiation models, we demonstrate that CQ activates TREG cell differentiation and induces Foxp3 gene expression in a Nurr1-dependent manner. Remarkably, CQ appears to induce Nurr1 function by two distinct mechanisms: firstly, by direct binding to Nurr1’s ligand-binding domain and promoting its transcriptional activity and secondly by upregulation of Nurr1 expression through the CREB signaling pathway. In contrast, CQ suppressed gene expression and differentiation of pathogenic TH17 cells. Importantly, using a valid animal model of inflammatory bowel disease (IBD), we demonstrated that CQ promotes Foxp3 expression and differentiation of TREG cells in a Nurr1-dependent manner, leading to significant improvement of IBD-related symptoms. Taken together, these data suggest that CQ ameliorates autoimmune diseases via regulating Nurr1 function/expression and that Nurr1 is a promising target for developing effective therapeutics of human inflammatory autoimmune diseases.
Subject terms: Autoimmunity, NMR spectroscopy
Introduction
Originally developed as anti-malarial drugs, chloroquine (CQ) as well as hydroxychloroquine (HCQ) have become part of the standard components of treatment for patients suffering from autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE)1–3. Numerous investigations into CQ/HCQ’s potential roles led to the proposition of diverse candidate mechanisms of action, including inhibition of immunological processes such as antigen presentation, proinflammatory cytokine expression, and inhibition of Toll-like receptors and NLRP3 inflammasome4–7. In particular, CQ was recently shown to increase the number of TREG cells and rebalance TH17/TREG-mediated immunity, strongly suggesting that CQ/HCQ regulate autoimmune disease by modulating T cell subset function and/or differentiation8,9. Despite these progresses, neither precise mechanisms nor the identity of molecular targets underlying CQ/HCQ’s roles in autoimmune diseases are well understood.
Nurr1 is an orphan nuclear receptor that plays a key role in the development and maintenance of midbrain dopamine neurons10,11. Moreover, Nurr1 was reported to protect midbrain dopamine neurons by its anti-inflammatory effects via recruiting the CoREST corepressor complex and removing NF-κB from the pro-inflammatory gene promoters in microglia and astrocytes12. Interestingly, Nurr1 was also found to bind to the regulatory regions of the Foxp3 gene and directly regulates its expression13. Furthermore, ectopic expression of Nurr1 in naïve CD4+ T cells resulted in induction of the TREG cell developmental program and inactivation of Nurr1 and other NR4A members led to severe interference with T cell development, strongly supporting Nurr1’s critical roles in T cell homeostasis14.
Recently, we reported that the transcriptional activity of Nurr1 can be regulated by CQ and amodiaquine through Nurr1’s ligand-binding domain (LBD)15, prompting us to hypothesize that CQ could modulate autoimmune diseases via regulation of Nurr1’s function in T cells. To address this hypothesis, we used in vitro T cell differentiation models and investigated the potential mechanisms of CQ’s functional effects. We found that CQ not only directly binds to Nurr1-LBD to increase its transcriptional activity, but also it can increase Nurr1 expression in T cells through CREB, thereby enhancing Foxp3 expression and inducing TREG cells differentiation. In contrast, CQ showed inhibitory effects on gene expression and differentiation of pathogenic TH17 cells, suggesting that CQ exhibits T cell subset-specific functional effects. In addition, we investigated a mouse model of inflammatory bowel disease (IBD) which is a chronic autoimmune disease of the colon and small intestine characterized by immune-mediated inflammation, diarrhea, rectal bleeding, swollen and damaged tissues of the digestive tract16,17. The dextran sulfate sodium (DSS)-induced mouse is a well-established model for studying IBD pathogenesis and developing novel therapies18. In particular, we chose this animal model to study the functional link between CQ and Nurr1 in autoimmune diseases because T cells’ essential roles are well validated for the development and continuation of the IBD disease process19,20. Using this DSS-induced mouse model, we showed that CQ can effectively improve symptoms of IBD in a Nurr1-dependent manner. Based on these data, we propose that targeting the CQ-Nurr1 interaction is a fundamental and effective strategy for the development of therapeutic agents for autoimmune diseases.
Results
CQ regulates TREG cell differentiation through a Nurr1-dependent mechanism
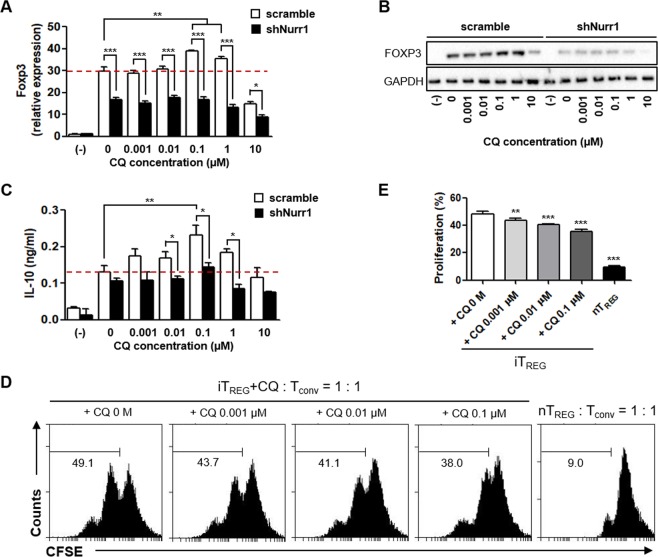
To address whether CQ regulates TREG differentiation in a Nurr1-dependent manner, naïve CD4+CD25−CD62Lhigh T cells were isolated from C57BL/6 mice and transfected with a lentiviral shNurr1 or scramble vector, and then activated with anti-CD3 and CD28 antibodies under induced TREG (iTREG)-polarizing condition in the presence of increasing doses of CQ. CQ treatment (up to 1 µM) increased Foxp3 and IL-10 expression (Fig. 1A–C) in a similar pattern to Nurr1 (Supplementary Fig. S1A). In addition, CQ treatment dose-dependently enhanced TREG’s suppressive activity as demonstrated in a TREG suppression assay (Fig. 1D,E). When 10 µM CQ was used, expression of these genes (including Nurr1) was down-regulated, probably due to CQ’s cytotoxic effects. Indeed, treatment of mouse primary naïve T cells with CQ at concentrations of 10 and 100 µM resulted in significant reduction in the total cell number and specific gene expression (Supplementary Fig. S2A–C). In line with these results, it has been reported that high concentrations of CQ inhibit the activities of human CD4+ T cells21 as well as other cell types such as monocytes/macrophages22,23. Knocking down Nurr1 expression by transfecting differentiating T cells with lenti-shNurr1 plasmid (Supplementary Fig. S1A), inhibited up-regulation of Foxp3 gene and protein expression by CQ treatment (Fig. 1A,B). Thus, these data suggest that at concentrations ranging from 0.001 to 1 µM CQ regulates TREG cell differentiation and increases expression of the Foxp3 gene in a Nurr1-dependent manner but shows cytotoxicity at concentration above 10 µM. In addition, Nurr1 knock-down inhibited CQ’s up-regulation of IL-10 expression (Fig. 1C). However, we observed modest up-regulation of IL-10 expression at 0.1 µM CQ, which may be due to incomplete knock-down of Nurr1. Alternatively, additional factor(s) may be involved in CQ’s up-regulation of IL-10 gene expression.
Figure 1.
Nurr1-dependent regulation of iTREG differentiation by CQ. Mouse primary naïve CD4+CD25−CD62Lhigh T cells were transfected with lenti-scramble- or lenti-shNurr1-plasmid. Cells were treated with CQ (0.001~10 μM) and stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 96 h under iTREG-polarizing conditions. (A,B) The level of Foxp3 mRNA (A) or protein (B) expression was determined by quantitative real-time PCR or western blot, normalized with GAPDH. (C) The level of IL-10 in the culture media was analyzed by ELISA. (D) In vitro TREG suppression assays based on CFSE dilution by Tconv cells proliferating in the presence of CQ treated iTREG cells for 48 h and analyzed with flow cytometry. (E) Quantitation of the percentage ± SEM of proliferation of Tconv in the presence of iTREG with or without CQ treatment. These experiments were repeated three times in triplicate using independently prepared samples. Each error bar represents means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
CQ regulates pathogenic TH17 cell differentiation through a Nurr1-independent mechanism
Next, we examined the effect of CQ on pathogenic TH17 (pTH17) cell differentiation, which have opposite effects from TREG. CQ significantly decreased the expression of key marker genes of pTH17 (i.e., RORγt, IL-23R, and IL-17A) under pTH17-polarizing condition in a concentration-dependent manner (Fig. 2A–C). When Nurr1 expression was knocked down (Supplementary Fig. S1B), expression of IL-17A protein was reduced as was the number of CD4+ IL-17A+ T cells (Supplementary Fig. S3A–C), which is in agreement with the previous Nurr1 knockout study24. However, Nurr1 knockdown did not affect the expression of RORγt and IL-23R (Fig. 2A,B), suggesting that downregulation of RORγt and IL-23R genes is Nurr1-independent. We next tested how CQ treatment affects the expression of Foxp3 and Nurr1 during the pTH17 differentiation process and regulates the pathogenic properties of pTH17 cells. Foxp3 expression was unaffected by treatment with CQ and by Nurr1 knockdown (Fig. 2D). In agreement with these data, previous work showed that Foxp3 expression is very limited under pathogenic pTH17 differentiation conditions due to the lack of chromatin remodeling of Foxp3 via TGFβ signaling25,26. In addition, under T cell activation conditions, Foxp3 expression was not induced in spite of high Nurr1 expression resulting from CQ treatment (Supplementary Fig. S4A,B). Finally, we examined the effects of CQ treatment on gene expression during TH1 and TH2 cell differentiation where TGFβ signaling is also absent. As shown in Supplementary Fig. S4C,D, expression of Nurr1 and Foxp3 as well as the marker gene of TH1 and TH2 cell (Tbet and GATA3, respectively) was unaffected by CQ treatment. Taken together, the effect of CQ-Nurr1 interaction and function appears to be T cell subset-specific and was most evident during TREG cell differentiation.
Figure 2.
Nurr1-independent regulation of pTH17 differentiation by CQ. Mouse primary naïve CD4+CD25−CD62Lhigh T cells were transfected with lenti-scramble- or lenti-shNurr1-plasmid. Cells were treated with CQ (0.001~10 μM) and stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 72 h under pTH17-polarizing conditions. (A–C) The levels of RORγt (A), IL-23R (B) or IL-17A (C) mRNA expression were determined by quantitative real-time PCR and normalized with GAPDH. (D) The levels of Foxp3 mRNA expression were determined by quantitative real-time PCR and normalized with GAPDH. These experiments were repeated three times in triplicate using independently prepared samples. Each error bar represents means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
CQ physically binds to Nurr1
Our findings strongly suggest that CQ acts through Nurr1 in order to effect its anti-inflammatory function in autoimmune diseases. To further understand CQ’s regulation of Nurr1 function we investigated the interaction between CQ and Nurr1 using nuclear magnetic resonance spectroscopy (NMR). Upon addition of CQ, a number of amino acid residues on Nurr1-LBD showed significant chemical shift perturbations on two-dimensional 1H-15N Heteronuclear Single Quantum Correlation (HSQC) spectra of the 15N-labeled Nurr1-LBD. These perturbations were primarily located at the C-terminal helix α12 including S441, I573, A586, I588, K590, L593, D594, T595, L596, and F598 (Fig. 3A,B). Overlay of the free and ligand-bound spectra of Nurr1-LBD revealed that the perturbed residues are mainly located in the helix α12 region (I573, A586, I588, K590, L593, D594, T595, L596, F598) and also S441 in helix α4 (Fig. 3C–E). We used site directed mutagenesis to determine the functional contribution of these residues in HEK293 cells. CQ-induced Nurr1 transcriptional activity was greatly affected by the following substitutions S441A, I573A, I588A, K590A, L593A, D594A, T595A, L596A, and F598A while mutations of other residues had no effect (Fig. 3F). These results strongly suggest that amino acid residues S441, I573, I588, D594, T595, L596, and F598 contribute to the physical interaction between Nurr1 and CQ and affect CQ-induced transcriptional activation of Nurr1.
Figure 3.
CQ interacts with Nurr1-LBD and directly regulates Nurr1 transcriptional activity. NMR titration experiments of Nurr1-LBD with CQ. (A) Overlay of 1H-15N HSQC spectra of uniformly 15N labeled Nurr1-LBD (red) and Nurr1-LBD in the presence of CQ at molar ratio of 1 to 2.5 (green) and 1 to 5 (blue). (B) Expanded views of chemical shift perturbations upon CQ binding. The perturbations of chemical shifts from free Nurr1-LBD (red) to CQ bound forms (green and blue) are indicated by arrows. (C) Chemical Shift Perturbation Plot of Nurr1-LBD upon CQ binding at molar ratio of 1 (Nurr1-LBD) to 5 (CQ). The difference in chemical shifts was calculated using the following formula, Δδ = [(1Hfree − 1Hbound)2 + (15Nfree − 15Nbound)2)]1/2. Interaction site mapping of Nurr1-LBD and CQ based on the NMR data. (D) Surface mapping of CQ binding site and interaction residues on Nurr1-LBD based on 1H-15N HSQC titration data. Perturbed amino acid residues are displayed according to their chemical shift perturbation: red (Δδ > 0.1), blue (0.8 < Δδ < 0.1). (E) Ribbon representation of mapping data in Fig. 3D. Perturbed amino acid residues were displayed by stick representation and chemical shift values were used in the same manner as in Fig. 3D. (F) Impact of Nurr1-LBD point mutation on CQ’s effect on Nurr1 transcriptional activity. Luciferase activities were diminished in full length mutant Nurr1 transfected HEK293 cell lines compared to wild-type Nurr1 transfected control. Each error bar represents means ± s.e.m. ***P < 0.001. NT, non-treated condition.
CQ upregulates Nurr1 expression through the CREB signaling pathway
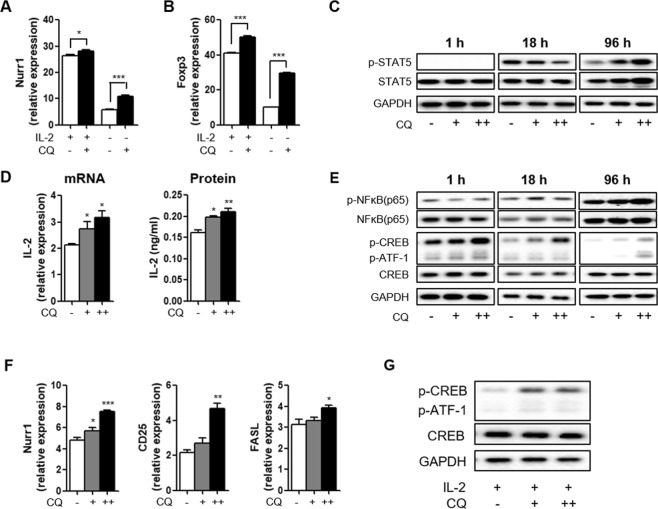
The above NMR and site-directed mutational studies suggest that CQ can enhance the transcriptional activity of Nurr1, leading to upregulation of Foxp3 gene expression in TREG cells. Interestingly, our results also showed that CQ treatment increases Nurr1 expression (Supplementary Fig. S1A), suggesting that CQ regulates both the function and the expression of Nurr1. To test this possibility, we used in vitro iTREG-polarizing conditions in the presence and the absence of IL-2, which is critical for this differentiation process via regulating Foxp3 expression27. Absence of IL-2 prominently diminished Nurr1 expression (Fig. 4A). However, in the absence of IL-2, treatment with CQ significantly increased both Nurr1 expression (approximately 1.8-fold) and Foxp3 expression (approximately 3.0-fold) (Fig. 4A,B). Based on these results, we tested whether CQ treatment can enhance IL-2-specific signaling pathway. STAT5 is activated through the IL-2/IL-2R-signaling pathway resulting in its phosphorylation (p-STAT5). We did not observe changes in the levels of p-STAT5 at 1 or 18 h post CQ treatment suggesting that CQ does not directly affect the IL-2/IL-2R-signaling pathway. However, at 96 h after CQ treatment we observed increased levels of p-STAT5 (Fig. 4C), suggesting that CQ alters IL-2 expression and then p-STAT5 levels. Indeed, we found that CQ treatment increased the expression of IL-2 at both the mRNA and the protein levels (Fig. 4D).
Figure 4.
Regulation of Nurr1 expression by CQ. Mouse primary naïve CD4+CD25−CD62Lhigh T cells were treated with 100 nM (+) or 1 μM (++) CQ and stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 1–96 h under iTREG-polarizing conditions (with or without IL-2). (A,B) The level of Nurr1 (A) and Foxp3 (B) mRNA expression were analyzed by quantitative real-time PCR and normalized with GAPDH. (C) The expression of p-STAT5, STAT5, and GAPDH proteins were confirmed by western blot after 1, 18, and 96 h treatment with CQ under iTREG-polarizing condition without IL-2 treatment. (D) The level of IL-2 mRNA and protein expression were analyzed by quantitative real-time PCR and ELISA, respectively. (E) The expression of p-NFkB(p65), NFkB(p65), p-CREB, CREB, and GAPDH proteins were confirmed by western blot after 1, 18, and 96 h treatment with CQ under iTREG-polarizing condition without IL-2 treatment. (F) The level of Nurr1, CD25, and FASL mRNA expression were determined by quantitative real-time PCR and normalized with GAPDH. (G) Mouse primary naïve CD4+CD25−CD62Lhigh T cells were treated with 100 nM (+) or 1 μM (++) CQ and stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 96 h under iTREG-polarizing conditions (with IL-2). The expression of p-CREB, CREB, and GAPDH proteins were confirmed by western blot. These experiments were repeated more than twice in triplicate using independently prepared samples. Each error bar represents means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
To investigate how CQ treatment regulates expression of IL-2 and Nurr1 genes, we next tested whether CQ impacts the activities of candidate transcription factors such as NFkB and CREB, which are known to affect IL-2 and Nurr1 expression28,29. CQ treatment did not alter the levels of NFkB or of its active phosphorylated form (p-NFkB (p65)) (Fig. 4E). In contrast, CQ treatment increased the phosphorylated form of CREB (p-CREB), in a dose-responsive manner during iTREG differentiation (Fig. 4E). However, as mentioned above, at high concentration, CQ disrupts the activity of p-CREB (Supplementary Fig. S2D). In addition, CQ treatment also significantly up-regulated expression of Nurr1, CD25, and FASL genes, which are known targets of p-CREB (Fig. 4F). These results suggest that CQ can induce IL-2 production but also affect CD25 production, leading to increased IL-2/IL-2R-signaling pathway. We also confirmed that CQ treatment increased the level of p-CREB, in the presence of IL-2 during iTREG differentiation (Fig. 4G). Taken together, these data suggest that CQ treatment activates the CREB pathway, leading to upregulation of Nurr1 and IL-2 expression, promoting iTREG differentiation and associated gene expression.
CQ suppresses the progression of IBD in DSS-induced mouse model
We used the DSS-induced colitis mouse model to study how CQ may modulate pathogenic progression and whether it involves Nurr1. Mice were infected with scrambled- or shNurr1-lentivirus and treated with CQ at a concentration known to be effective in this model30. We confirmed the ability of shNurr1-lentivirus to knockdown Nurr1 both in vitro and in vivo (Supplementary Fig. S1C,D). We studied five different groups of mice: No DSS; scrambled-lentivirus infected-DSS fed mice with vehicle injection (Scr + veh) or with CQ injection (Scr + CQ); and shNurr1-lentivirus infected-DSS fed mice with vehicle injection (shNurr1 + veh) or with CQ injection (shNurr1 + CQ) group. All animals treated with DSS showed reduced body weight, shortened colons, and splenomegaly. These changes were significantly attenuated in the CQ-treated group (Fig. 5A,B; Supplementary Fig. S5A,B). Clinical scores were also increased in DSS fed mice, which were significantly reduced in the CQ-treated groups (Fig. 5C). Mice fed with DSS and infected with shNurr1-lentivirus failed to respond to CQ treatment, indicating a relationship between CQ and Nurr1 in the mitigation of IBD in this model (Fig. 5A–C). Histological examination showed that CQ inhibited CD4+ T cells infiltration, inflammatory cells and cytokine, and also reduced epithelial cell destruction with goblet cell depletion (Fig. 5D; Supplementary Fig. S5C–E). Notably, this improvement by CQ was not observed in shNurr1-lentivirus infected-DSS fed mice, as evident in comparison of Scr + veh vs. shNurr1 + veh and Scr + CQ vs. shNurr1 + CQ groups. Furthermore, Foxp3 expression and Foxp3-positive cells were significantly up-regulated by CQ treatment in the mesenteric lymph node (MLN) CD4+ T cells and colon tissues, respectively (Fig. 5E–G; Supplementary Fig. S5F). Knocking down Nurr1 in shNurr1-lentivirus infected-DSS fed mice prevented the accumulation of Foxp3-positive cells in the MLN and colon tissues further supporting the conclusion that Foxp3 upregulation and functional improvement in this mouse model is Nurr1-dependent.
Figure 5.
Nurr1-dependent attenuation of DSS-induced colitis by CQ. Male mice were infected with none, scrambled-lentivirus (Scr) or shNurr1-lentivirus (shNurr1) 7 days prior to treatment with water (No DSS) or 3% DSS for 8 days. During the 8 days, each group, Scr + CQ and shNurr1 + CQ, received 50 mg/kg/day of CQ intraperitoneally. Mice were sacrificed at day 8. (A) Body weight change after DSS induction of colitis was evaluated and expressed as a percentage of the initial weight. (B) Colon length was measured. (C) Histological colitis scores were recorded. (D) Representative histologic images of H&E-stained colon sections. (E) MLN CD4+ T cells’ Foxp3 levels were analyzed by flow cytometry. (F) Quantification of results in E. (G) Representative histologic images of Foxp3/Hoechst-stained colon sections. Data are representative of two experiments with ten mice per group. Each error bar represents means ± s.e.m. *P < 0.05.
Discussion
Autoimmune diseases are associated with an imbalance of pathogenic autoreactive effector T cells and protective regulatory T cells. Many immunotherapies for autoimmune diseases have a common goal of recovering self-tolerance and immune homeostasis through a variety of strategies to regulate immune responses toward dominant TREG-mediated regulation31,32. Although a variety of drugs with the potential to modify TREG- and TH17-responses have been developed and approved for the treatment of autoimmune diseases, others are currently being tested in clinical trials. For examples, Norisoboldine, a natural aryl hydrocarbon receptor agonist, promotes TREG differentiation and inhibits collagen-induced arthritis through regulating the balance between TREG and TH17 cells33. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, also attenuated atherosclerotic lesions affecting the TH17/TREG balance in an AMPK-dependent mechanism34. Low-dose IL-2, a growth factor for T cell proliferation, plays an essential role for TREG differentiation, maintenance and expansion, and a clinical trial is underway to demonstrate its potential as a therapeutic agent for 11 autoimmune diseases35. Recently, attempts have been made to find a druggable target that would inhibit the function of TH17 cells in order to treat autoimmune diseases. Digoxin, SR1001, or tRORγt-TMD was identified as inverse agonists that interact physically with the putative RORγt-LBD or competitive inhibition of endogenous RORγt36–38. As such, a number of drug candidates are currently undergoing clinical studies to treat autoimmune diseases, but they have yet to be approved. Here, we demonstrated that CQ, an FDA-approved drug, can regulate the balance of TREG and TH17 cells in a Nurr1-dependent and -independent mechanisms, respectively.
Chloroquine (CQ), developed as an anti-malarial drug almost a century ago, inhibits the parasitic enzyme heme polymerase that converts the toxic heme into non-toxic hemozoin, resulting in the accumulation of toxic heme within the parasite39. It was demonstrated that CQ decreases TH17-related cytokines from PBMCs of SLE and RA patients40 but no mechanism of action was clearly identified. Using high-throughput screening assay systems, we recently identified CQ and two additional FDA-approved drugs (i.e., amodiaquine, and glafenine) that appear to activate the transcriptional activity of Nurr1 via its ligand-binding domain15. In the present study, we provide several lines of evidence that support the notion that CQ modulates autoimmune diseases via regulation of Nurr1’s function in TREG cells. First, using NMR spectroscopy we observed that CQ binds to the helix α12 region located at the C-terminal of Nurr1, and confirmed the importance of these interactions using site-directed mutagenesis studies. Nurr1 is considered to be an orphan receptor because its activity appears to be ligand independent and its structure is considered locked in a constitutively active form41. However, our results show that the transcriptional activity of Nurr1 can be regulated by CQ through direct binding to Nurr1-LBD, as validated by mutational studies. Second, we demonstrated that CQ increases Nurr1 expression in T cells through CREB, resulting in enhanced Foxp3 expression and TREG cell differentiation. Our results are in line with previous findings showing that ectopic expression of Nurr1 in early phases of T cells differentiation activates Foxp3 expression driving the TREG cell developmental program14 and further we identified as-yet unidentified mechanism explaining how CQ increases Nurr1 expression and contributes to TREG cell differentiation. Third, CQ showed inhibitory effects on gene expression and differentiation of pathogenic TH17 cells, suggesting that CQ exhibits T cell subset-specific functional effects. However, by confirming that the high concentration of CQ plays a role in inhibiting the function of all T cell subsets including TREG, we suggest that there is an effective concentration range for each cell type. It may also be a mechanism related to autophagy, a well-known function of CQ. But it has not been elucidated yet. Finally, using the DSS-induced mouse as an IBD model, we found that CQ can effectively improve symptoms of IBD in a Nurr1-dependent manner, strongly suggesting that the CQ-Nurr1 axis is underlying the CQ’s effect in IBD models.
Various autoimmune disease such as colitis, RA, and SLE are known to be caused by different cell types and mechanisms42. Nevertheless, from a T cell perspective, they are commonly induced by TH17 cells and treated by TREG cells. Thus, while further studies are warranted to elucidate the detailed mechanisms of CQ in animal models of other forms of autoimmune diseases, our study is the first to offer experimental evidence that CQ’s mechanism of action in the treatment of autoimmune diseases is through its interaction with Nurr1. Our goal here was to identify the mechanism of action of CQ’s in ameliorating autoimmune diseases. Having demonstrated a Nurr1-dependent mechanism of action supports efforts to develop other Nurr1 targeting drugs offering less off target effects. For instance, since prolonged treatment with CQ impairs autophagy and contributes to undesirable side effecs43 our results support future efforts to develop better agonists/activators of Nurr1 with less side effects for better treatment of autoimmune diseases such IBD.
In sum, for the first time to our knowledge, we demonstrated that CQ can induce Nurr1 function by two mechanisms: (1) direct binding to Nurr1’s LBD and promoting its transcriptional activity and (2) upregulating Nurr1’s expression, leading to induction of Foxp3 expression and TREG cell differentiation. Since TREG cells are critical to maintain tolerance to self-antigens and for protection against autoimmune diseases44, we propose that the induction of Nurr1 function/expression by CQ underlies, at least in part, its effectiveness in the treatment of autoimmune diseases. Importantly, CQ’s functional effect is T cell subset-specific; while it upregulates TREG differentiation and anti-inflammatory cytokine expression it downregulates TH17 differentiation and pro-inflammatory cytokine expression, leading to significant protective effects in autoimmune diseases in a Nurr1-dependent manner, as evidenced by our in vivo analyses using the DSS-induced colitis mouse model. Taken together, these data show a preclinical “proof of concept” that Nurr1 is a viable effective target for the development of mechanism-based therapies for autoimmune diseases.
Methods
Animals
C57BL/6 (8–10 weeks of age, male and female) mice were purchased from The Jackson Laboratory and housed under pathogen-free condition at the Mailman Research Center Animal Care Facility of McLean Hospital. All animal studies were performed in compliance with National Institutes of Health guidelines and were approved by McLean Hospital’s Institutional Animal Care and Use Committee (2015N000002).
Cell sorting and culture condition
Mouse naïve CD4+ T cells were isolated from spleens from C57BL/6 mice on a magnetic-activated cell sorter (MACS) column using CD4, CD25, and CD62L microbeads (Miltenyi Biotec). To isolate CD4+ CD25− CD62L+ T cells, we performed a three-step purification using CD4-negative beads, CD25-positive beads, and CD62L-positive beads. Primary naïve CD4+CD25−CD62L+ T cells (naïve CD4+ T cells) were maintained in complete medium (RPMI1640 containing 10% heat-inactivated FBS, 100 μg/ml penicillin/streptomycin, and 50 mM 2-mercaptoethanol).
T cell differentiation condition
Naïve CD4+ T cells were maintained in complete RPMI1640 medium and stimulated with 1 μg/ml plate-bound anti-CD3 (553057, BD) and 0.5 μg/ml soluble anti-CD28 (553294, BD) under conditions formulated to obtain the following cell types: pathogenic TH17 (IL-23 [20 ng/ml], IL-6 [30 ng/ml], and IL-1β [20 ng/ml] with anti-IL-4/IFN-γ (504108/505706, Biolegend) antibody [2 μg/ml, each]), TH1 (IL-12 [10 ng/ml] and anti-IL-4 antibody), TH2 (IL-4 [40 ng/ml] and anti-IFN-γ antibody), and iTREG (TGF-β1 [5 ng/ml] with or without IL-2 [50 U/ml] with anti-IL-12/IFN-γ antibody [2 μg/ml, each]).
Measurement of cytokines
After 72–96 h incubation, culture supernatants from stimulated CD4+ T cells were analyzed by ELISA for murine IL-2, IL-10, and IL-17A in accordance with the manufacturer’s instructions (eBioscience). After 8 days, DSS mice were euthanized and colon sections were dissected (1.0 cm). Same length of colon sections from each mouse were cultured in complete DMEM media for 24 h and IFN-γ secretion was measured by ELISA from each supernatant.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from cells with a GeneJET RNA Purification Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. cDNA synthesis was performed with an iScriptTM cDNA Synthesis Kit (Bio-Rad). The following primers were used: RORγt forward: 5′-TGCGACTGGAGGACCTTCTA-3′; reverse: 5′-AGACTGTGTGGTTGTTGGCA-3′, IL17A forward: 5′-CTCCAGAAGGCCCTCAGACTAC-3′; reverse: 5′-AGCTTTCCCTCCGCATTGACACAG-3′, Foxp3 forward: 5′-CCCAGGAAAGACAGCAACCTT-3′; reverse: 5′-TTCTCACAACCAGGCCACTTG-3′, Nurr1 forward: 5′-TTCCAATCCGGCAATGACCA-3′; reverse: 5′-TTACCCTCCACTGGGTTGGA-3′, IL-23R forward: 5′-GCCAAGAGAACCATTCCCGA-3′; reverse: 5′-TCAGTGCTACAATCTTCAGAGGACA-3′, CD25 forward: 5′-AAGTGTGGGAAAACGGGGTG-3′; reverse: 5′-GTGGGTTGTGGGAAGTCTGT-3′, FASL forward: 5′-TGGTGGCTCTGGTTGGAATG-3′; reverse: 5′-GGGTTGGCTATTTGCTTTTCA-3′ and GAPDH forward: 5′-TCAACAGCAACTCCCACTCTTCCA-3′; reverse: 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′. qRT-PCR reactions were performed using the CFX ConnectTM Real-Time System (Bio-Rad, Hercules, CA). Calculations of relative expression were performed by using the comparative Ct method, using the naïve CD4+ T cell as a reference control (value = 1).
TREG suppression assays
Conventional CD4+ T cells (Tconv) were labeled with CellTraceTM CFSE (Life Technologies, InvitrogenTM) and stimulated with plate-bound anti-CD3 and soluble CD28 antibodies. The same number of naïve CD4+ T cells were differentiated into iTREG in the presence of different concentrations of CQ for 96 h (iTREG + CQ, unsorted cells). The resulting iTREG + CQ unsorted cells were added to wells of a 96 well plates seeded with CFSE-labeled Tconv cells. The cells were kept in complete medium for 48 h. Natural TREG (nTREG, CD4+ CD25+) cells were isolated by MACS and used as a positive control. CFSE dilution of Tconv cells was analyzed by flow cytometry with gating on CFSE-stained CD4+ T cells.
Nuclear magnetic resonance (NMR) data acquisition and assignments
For data acquisition samples contained 0.5–0.6 mM protein in 20 mM sodium phosphate, pH 7.0 with 50 mM NaCl and 0.01% NaN3 in 90% H2O/10% D2O. Backbone resonance assignments were made using transverse relaxation-optimized spectroscopy. TROSY-based HSQC, HNCACB, HN(CO)CACB and HNCA spectra were obtained using fully deuterated and uniformly 13C/15N-labeled Nurr1-LBD45. Post data acquisition, sequence-specific resonance assignments were performed and ~ 94% completeness of all the main backbone 1HN, 15N, Cα and Cβ atoms were achieved. Molecular interactions between Nurr1-LBD and CQ were determined using two-dimensional TROSY-HSQC spectra of uniformly 15N-labeled Nurr1-LBD (0.1–0.2 mM) and 50 mM stock solution of CQ in DMSO changing the molar ratio (Nurr1-LBD: CQ) from 1: 0 to 1: 5. Initial NMR spectrum of the free protein was recorded using 0.2 mM 15N-labeled Nurr1-LBD after which CQ was added in accordingly. CQ binding sites were mapped on the crystal structure of Nurr1-LBD (PDB code: 1OVL) by analyzing chemical shift perturbations before and after the addition of CQ. All NMR experiments were performed on a Bruker Avance 700 MHz spectrometer equipped with a 5 mm triple resonance, z-axis-gradient cryogenic probe at 298 K. NMRPipe46 was used to process all NMR spectra which were then analyzed using SPARKY 3.113 program (T. D. Goddard and D. G. Kneller, SPARKY 3, University of California).
Luciferase reporter assays
HEK293 cells were transfected with the following plasmids: pCMV-fNurr1 (mouse full-length Nurr1) or pCMV-fNurr1(mt) which contains mutations in the LBD domain and p4xNL3-Luc. The reporter plasmid, p4xNL3-Luc, contains 4 copies of the NBRE-like NL3 motif inserted upstream of luciferase. Relative luciferase activity was measured using a luciferase assay kit and normalized with β-galactosidase activity.
Immunoblot analysis
Cells were lysed in RIPA lysis buffer (Sigma) (with protease inhibitors) and protein concentration determined using the BCA assay (Thermo Fisher Scientific). An equal volume of 6X loading buffer was added to each sample, which were then boiled for 10 min and loaded onto a 4–12% Bis-Tris Plus gel. Proteins were electrophoresed and then transferred to polyvinylidene difluoride membrane. The membrane was probed with mouse anti-Foxp3 (ab36607, Abcam), anti-pCREB (9198, CST), anti-CREB (9197, CST), anti-pSTAT5 (9359, CST), anti-STAT5 (94205, CST), anti-pNFkB(p65) (3033, CST), anti-NFkB(p65) (8242, CST) or anti-GAPDH (sc-32233, SCBT) antibody diluted 1:1000 in blocking solution (PBS containing 0.1% BSA) and subsequently incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham). Bound antibodies were visualized using ECL (Amersham).
Lentivirus production and transduction
Scrambled shRNA (#RHS6848) and specific Nurr1 shRNA cloned into pLKO.1 lentiviral vector were purchased from GE Dharmacon. Knockdown of Nurr1 expression levels was verified by quantitative real-time PCR. Lentiviruses were produced by transfecting HEK293 cells with shRNA plasmid (scrambled or Nurr1) and two helper plasmids (psPAX2 and pMD2.G) using PolyJetTM Reagent (SignaGen Laboratories). The virus-containing medium was harvested 48 h after transfection and then centrifuge at 1,000 rpm for 10 min, and subsequently filtered through a 0.45 μm filter (Millipore). Thereafter, the supernatant was combined with Lenti-X concentrator (Clontech Laboratories) and the mixture was incubated at 4 °C overnight for concentration. After centrifugation (3,000 rpm, 45 min) at 4 °C, the supernatant was carefully removed and then the pellet was suspended in PBS. Finally, the viral titer was determined using the QuickTiter™ Lentivirus Titer Kit (Cell Biolabs) according to the manufacturer’s instruction. Lentivirus transduction into primary T cell was performed with Lentiboost (Sirion Biotech)47 at multiplicity of infection (MOI) of 50 or 100, based on toxicity optimization. After 24 h in culture, cells were washed and then stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibodies. After 96 h, cells were harvested and further prepared for RNA and protein isolation.
Colitis development and histological examination
Mice were infected with ~5 × 108 TU/mouse of scrambled-lentivirus (Scr) or shNurr1-lentivirus (shNurr1) 7 days before treatment. Mice received normal water (No DSS) or 3% dextran sulfate sodium (DSS; MP Biomedicals) water for 8 days and daily injection of CQ (50 mg/kg, ip). Body weight, stool consistency, and blood in the stool were monitored daily. Scoring was as follows: Normal stool with negative hemoccult: 0; Soft stools with positive hemoccult: 1; Very soft stools with traces of blood: 2; Diarrhea with visible rectal bleeding and blood around anus: 348. After 8 days, animals were euthanized, colon length was measured, and colon tissue sections were stained with H&E or Alexa Fluor 488-CD4/Alexa Fluor 568-Foxp3 antibodies.
Flow cytometry analysis
Cells were isolated from mesenteric lymph node and stained with CD4 antibody (17-0042, eBioscience). To facilitate intracellular staining for Foxp3 (563101, BD), cells were fixed and permeabilized using Foxp3 fixation/permeabilization buffers (BD Biosciences) according to the manufacturer’s instructions.
Statistical analysis
Microsoft Excel software (Microsoft Corp.) and GraphPad Prism software were used for all statistical analyses and the specific tests used are described in the figure legends. Student’s t-test was used when comparing two groups or within a group while multigroup comparisons were performed using two-way ANOVA followed by Bonferroni post-hoc test, or one-way ANOVA followed by Tukey’s test.
Supplementary information
Acknowledgements
This work was supported by NIH grants (NS070577, NS084869, and OD024622). We would like to thank all members of the molecular neurobiology laboratory past and present who participated in the project.
Author contributions
T.Y.P., H.S.Y., P.L. and K.S.K. conceived the project and designed the experiments. T.Y.P., Y.J., W.K., J.S., H.T.T. and C.H.K. performed experiments and analyzed the data. T.Y.P., P.L. and K.S.K. wrote the manuscript and all authors contributed to manuscript editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pierre Leblanc and Kwang-Soo Kim.
Contributor Information
Pierre Leblanc, Email: pleblanc@mclean.harvard.edu.
Kwang-Soo Kim, Email: kskim@mclean.harvard.edu.
Supplementary information
is available for this paper at 10.1038/s41598-019-52085-w.
References
- 1.O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N. Engl. J. Med. 2004;350:2591–2602. doi: 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat. Rev. Nephrol. 2011;7:718–729. doi: 10.1038/nrneph.2011.150. [DOI] [PubMed] [Google Scholar]
- 3.O’Dell JR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N. Engl. J. Med. 2013;369:307–318. doi: 10.1056/NEJMoa1303006. [DOI] [PubMed] [Google Scholar]
- 4.Haladyj E, Sikora M, Felis-Giemza A, Olesinska M. Antimalarials – are they effective and safe in rheumatic diseases? Reumatologia. 2018;56:164–173. doi: 10.5114/reum.2018.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen NJ, Schleich MA, Karp DR. Multifaceted effects of hydroxychloroquine in human disease. Semin. Arthritis. Rheum. 2013;43:264–272. doi: 10.1016/j.semarthrit.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Taherian E, Rao A, Malemud CJ, Askari AD. The biological and clinical activity of anti-malarial drugs in autoimmune disorders. Curr. Rheumatol. Rev. 2013;9:45–62. doi: 10.2174/1573397111309010010. [DOI] [PubMed] [Google Scholar]
- 7.Thomé R, Lopes SC, Costa FT, Verinaud L. Chloroquine: modes of action of an undervalued drug. Immunol. Lett. 2013;153:50–57. doi: 10.1016/j.imlet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 8.An N, et al. Chloroquine autophagic inhibition rebalances Th17/Treg-mediated immunity and ameliorates systemic lupus erythematosus. Cell. Physiol. Biochem. 2017;44:412–422. doi: 10.1159/000484955. [DOI] [PubMed] [Google Scholar]
- 9.Thomé R, et al. Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS One. 2013;8:e65913. doi: 10.1371/journal.pone.0065913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zetterström RH, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 11.Kadkhodaei B, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J. Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saijo K, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekiya T, et al. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat. Commun. 2011;2:269. doi: 10.1038/ncomms1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekiya T, et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013;14:230–237. doi: 10.1038/ni.2520. [DOI] [PubMed] [Google Scholar]
- 15.Kim CH, et al. Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2015;112:8756–8761. doi: 10.1073/pnas.1509742112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham C, Cho JH. Inflammatory bowel disease. N. Engl. J. Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 18.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu. Rev. Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389–400. doi: 10.1016/j.immuni.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ML, Sundrud MS. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm. Bowel. Dis. 2016;22:1157–1167. doi: 10.1097/MIB.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt RL, et al. Chloroquine inhibits human CD4+ T-cell activation by AP-1 signaling modulation. Sci. Rep. 2017;7:42191. doi: 10.1038/srep42191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber SM, Chen JM, Levitz SM. Inhibition of mitogen-activated protein kinase signaling by chloroquine. J. Immunol. 2002;168:5303–5309. doi: 10.4049/jimmunol.168.10.5303. [DOI] [PubMed] [Google Scholar]
- 23.Jang CH, Choi JH, Byun MS, Jue DM. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford) 2006;45:703–710. doi: 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- 24.Raveney BJ, Oki S, Yamamura T. Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signaling. PLoS One. 2013;8:e56595. doi: 10.1371/journal.pone.0056595.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signaling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Zhang P, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 27.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 28.Moulton VR, Tsokos GC. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis. Res. Ther. 2011;13:207–216. doi: 10.1186/ar3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEvoy AN, et al. Activation of nuclear orphan receptor Nurr1 transcription by NF-kappa B and cyclic adenosine 5′-monophosphate response element-binding protein in rheumatoid arthritis synovial tissue. J. Immunol. 2002;168:2979–2987. doi: 10.4049/jimmunol.168.6.2979. [DOI] [PubMed] [Google Scholar]
- 30.Nagar J, et al. Therapeutic potential of chloroquine in a murine model of inflammatory bowel disease. Int. Immunopharmacol. 2014;21:328–335. doi: 10.1016/j.intimp.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Bluestone JA, Bour-Jordan H, Cheng M, Anderson M. T cells in the control of organ-specific autoimmunity. J. Clin. Invest. 2015;125:2250–2260. doi: 10.1172/JCI78089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, et al. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528:225–230. doi: 10.1038/nature16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong B, et al. Norisoboldine ameliorates collagen-induced arthritis through regulating the balance between Th17 and regulatory T cells in gut-associated lymphoid tissues. Toxicol. Appl. Pharmacol. 2015;282:90–99. doi: 10.1016/j.taap.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Tian Y, et al. Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanism. Cardiovasc. Diabetol. 2017;16:140. doi: 10.1186/s12933-017-0623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenzwajg M, et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann Rheum Dis. 2019;78:209–217. doi: 10.1136/annrheumdis-2018-214229. [DOI] [PubMed] [Google Scholar]
- 36.Huh JR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solt LA, et al. Supression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park TY, et al. RORγt-specific transcriptional interactomic inhibition suppresses autoimmunity associated with TH17 cells. Proc. Natl. Acad. Sci. USA. 2014;111:18673–18678. doi: 10.1073/pnas.1413687112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fong KY, Wright DW. Hemozoin and antimalarial drug discovery. Future. Med. Chem. 2013;5:1437–1450. doi: 10.4155/fmc.13.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva JC, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo) 2013;68:766–771. doi: 10.6061/clinics/2013(06)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2010;30:1535–1541. doi: 10.1161/ATVBAHA.109.191163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat. Immunol. 2017;18:716–724. doi: 10.1038/ni.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17:277–285. doi: 10.1038/ni.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyara M, Ito Y, Sakaguchi S. TREG-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol. 2014;10:543–551. doi: 10.1038/nrrheum.2014.105. [DOI] [PubMed] [Google Scholar]
- 45.Pervushin K, Riek R, Wider G, Wüthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaglio F, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 47.Delville M, et al. A nontoxic transduction enhancer enables highly efficient lentiviral transduction of primary murine T cells and hematopoietic stem cells. Mol. Ther. Methods. Clin. Dev. 2018;10:341–347. doi: 10.1016/j.omtm.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014;104:Unit-15.25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.